Abstract
Chronic granulomatous disease (CGD) is an inherited disorder characterized by recurrent infections and deregulated inflammatory responses. CGD is caused by mutations in subunits of the NADPH oxidase, an enzyme that generates reactive oxygen species in phagocytes. To elucidate the contribution of the proinflammatory protease caspase-1 to aberrant inflammatory reactions in CGD, we analyzed cells isolated from patients with defects in the phagocyte oxidase subunits p22phox, p47phox or gp91phox. We report that mononuclear phagocytes from CGD patients activated caspase-1 and produced biologically active interleukin-1β (IL-1β) in response to danger signals. Notably, caspase-1 activation and IL-1β secretion from CGD monocytes was elevated in asymptomatic patients and strongly increased in patients with noninfectious inflammatory conditions. Treatment with IL-1 receptor antagonist reduced IL-1 production in monocytes ex vivo and during medical therapy. Our results identify phagocyte oxidase defective monocytes as a source of elevated IL-1 and provide a potential therapeutic option to ameliorate inflammatory conditions associated with CGD.
Introduction
Chronic granulomatous disease (CGD) is a genetically heterogeneous primary immunodeficiency caused by defects in phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits.1 This phagocyte oxidase generates superoxide by transferring electrons from NADPH to molecular oxygen and consists of the catalytic subunit gp91phox, structurally stabilized by p22phox, and of the regulatory subunits p47phox, p40phox, p67, and RAC.2 Loss-of-function mutations in any of these components abrogate oxidase activity and compromise host immunity against certain bacteria and fungi. However, there is now increasing evidence for excessive inflammation in CGD even in the absence of infectious agents with increased frequency and severity of granulomatous inflammatory reactions, notably colitis.3–5
Inflammasomes are implicated in host protection and a variety of inflammatory diseases by regulating the maturation of the caspase-1 dependent cytokines interleukin-1β (IL-1β) and IL-18 in response to a broad range of danger signals.6 However, the function of the phagocyte oxidase during caspase-1–mediated proinflammatory responses is controversial, and the implications of such responses for CGD pathology are poorly understood.7
Here we evaluated the contribution of the phagocyte oxidase to the exuberant inflammatory responses associated with CGD, by analyzing inflammasome activation in cells from CGD patients. We show that mononuclear phagocytes from CGD patients activate caspase-1 and produce biologically active IL-1β in response to danger signals. Notably, IL-1 release from monocytes was elevated in CGD patients and could be controlled by IL-1 receptor antagonist (IL-1Ra) ex vivo and during treatment with anakinra. Our results implicate caspase-1–mediated inflammation in CGD pathogenesis and may help designing therapies to ameliorate inflammatory conditions in these patients.
Methods
Human monocytes and macrophages
All experiments with human samples were done after informed consent was received from the patients and parents in accordance with the Declaration of Helsinki, as part of a protocol approved by the institutional review board of the University Children's Hospital Zurich. Blood from CGD patients, heterozygous carriers, and healthy donors (supplemental Tables 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) was drawn on ethylenediaminetetraacetic acid (EDTA) and diluted in phosphate-buffered saline (PBS) supplemented with 2mM EDTA, pH 7.2 (PBS/EDTA). Up to 30 mL of blood were layered on 15 mL of Histopaque (density = 1.077 g/mL) and centrifuged at 400g and 21°C for 30 minutes. The resulting interphase containing peripheral blood mononuclear cells (PBMCs) was isolated and washed with PBS/EDTA at 400g and 21°C for 10 minutes. Platelets were removed by repeated centrifugation at 200g for 15 minutes. Monocytes were isolated from PBMCs by depletion of nonmonocytes (negative selection) using the Monocyte Isolation Kit II (Miltenyi Biotec; 130-091-153) according to the manufacturer's instructions. Monocytes were then cultivated in RPMI containing penicillin/streptomycin and 5% human serum (Sigma-Aldrich). Monocytes were used for experiments the next day. For experiments with monocyte-derived macrophages, monocytes were differentiated into macrophages in RPMI containing penicillin/streptomycin and 5% human serum with 5 ng/mL macrophage colony stimulating factor (M-CSF; Miltenyi Biotec) for 6 days. Purity of monocytes and macrophages was more than 96% as determined by fluorescent-activated cell sorter using anti-CD14 antibody (Miltenyi Biotec; 130-080-701).
Caspase-1 activation assays
Monocytes were stimulated with 500 ng/mL lipopolysaccharide (LPS; from Salmonella typhimurium; Alexis Biochemicals) or 100 μg/mL monosodium urea (MSU). Monocyte-derived macrophages were primed with 500 ng/mL LPS for 3 hours before addition of the indicated inflammasome activators: adenosine triphosphate (ATP; 2mM) from Roche; nigericin (2μM) and silica (100 μg/mL) from Sigma-Aldrich; MSU (100 μg/mL) and prepared as described.8 Caspase-1 activity was assessed by fluorescent-activated cell sorter using caspase-1 FLICA (Immunochemistry Technologies) according to the manufacturer's instructions. Human mature IL-1β, IL-1α, tumor necrosis factor, and IL-6 were determined in cell supernatants and IL-1β precursor in cell lysates by enzyme-linked immunosorbent assay (BD Biosciences and R&D Systems, respectively). To interfere with IL-1 ex vivo, 1 to 10μM YVAD-cmk (Alexis Biochemicals) or 1 to 10 ng/mL anakinra (Amgen/Biovitrum) was used.
Medical treatment with IL-1Ra
Treatment of a patient with IL-1Ra was performed after informed consent was received from the patients and parents by daily subcutanous injection of 15 mg of anakinra for 8 days, before conditioning for bone marrow transplantation.
Results and discussion
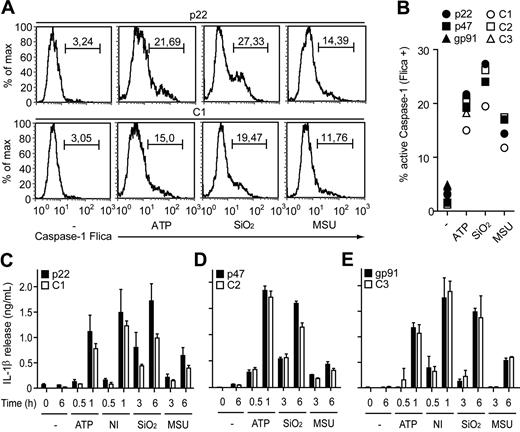
To analyze the impact of the phagocyte oxidase on caspase-1–mediated inflammation we tested human mononuclear phagocytes from asymptomatic CGD patients, heterozygous carriers, and healthy controls for their responses to inflammasome activation (supplemental Tables 1-2). Interestingly, macrophages with a defect in any of the phagocyte oxidase subunits p22phox, p47phox, or gp91phox responded to danger signals such as extracellular ATP, the pore-forming toxin nigericin, crystals of uric acid (monosodium urea, MSU), or silica with robust caspase-1 activation (Figure 1A-B). CGD macrophages also secreted biologically active IL-1β in response to these activators at levels comparable with healthy controls, indicating that a functional phagocyte oxidase is dispensable for caspase-1–mediated inflammatory responses in human macrophages (Figure 1C-E).
Macrophages from CGD patients activate caspase-1 and secrete mature IL-1β. (A-B) Caspase-1 activation in monocyte-derived macrophages determined by a fluorescent inhibitor of active caspase-1 (FLICA). (A) Lipopolysaccharide (LPS)–primed (−) macrophages from a chronic granulomatous disease (CGD) patient (p22) and a healthy control (C1) stimulated for 1 hour with adenosine triphosphate (ATP) or for 6 hours with silica crystals (SiO2) or monosodium urea (MSU) crystals. Numbers above bracketed lines indicate percentage of cells with active caspase-1. (B) Active caspase-1 in macrophages from 3 CGD patients with the indicated mutations and 3 healthy controls (C1-C3) quantified by caspase-1 FLICA. (C-E) Macrophages from the indicated CGD patients and healthy donors stimulated with LPS plus ATP, nigericin (NI), SiO2, or MSU. The production of mature interleukin-1β (IL-1β) at the indicated time points was determined by enzyme-linked immunosorbent assay. Data are representative of 6 experiments with cells from at least 5 different CGD patients (error bars indicate SEM of triplicate wells).
Macrophages from CGD patients activate caspase-1 and secrete mature IL-1β. (A-B) Caspase-1 activation in monocyte-derived macrophages determined by a fluorescent inhibitor of active caspase-1 (FLICA). (A) Lipopolysaccharide (LPS)–primed (−) macrophages from a chronic granulomatous disease (CGD) patient (p22) and a healthy control (C1) stimulated for 1 hour with adenosine triphosphate (ATP) or for 6 hours with silica crystals (SiO2) or monosodium urea (MSU) crystals. Numbers above bracketed lines indicate percentage of cells with active caspase-1. (B) Active caspase-1 in macrophages from 3 CGD patients with the indicated mutations and 3 healthy controls (C1-C3) quantified by caspase-1 FLICA. (C-E) Macrophages from the indicated CGD patients and healthy donors stimulated with LPS plus ATP, nigericin (NI), SiO2, or MSU. The production of mature interleukin-1β (IL-1β) at the indicated time points was determined by enzyme-linked immunosorbent assay. Data are representative of 6 experiments with cells from at least 5 different CGD patients (error bars indicate SEM of triplicate wells).
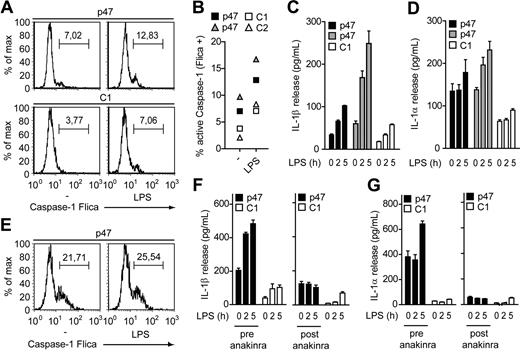
Because monocytes have a specific function in innate immunity and activate caspase-1 by a unique mechanism such as a single Toll-like receptor 4 (TLR4) stimulation, we next analyzed IL-1 production in CGD monocytes.9 Notably, caspase-1 activation and IL-1 secretion were elevated in unstimulated CGD monocytes and in response to LPS or MSU (Figure 2A-D, supplemental Figures 1-2). Consistently, IL-1 levels were substantially reduced ex vivo by inhibiting caspase-1 with a specific inhibitor or by blocking IL-1 signaling with the naturally occurring IL-1Ra (supplemental Figures 1,3).10,11
Elevated IL-1 secretion from CGD monocytes can be counteracted with IL-1Ra. (A-B) Caspase-1 activation in monocytes determined by caspase-1 FLICA. (A) Unstimulated (−) and LPS-treated (6 hours) monocytes from an asymptomatic CGD patient (p47) and a healthy control (C1). (B) Active caspase-1 in monocytes from 2 asmyptomatic CGD patients with the indicated mutation and 2 healthy controls (C1, C2) quantified by caspase-1 FLICA. (C-D) IL-1β (C) and IL-1α (D) release from monocytes of 2 asymptomatic CGD patients and a healthy control (C1) treated with LPS for the indicated time points determined by enzyme-linked immunosorbent assay. (E) Caspase-1 activation in unstimulated and LPS-treated (6 hours) monocytes from a symptomatic CGD patient with colitis determined by caspase-1 FLICA. (F-G) IL-1β (F) and IL-1α (G) release from monocytes of the indicated symptomatic CGD patient before (pre-anakinra) and after (post-anakinra) treatment with anakinra compared with a healthy control (C1). Data are representative of 4 (A-D) or 3 (E) experiments with cells from at least 2 different CGD patients (error bars indicate SEM of triplicate wells).
Elevated IL-1 secretion from CGD monocytes can be counteracted with IL-1Ra. (A-B) Caspase-1 activation in monocytes determined by caspase-1 FLICA. (A) Unstimulated (−) and LPS-treated (6 hours) monocytes from an asymptomatic CGD patient (p47) and a healthy control (C1). (B) Active caspase-1 in monocytes from 2 asmyptomatic CGD patients with the indicated mutation and 2 healthy controls (C1, C2) quantified by caspase-1 FLICA. (C-D) IL-1β (C) and IL-1α (D) release from monocytes of 2 asymptomatic CGD patients and a healthy control (C1) treated with LPS for the indicated time points determined by enzyme-linked immunosorbent assay. (E) Caspase-1 activation in unstimulated and LPS-treated (6 hours) monocytes from a symptomatic CGD patient with colitis determined by caspase-1 FLICA. (F-G) IL-1β (F) and IL-1α (G) release from monocytes of the indicated symptomatic CGD patient before (pre-anakinra) and after (post-anakinra) treatment with anakinra compared with a healthy control (C1). Data are representative of 4 (A-D) or 3 (E) experiments with cells from at least 2 different CGD patients (error bars indicate SEM of triplicate wells).
Based on these findings, we wondered whether the hyperinflammatory condition in symptomatic CGD patients involves a deregulation of IL-1 and analyzed monocytes from patients with granulomatous colitis, a common gastrointestinal manifestation in CGD that involves inflammation in the absence of infectious agents.5 Intriguingly, both, unstimulated and LPS-stimulated monocytes from symptomatic CGD patients showed strongly increased caspase-1 and IL-1 levels, indicating that caspase-1 is active during severe colitis in CGD despite the loss of phagocyte oxidase function (Figure 2E, supplemental Figure 4).12 Symptomatic CGD patients with clinical symptoms distinct from colitis also show increased IL-1 production and could be counteracted by inhibition of caspase-1 or IL-1 signaling ex vivo (supplemental Figure 4). In agreement with previous studies, further analysis confirmed a differential preactivation of CGD monocytes as characterized by increased levels of tumor necrosis factor, IL-6, and IL-1β precursor that may reflect the clinical situation of the individual patient (supplemental Figure 5).13
Our observations prompted us to test IL-1Ra (anakinra/Kinert) for the treatment of noninfectious colitis in CGD. Interestingly, within 1 week of daily administration of anakinra, IL-1 production in monocytes dropped substantially, suggesting that deregulated IL-1 production is associated with the aberrant inflammatory manifestation associated with CGD (Figure 2F-G). However, clinical improvement only consisted of reduced frequency of abdominal pain crises and not of a reduced frequency of stools per day, possibly due to slow resolution of the chronic inflammatory lesions during the short time of treatment with anakinra.
The impact of the phagocyte oxidase during inflammasome activation is controversial due to use of different model systems. Because phagocytosis activates the phagocyte oxidase, it was suggested that a functional phagocyte oxidase is particularly important for caspase-1 activation induced by particles.6,14 Our data indicate that both inflammasome stimuli that require phagocytosis, such as MSU or silica, as well as those that do not require phagocytosis, like ATP or nigericin, activate caspase-1 independently of the phagocyte oxidase. Our study thus confirms that macrophages from CGD patients respond to inflammasome activation similarly to macrophages from gp91phox deficient mice, which also activate caspase-1 robustly.8,15,16 Notably, the results presented here contrast with those of previous studies based on pharmacologic inhibition of the phagocyte oxidase or p22phox knockdown in THP-1 cells.17–19 In fact, pharmacologic inhibition and enzymatic degradation of reactive oxygen species can act oppositionally on inflammasome activation, warranting a careful consideration for the use of these inhibitors.15,19 Accordingly, enzymes that maintain the redox homeostasis rather potentiate IL-1β processing and inflammation.15,20 The discrepancy of results obtained by individual experimental designs underscores the importance of studying human pathology using unmanipulated primary cells from healthy donors and diseased patients.
More importantly, our study together with a recent report by van de Veerdonk et al21 identifies CGD monocytes as a source of increased IL-1 and is consistent with the observation that inflammatory markers are elevated in CGD.13,22,23 Along with other recent findings, our results extend the initial conception of the phagocyte oxidase primarily as a source of harmful mediators by suggesting an anti-inflammatory function via inflammasome regulation in monocytes.5,24,25 Because IL-1 is a pleiotropic cytokine involved in the induction of other inflammatory molecules and the regulation of lymphocyte function, our data open up new potential strategies for therapeutic intervention by targeting IL-1 for inflammatory noninfectious complications in CGD.11
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to all CGD patients, relatives, and blood donors for their participation in this study. We further thank the members of the Zychlinsky laboratory for discussion and Matteo Bianchi for technical assistance.
This work was supported by a grant of the Chronic Granulomatous Disorder Research Trust, United Kingdom (J.R.) and a grant from the Stiftung für wissenschaftliche Forschung an der Universität Zürich/Baugarten Stiftung (R.A.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
National Institutes of Health
Authorship
Contribution: F.M. conducted all experiments, wrote the manuscript, and conceived the research with A.Z.; and J.R. designed the clinical anakinra treatment, contributed to the writing of the manuscript, and attended the patients together with R.A.S., D.M., and A.F.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arturo Zychlinsky, Department of Cellular Microbiology, Max Planck Institute for Infection Biology, Chariteplatz 1, Berlin 10117, Germany; e-mail: zychlinsky@mpiib-berlin.mpg.de; or Janine Reichenbach, Division of Immunology/Haematology/BMT, University Children's Hospital Zurich, Steinwiesstrasse 75, 8032 Zurich, Switzerland; e-mail: janine.reichenbach@kispi.uzh.ch.
References
Author notes
J.R. and A.Z. contributed equally to the work.