Abstract
Pulmonary arterial hypertension (PAH) is suspected to be a strong mortality determinant of hemolytic disorders. However, direct contribution of acute intravascular hemolysis to fatal PAH has not been investigated. The roles of nitric oxide (NO) insufficiency and platelet activation in hemolysis-associated fatal PAH have been suspected but not been experimentally studied. We recently generated a unique intravascular hemolysis mouse model in which the membrane toxin, intermedilysin (ILY), exclusively lyses the erythrocytes of transgenically expressing human CD59 mice (ThCD59RBC), thereby inducing ILY-dose–dependent massive hemolysis. Using this murine hemolysis model, we found that the acute increase in pulmonary arterial pressure leading to right ventricle failure caused sudden death. Reduced NO bioavailability and massive platelet activation/aggregation leading to the formation of massive thrombosis specifically in the pulmonary microvasculature played the critical roles in pathogenesis of acute hemolysis-associated fatal PAH. Therapeutic interventions enhancing NO bioactivity or inhibiting platelet activation prevented sudden death or prolonged survival time via the suppression of the acute increase in pulmonary arterial pressure and improvement of right ventricle function. These findings further highlight the importance of the inhibition of platelet activation and the enhancement of NO bioavailability for the treatment and prevention of hemolysis-associated (fatal) PAH.
Introduction
Intravascular hemolysis presents with pleiotropic clinical manifestations including anemia, abdominal pain, dysphagia, erectile dysfunction, pulmonary arterial hypertension (PAH), renal failure, and platelet activation and thrombosis, all of which are considered direct sequelae of erythrocyte lysis.1,2 Another known consequence of intravascular hemolysis is unexpected sudden death,2–5 which accounts for 12% to 26% of all deaths in patients with sickle cell disease (SCD),6–10 and is frequently associated with other hemolytic diseases such as thalassemia, hemolytic uremic syndrome (HUS), paroxysmal nocturnal hemoglobinuria (PNH), ABO mismatch transfusion reaction, and autoimmune hemolytic anemia.11 Although the mechanism(s) of hemolysis-associated sudden death is poorly defined, PAH with characteristic histopathologic findings including plexiform, concentric medial hyperplastic pulmonary vascular lesions, and in situ pulmonary arterial thrombosis5,12–14 is suspected to be a strong, albeit still unproven, cause of death in acute hemolytic disorders.5,12–15
Nitric oxide (NO) insufficiency is suspected to play an important role in the pathogenesis of hemolysis-mediated PAH.1 NO produced by the endothelium plays a fundamental role in regulating basal vasomotor tone, blood pressure, and organ blood flow distribution; it also inhibits platelet activation and expression of molecules that promote adhesion of platelets and leukocytes to the endothelium.1,16,17 NO-induced vasodilation derives from its stimulatory effect on soluble guanylyl cyclase with subsequent formation of cyclic GMP (cGMP) causing vascular smooth muscle relaxation. NO bioavailability can be dramatically reduced by the release of cell free hemoglobin. The rate of NO reacting with hemoglobin encapsulated within erythrocytes is reduced by nearly 3 orders of magnitude by major diffusion barriers imposed by a cell-free zone along the endothelium in laminar flowing blood and the unstirred layer, an area of nonflowing blood around the external surface of the erythrocytes.18 The release of hemoglobin into plasma during intravascular hemolysis removes these diffusion barriers, increasing the scavenging of NO. The reduced NO bioavailability leads to a clinical state of endothelial dysfunction, smooth muscle constriction, and increased peripheral resistance, which in the pulmonary circulation are likely involved in the development of hemolysis-associated PAH.12 Platelet activation and thrombus formation is another consequence of intravascular hemolysis. Increased platelet activation, well established in patients with hemolytic anemias such as SCD, β-thalassemia, and PNH,19,20 is another pathogenic factor that contributes to the clinical manifestations of these disorders including PAH.20,21 For example, new and old thrombi in the pulmonary vasculature were found by autopsy in up to 75% of SCD.4,22 Despite strong clinical evidence, investigations on either the role of platelet activation in hemolysis-associated PAH or effective therapies have been hampered by practical and ethical challenges and the lack of adequate animal models. As a consequence, current treatment for PAH associated with hemolytic disorders is based on limited evidence or expert opinion.15 These considerations highlight the need for reliable and reproducible animal models of hemolysis-associated fatal PAH. To date, several animal models, such as the free water-induced intravascular hemolysis dog model and the SCD and incompatible blood transfusion mouse models, have provided many insights into the pathogenesis of hemolysis and hemolysis-associated PAH.23–25 However, they have not been used to investigate the direct contribution of hemolysis-associated fatal PAH to the pathogenesis of sudden death in hemolytic disorders. Moreover, the precise underlying mechanisms of NO insufficiency and platelet activation in hemolysis-associated PAH and/or sudden death still require further investigation.
Recently, we generated an intravascular hemolysis model in which varying degrees of hemolysis can be conditionally induced by the administration of intermedilysin (ILY) to transgenic mice expressing human CD59 (hCD59) only in their erythrocytes (ThCD59RBC).26 ILY, a bacterial pore-forming toxin, is potently hemolytic to human and to mouse erythrocytes transgenically expressing hCD59, but not at all hemolytic in other animal species including wild-type (WT) mice,26,27 through specific interaction of ILY with hCD59.26,28 Using this model, we document here that reduced NO bioavailability and platelet activation/aggregation play a critical role in the pathogenesis of hemolysis-associated fatal PAH.
Methods
Animal studies were approved by Harvard Medical School Institutional Animal Care and Use Committee.
Preparation of recombinant ILY
We purified His-tagged recombinant ILY as described previously.28
Hypotonics, pneumolysin, and melittin-mediated hemolysis on ThCD59RBC erythrocytes
Pneumolysin (PLY) and melittin were purchased from Dr James C. Paton's laboratory (School of Molecular and Biomedical Science, University of Adelaide) and Calbiochem, respectively. The erythrocytes were collected by phlebotomy through the vena cava with EDTA (5mM; ethylenediaminetetraacetic acid) as an anticoagulant, were washed twice with phosphate-buffered saline (PBS), and were resuspended in 150mM sodium chloride. The erythrocytes (vol/vol, 1%) were incubated in serially diluted sodium chloride (in double-distilled H2O), PLY (in PBS), or melittin (in PBS) in 200 μL of total volume at 37°C for 30 minutes. After centrifugation, 150 μL of supernatants were used to read optical density at 414 nm (OD414). The supernatants from the erythrocytes incubated with 150mM sodium chloride or double-distilled H2O were used as blank or total lysis control, respectively. The percentage hemolysis was calculated as [(experimental OD414 − blank OD414)/(total lysis OD414 − blank OD414)] × 100.
Administration of ILY and evaluation of mouse death
The volume of ILY injected was 5 μL/g body weight, and the concentration of ILY in the injection buffer (PBS) was adjusted according to experimental objectives so that the volume remained constant. The tail vein injection was used to administer ILY with constant velocity, which also applied to jugular vein injection except this treatment was used for the right ventricular systolic pressure (RVSP) measurement and echocardiographic analysis as described below. The constant velocities of rapid and slow tail-vein injection were 10 μL/s and 1.67 μL/s, respectively. We observed the number of surviving and dead mice and the survival time in the mice that died within 15 minutes after ILY injection.
Pretreatment with sildenafil and antithrombotic agents
Before the administration of ILY, we pretreated mice orally with sildenafil (100 μg/g/day; Pfizer) through gavage for 10 days, clopidogrel (30 μg/g; Sigma-Aldrich) orally through gavage at 1 hour, integrilin (10 μg/g; Schering Corporation) by intravenous injection at 5 minutes, neuraminidase (type VI, Clostridium perfringens; dissolved in sterile 0.9% NaCl, 0.05 U/mouse; Sigma-Aldrich) intraperitoneally at 24 hours, sodium heparin (5 U/g; American Pharmaceutical Partners), or hirudin (1 mg/kg; Cabiochem) by intravenous injection at 5 minutes.
Right heart pressure measurements
Mice were anesthetized with a ketamine (90 mg/kg) and xyaline (10 mg/kg). An incision was made on the ventral side of the neck to expose the right jugular vein. An Ultra-Miniature Mikro-Tip Pressure Transducer 1.4F Catheter (Millar Instruments) was inserted into the right jugular vein and advanced into the right ventricle. Right ventricular pressures were measured for approximately 5 minutes using the MPVS 400 system (Millar Instruments) to establish the baseline. The left jugular vein was exposed, and a cannula was inserted and secured in the vein. ILY and vehicle were administered via the left jugular vein while monitoring the changes in right ventricular pressure to the treatment. RVSP was used as a surrogate for pulmonary artery systolic pressure.
Systemic blood pressure measurements
Systemic blood pressure was measured by tail-cuff plethysmography as described previously.29 Mice were anesthetized with ketamine (90 mg/kg) and xyaline (10 mg/kg). Systemic blood pressure was measured for approximately 5 minutes using the BP-2000 Series II Blood Pressure Analysis System (Visitech Systems) to establish the baseline. The left jugular vein was exposed, and a cannula was inserted and secured in the vein. ILY and vehicle were administered into the left jugular vein while monitoring the changes in systemic pressure to the treatment.
Echocardiographic analysis
Mice were anesthetized with a 100% O2 plus 2% isoflurane mixture. Imaging analysis was carried out using a VEVO 2100 System with a 55-MHz probe, and the VEVO Integrated Rail System (Visualsonics). Images were taken along the parasternal long-axis view in B-Mode. Changes in the right ventricle (area) at end-diastole were monitored as ILY was injected into the left jugular vein.
Hematocrit, plasma free hemoglobin, NO metabolites, sP-selectin, and thrombin-antithrombin III complexes measurement
We collected the blood and plasma for the measurements from the ThCD59RBC immediately after ILY140FI and 15 minutes after ILY140SI or ILY70FI, as well as from the WT immediately after ILY140FI. We measured these parameters as described previously.26
Evans blue aortic staining
We measured these parameters as described previously.26
Platelet count
To exclude the interference of the released erythrocyte fragments in the regular complete blood count procedure, we used a BD Unopette System Test to count platelets in a hemacytometer by microscopy, according to the manufacturer's instructions.
Tissue histology
Lung, liver, kidney, and heart sections were processed and stained with an anti–P-selectin (C20; Santa Cruz Biotechnology), rabbit anti–integrin αIIb30 (sc-15 328; Santa Cruz Biotechnology), or rabbit anti–von Willebrand factor (VWF)26 (Dako) antibody, 1:50 dilution, followed by the goat avidin-biotin complex (ABC) staining system (sc-2023 or sc-2018; Santa Cruz Biotechnology). Heart sections were stained with mouse anti-hCD59 monoclonal antibody Bric 229 (International Blood Group Reference Laboratory) and fluorescein isothiocyanate–conjugated horse anti–mouse secondary antibody as previously described.26
Detection of circulating endothelial cells
We detected circulating endothelial cells as described previously.26
cGMP measurement
The cGMP Enzyme Immunoassay kit (Sigma-Aldrich) was used to measure the cGMP levels in the lung following the manufacturer's instruction. Briefly, lung samples were harvested and stored in liquid nitrogen. The tissues were weighed and then homogenized in 10 volumes of 0.1M HCl and further centrifuged at 6000g at room temperature. The supernatant was collected and diluted by 0.1M HCl (1:1, vol/vol) for protein concentration measurement, and the cGMP level was determined quantitatively by the following competitive immunoassay as recommended by the manufacturer.
Statistical analysis
Experimental results are shown as the mean plus or minus SEM. The comparison between 2 or 3 groups was examined with a nonparametric Mann-Whitney test or nonparametric Kruskal-Wallis test, respectively. The comparison of maximal right ventricle area among the groups and the elapsed time until maximum right ventricle dilation was analyzed by one-way analysis of variance (ANOVA) followed by a pairwise Student-Newman-Keuls method and t test, respectively. The difference in the mortality rate among groups was analyzed by χ2 test. All statistical tests with P less than .05 were considered significant.
Results
Further characterization of the ILY-mediated hemolysis model
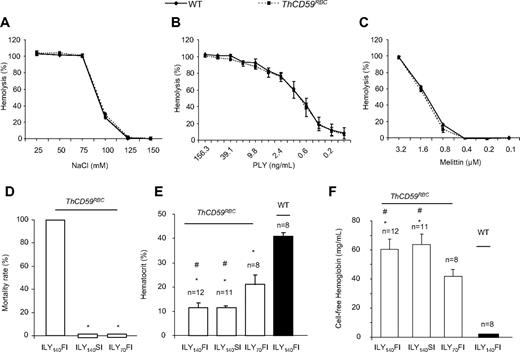
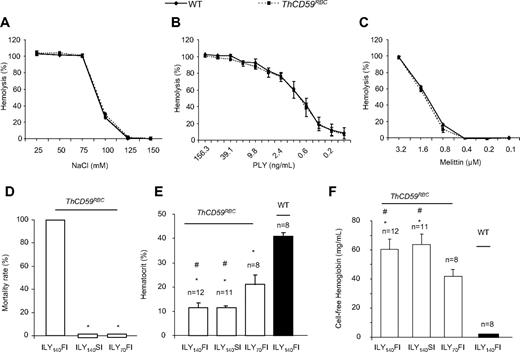
We have previously reported the generation of an ILY-mediated rapid, specific, and conditional erythrocyte ablation model using ThCD59RBC mice that specifically express hCD59 on erythrocytes under the control of the α-hemoglobin promoter.26 Intravenous injection of ILY in ThCD59RBC mice induced acute intravascular hemolysis, leading to reduced NO bioavailability, increased platelet activation, and rapid death. The specificity of ILY-mediated intravascular hemolysis in ThCD59RBC mice was demonstrated by the absence of hemolysis in WT mice even after injecting up to a 500-fold higher dose of ILY, and by abrogation of the ILY lytic effect on ThCD59RBC by pretreatment with anti-ILY–specific antibodies.26 Here, we further demonstrate that ThCD59RBC erythrocytes exhibit osmotic fragility and increase sensitivity to colloidosmotic lysis induced by either pneumolysin or melittin that are similar to WT erythrocytes (Figure 1A-C). These results confirm that the expression of hCD59 in ThCD59RBC erythrocytes does not itself confer a generally increased susceptibility to lytic challenges and that the lytic effect of ILY is limited to mouse erythrocytes expressing hCD59 transgenically.
Further characterization of ILY-mediated hemolysis mouse model. (A-C) ThCD59RBC erythrocyte susceptibility to lysis by different concentrations of NaCl (A), mellitin (B), or PLY (C) was similar to that of WT erythrocytes. Erythrocytes were incubated in serial concentrations of NaCl, mellitin, or PLY at 37°C for 30 minutes. Results represent mean ± SEM (n = 3, P > .05). (D) ILY-mediated hemolysis-associated sudden death. ILY140FI induced 100% (12/12) mortality, but ILY140SI (0/12) and ILY70FI (0/8) did not cause death in any mouse. *P < .001 vs the mice with ILY140FI. (E-F) Hematocrit- and plasma-free hemoglobin levels in the ThCD59RBC and WT mice administered ILY injection. *P < .001 vs WT mice and #P < .01 vs the ThCD59RBC mice administered ILY70FI. Results represent mean ± SEM.
Further characterization of ILY-mediated hemolysis mouse model. (A-C) ThCD59RBC erythrocyte susceptibility to lysis by different concentrations of NaCl (A), mellitin (B), or PLY (C) was similar to that of WT erythrocytes. Erythrocytes were incubated in serial concentrations of NaCl, mellitin, or PLY at 37°C for 30 minutes. Results represent mean ± SEM (n = 3, P > .05). (D) ILY-mediated hemolysis-associated sudden death. ILY140FI induced 100% (12/12) mortality, but ILY140SI (0/12) and ILY70FI (0/8) did not cause death in any mouse. *P < .001 vs the mice with ILY140FI. (E-F) Hematocrit- and plasma-free hemoglobin levels in the ThCD59RBC and WT mice administered ILY injection. *P < .001 vs WT mice and #P < .01 vs the ThCD59RBC mice administered ILY70FI. Results represent mean ± SEM.
Interestingly, we observed that the minimal dose of ILY needed to kill all mice with a single injection (1LD100) was highly dependent on the rate of injection. Injection of 140 ng/g (1LD100 for this lot) of ILY within 10 seconds (which we denote “fast ILY injection” or ILY140FI) induced 100% death in approximate 70 seconds. In contrast, injection of the same dose of ILY in 60 seconds (denoted “slow ILY injection” or ILY140SI) did not cause any mouse death (Figure 1D), even when the ultimate degree of hemolysis achieved was similar after ILY140FI and ILY140SI (Figure 1E-F). The injection of 70 ng/g ILY with the rapid (ie, within 10 seconds) injection (referred to as ILY70FI) did not induce death (Figure 1D) with lower overall extent of hemolysis compared with either ILY140FI or ILY140SI (Figure 1E-F).
Because hCD59 protein expressed in mouse erythrocytes under the control of the hemoglobin promoter can “jump” to the endothelium probably through its glycosyl-phosphatidylinositol (GPI) membrane anchor31 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), we investigated whether ILY would trigger off-target effects on the endothelium of ThCD59RBC mice. Intravenous administration of 60 ng/g ILY, a dose that induced massive intravascular hemolysis in ThCD59RBC mice, did not affect the plasma levels of VWF, a biomarker for endothelial dysfunction32,33 at 15 minutes, 3 hours, or 6 hours after injection, compared with the levels in similarly injected WT mice (supplemental Figure 2A). In addition, WT and ThCD59RBC mice injected with ILY140SI showed no difference in the number of circulating endothelial cells (data not shown) or in visible Evans blue staining of aortic walls (supplemental Figure 2B), as measured 2 hours after the injection. In contrast, injecting ILY into the transgenic mice that selectively express hCD59 on endothelial and hematopoietic cells (ThCD59END)26,33 caused death in all animals at a dose of 7.5 ng/g (15-fold lower than the LD100 in ThCD59RBC) and independently of the speed of injection. In addition, injection of a lower, sublethal dose of ILY into ThCD59END mice caused a significant increase in the number of circulating endothelial cells and in visible Evans blue staining of the aorta, both indicative of endothelial damage.26 We also demonstrated that hCD59 is absent in platelets from ThCD59RBC mice, confirming the specific expression of hCD59 on erythrocytes (supplemental Figure 2C). Taken together, these results indicate that ILY injection has minimal direct effect, if any, on endothelial cells and no direct effect on other non-hCD59 expressing cells, such as platelets, in the ThCD59RBC model.
Cause of sudden death in acute hemolysis
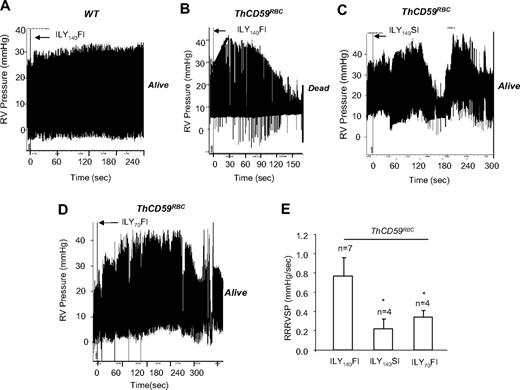
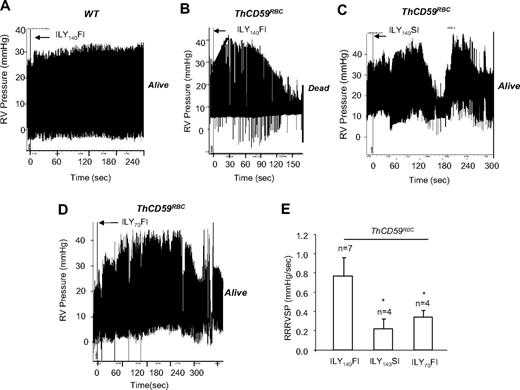
To assess the potential role of PAH as a determinant of mortality after acute hemolysis, as suspected for many hemolytic disorders,5,12–14 we measured the peak RVSP as a surrogate for the pulmonary artery systolic pressure by right-heart catheterization during ILY-mediated intravascular hemolysis. In ThCD59RBC mice, but not WT mice, ILY140FI induced rapid intravascular hemolysis with death (71.1 ± 30.6 seconds; n = 7) preceded by an abrupt increase in RVSP (9.86 ± 0.96 mm Hg increase) with 0.77 plus or minus 0.19 mm Hg/s rate of rise of RVSP (RRRVSP; Figure 2A-B,E). The RRRVSP is used as a measure of the dynamic change of RVSP. Injection of either ILY70FI or ILY140SI into ThCD59RBC mice, which were nonlethal, caused an increase in RVSP and a maximal RVSP comparable with ILY140FI but at a significantly slower RRRVSP (Figure 2C-E).
ILY140FI-induced death in ThCD59RBC mice associated with a significantly lower RRRVSP compared with ILY140SI or ILY70FI. (A-D) Representative tracings show the dynamic changes in RVSP in the WT (A) and ThCD59RBC mice with ILY140FI (B), and the ThCD59RBC mice with ILY140SI (C) and ILY70FI (D). (E) RRRVSP comparison among these mice. RRRVSP (mm Hg/s) = increased systolic pulmonary arterial pressure (in mm Hg)/time (in seconds; black line in panel B). RRRVSP t test. *P < .05 vs ThCD59RBC mice with ILY140FI. Results represent mean ± SEM.
ILY140FI-induced death in ThCD59RBC mice associated with a significantly lower RRRVSP compared with ILY140SI or ILY70FI. (A-D) Representative tracings show the dynamic changes in RVSP in the WT (A) and ThCD59RBC mice with ILY140FI (B), and the ThCD59RBC mice with ILY140SI (C) and ILY70FI (D). (E) RRRVSP comparison among these mice. RRRVSP (mm Hg/s) = increased systolic pulmonary arterial pressure (in mm Hg)/time (in seconds; black line in panel B). RRRVSP t test. *P < .05 vs ThCD59RBC mice with ILY140FI. Results represent mean ± SEM.
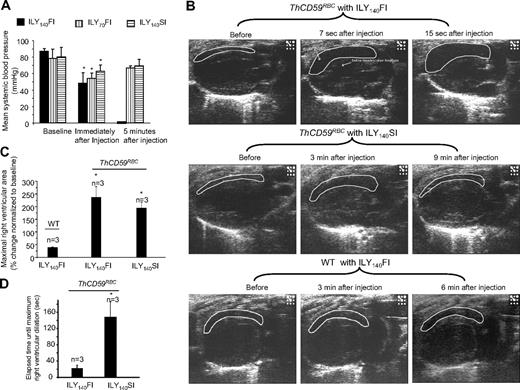
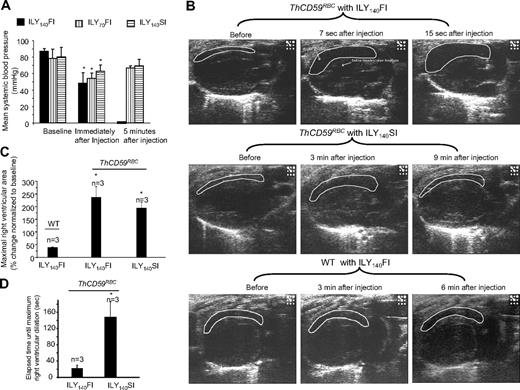
To investigate further the hemodynamic changes caused by acute hemolysis, we measured mean systemic arterial blood pressure (MABP) and monitored right and left heart function by echocardiography. In ThCD59RBC mice, ILY140FI, ILY140SI, and ILY70FI, resulted in an immediate decrease in MABP compared with baseline values before the injection (Figure 3A). The mice treated with ILY140FI that died had a greater increase in right ventricle (RV) diastolic area than those that survived with the ILY140SI or ILY70FI, as shown in the echocardiograms (Figure 3B-C and supplemental Videos 1-4). Importantly, the time interval needed to achieve maximal right ventricle dilation (end-diastolic volume) was significantly shorter in mice treated with the lethal ILY140FI dose than in those treated with ILY140SI (Figure 3D and supplemental Videos 1-4). Furthermore, values of MABP and right heart function in mice treated with either ILY140SI or ILY70FI returned to normal levels after a transient decrease (Figure 3A and supplemental Videos 1-4). No changes in hemodynamic or echo diagnostic parameters were observed in WT mice after ILY140FI. Taken together, these results indicate that the direct cause of death in this intravascular hemolysis model is an abrupt increase in pulmonary blood pressure (represented by RRRVSP) that leads to right ventricle failure with minimal or no changes in left ventricular function.
Systemic blood pressures and echocardiogram in ILY-treated mice. (A) Hemolysis in the ThCD59RBC induced by ILY injection with 3 different dosing schedules induced an immediate, rapid decrease in MABP. Five minutes later, the mice treated with either ILY140SI or ILY70FI had a gradual increase in the MABP, but all mice treated with ILY140FI died (and had no measurable MABP). *P < .05 (n = 4) compared with the respective baseline level of MABP. (B) Representative echocardiographic frames in the parasternal long-axis view show the dynamic changes in right ventricular volume in the ThCD59RBC mice treated with different ILY dosing schedules (the full echocardiogram videos are showed in supplemental Videos 1-4). *P < .01 vs WT with ILY140FI. (C) Increased percentage of maximal right ventricular area at end-diastole vs baseline right ventricular area in the mice treated with the ILY. *P < .05 vs ILY140FI. (D) Elapsed time until maximal right ventricular dilation was achieved in the mice treated with ILY140FI and ILY140SI. *P < .05 vs ILY140FI. Results represent mean ± SEM.
Systemic blood pressures and echocardiogram in ILY-treated mice. (A) Hemolysis in the ThCD59RBC induced by ILY injection with 3 different dosing schedules induced an immediate, rapid decrease in MABP. Five minutes later, the mice treated with either ILY140SI or ILY70FI had a gradual increase in the MABP, but all mice treated with ILY140FI died (and had no measurable MABP). *P < .05 (n = 4) compared with the respective baseline level of MABP. (B) Representative echocardiographic frames in the parasternal long-axis view show the dynamic changes in right ventricular volume in the ThCD59RBC mice treated with different ILY dosing schedules (the full echocardiogram videos are showed in supplemental Videos 1-4). *P < .01 vs WT with ILY140FI. (C) Increased percentage of maximal right ventricular area at end-diastole vs baseline right ventricular area in the mice treated with the ILY. *P < .05 vs ILY140FI. (D) Elapsed time until maximal right ventricular dilation was achieved in the mice treated with ILY140FI and ILY140SI. *P < .05 vs ILY140FI. Results represent mean ± SEM.
Reduced NO bioavailability and platelet activation in hemolysis-associated fatal PAH
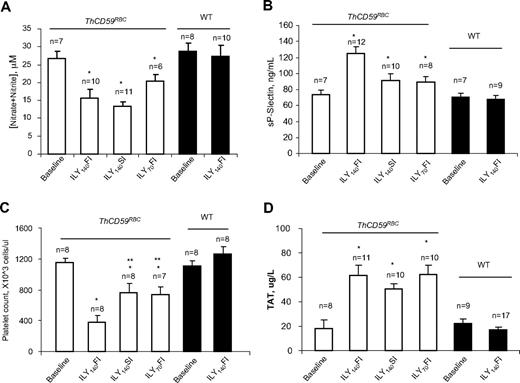
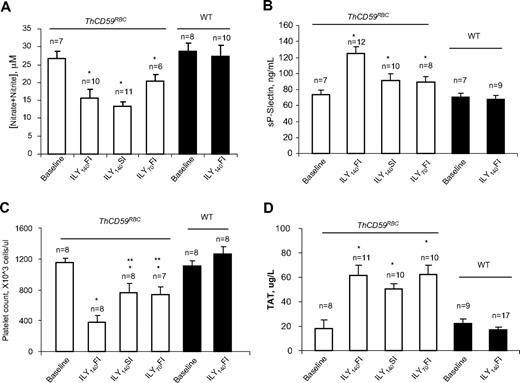
Reduced NO bioavailability due to the NO-scavenging effect of cell-free hemoglobin in acute hemolysis results in vascular smooth muscle constriction, platelet activation, and in situ thrombosis, inducing a further increase in pulmonary vascular resistance that potentiates the development of hemolysis-associated pulmonary hypertension and sudden death.12 We have previously reported that ILY injection in ThCD59RBC mice markedly decreased NO levels and increased platelet activation, as shown by an increase in serum soluble P-selectin (sP-selectin) levels.26 Here, we confirmed that the NO levels were significantly reduced in the ThCD59RBC mice injected with ILY compared with the WT mice (Figure 4A) and demonstrate that the levels of sP-selectin were significantly higher and the platelet counts significantly lower in the ThCD59RBC mice treated with ILY140FI than those treated with either ILY140SI or ILY70FI (Figure 4B-C). In addition, ILY140SI or ILY70FI injection induced significantly higher levels of sP-selectin and significantly lower platelet counts in ThCD59RBC than in WT mice (Figure 4B-C). Furthermore, ILY-induced hemolysis in ThCD59RBC mice was associated with an activation of the coagulation cascade, as demonstrated by an increase in the intravascular coagulation marker TAT (Figure 4D) that was not observed in WT mice.
NO bioavailability and platelet activation in ILY-mediated hemolysis mice. (A) NO (measured as nitrite and nitrate) was reduced in ILY-injected ThCD59RBC but not WT mice. *P < .05 vs WT. Values shown are mean ± SEM. (B-C) sP-selectin levels or platelet counts. *P < .05 vs WT with ILY140FI and **P < .05 vs ThCD59RBC with ILY140FI. (D) ILY resulted in increased TAT in ThCD59RBC but not in WT mice. Blood samples were collected 1 minute after ILY140FI and 15 minutes after ILY140SI and ILY70FI from ThCD59RBC and at 15 minutes after ILY140FI from WT mice. Results represent mean values ± SEM. *P < .01 vs WT with ILY140FI.
NO bioavailability and platelet activation in ILY-mediated hemolysis mice. (A) NO (measured as nitrite and nitrate) was reduced in ILY-injected ThCD59RBC but not WT mice. *P < .05 vs WT. Values shown are mean ± SEM. (B-C) sP-selectin levels or platelet counts. *P < .05 vs WT with ILY140FI and **P < .05 vs ThCD59RBC with ILY140FI. (D) ILY resulted in increased TAT in ThCD59RBC but not in WT mice. Blood samples were collected 1 minute after ILY140FI and 15 minutes after ILY140SI and ILY70FI from ThCD59RBC and at 15 minutes after ILY140FI from WT mice. Results represent mean values ± SEM. *P < .01 vs WT with ILY140FI.
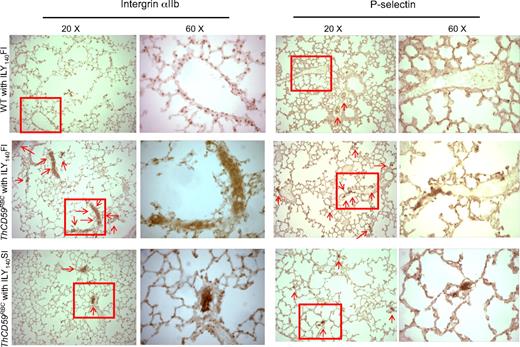
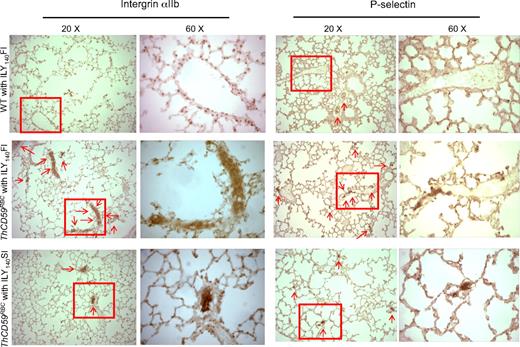
At the histologic level, we also observed more extensive P-selectin staining, indicative of platelet activation/aggregation,34 and integrin αIIb, a specific marker of platelet activation,30,35 in the small perialveolar pulmonary vessels in the ThCD59RBC mice treated with ILY140FI than in the ThCD59RBC mice treated with ILY140SI (Figure 5 and supplemental Figure 3). Moreover, platelet aggregates and thrombi specifically occurred in the pulmonary microvasculature, but not the vasculatures of other tissues such as kidney, liver, and heart of ThCD59RBC mice treated with ILY140FI (Figure 5; supplemental Figure 4). WT mouse lungs had no or very little staining. These results indicate that platelet activation/aggregation and thrombosis in the pulmonary vasculature correlates with the severity of hemolysis-associated PAH in this unique hemolytic model.
Representative images of integrin αIIb and P-selectin staining in lung. There is more extensive integrin αIIb and P-selectin staining (arrows) in small perialveolar blood vessels in the lungs of ThCD59RBC mice treated with ILY140FI than in those treated with ILY140SI. Platelet aggregates were adherent to the vessel walls in the ThCD59RBC mice treated with ILY140FI; this is evident in the 60× magnified images. WT mouse lungs had no or very little staining. Lower magnification (4× and 10×) images for P-selectin staining of the different mice are shown in supplemental Figure 3.
Representative images of integrin αIIb and P-selectin staining in lung. There is more extensive integrin αIIb and P-selectin staining (arrows) in small perialveolar blood vessels in the lungs of ThCD59RBC mice treated with ILY140FI than in those treated with ILY140SI. Platelet aggregates were adherent to the vessel walls in the ThCD59RBC mice treated with ILY140FI; this is evident in the 60× magnified images. WT mouse lungs had no or very little staining. Lower magnification (4× and 10×) images for P-selectin staining of the different mice are shown in supplemental Figure 3.
Sildenafil rescues hemolysis-associated fatal PAH
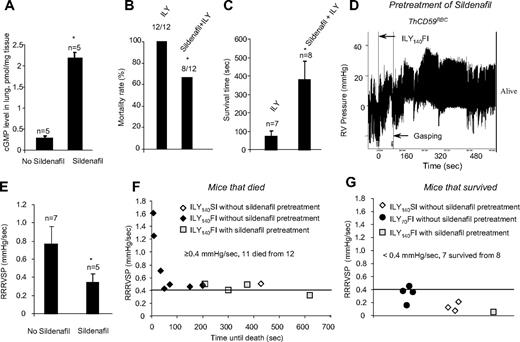
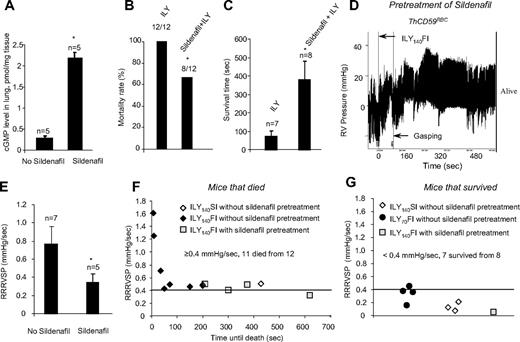
To investigate the role of NO in the pathogenesis of hemolysis-associated PAH, we pretreated ThCD59RBC mice with sildenafil for 10 days (100 mg/kg, oral daily dose). Sildenafil is a specific phosphodiesterase-5 (PDE-5) inhibitor that increases cGMP levels and thereby potentiates NO-dependent signaling.23 Sildenafil pretreatment resulted in a significant increase in the levels of cGMP in the lung of ThCD59RBC mice compared with no pretreatment (Figure 6A) and greatly reduced their mortality rate (Figure 6B) or prolonged their survival time in animals that died (Figure 6C) among the ThCD59RBC mice given the ILY140FI injection. This protective effect of sildenafil was associated with a significant decrease in RRRVSP (Figure 6D). A plot of individual RRRVSP versus survival time after injection of ILY140FI to ThCD59RBC mice shows that RRRVSP inversely correlates with the survival time, and that sildenafil pretreatment reduced the RRRVSP while prolonging the survival time (Figure 6E). Consistent with the mean RRRVSP results depicted in Figure 2, ThCD59RBC mice injected with either ILY140SI or ILY70FI had much lower values of RRRVSP, and all but one survived. A RRRVSP of 0.4 mm Hg/s represents a valid cutoff value to predict death or survival in ILY-treated ThCD59RBC mice: when RRRVSP was greater than or equal to 0.4 mm Hg/s, 11 of 12 mice died, and when RRRVSP was less than 0.4 mm Hg/s, 1 of 8 mice died. This analysis of individual mouse values combined with the effect of sildenafil strengthen our conclusion that the velocity of RVSP raise (ie, the RRRVSP) rather than the actual value of RVSP achieved by each mouse is the critical factor in determining death in ILY-mediated acute hemolysis. The protective effects of sildenafil indicate that the rapid increase in RVSP that critically determines hemolysis-associated death is likely a consequence of reduced NO bioavailability.
Sildenfil prevented death or prolonged the survival time in the ThCD59RBC mice treated with ILY140FI. (A) cGMP levels in the lung of the ThCD59RBC mice with or without sildenafil pretreatment. *P < .01 vs without sildenafil pretreatment. (B-C) Pretreatment with sildenafil reduced mortality (B) or prolonged survival time (C) in the ThCD59RBC mice treated with ILY140FI. *P < .05 vs ILY (or without sildenafil pretreatment). (D) Representative image shows the dynamic change of RVSP in the sildenafil-pretreated ThCD59RBC mice given ILY140FI. (E) Pretreatment with sildenafil suppressed the RRRVSP in the ThCD59RBC mice given ILY140FI. *P < .05 vs the mice without sildenafil pretreatment. (F) RRRVSP inversely correlated with the time until the death among ThCD59RBC mice with the different treatments. (G) There were low levels of RRRVSP in the mice that survived with the different treatments.
Sildenfil prevented death or prolonged the survival time in the ThCD59RBC mice treated with ILY140FI. (A) cGMP levels in the lung of the ThCD59RBC mice with or without sildenafil pretreatment. *P < .01 vs without sildenafil pretreatment. (B-C) Pretreatment with sildenafil reduced mortality (B) or prolonged survival time (C) in the ThCD59RBC mice treated with ILY140FI. *P < .05 vs ILY (or without sildenafil pretreatment). (D) Representative image shows the dynamic change of RVSP in the sildenafil-pretreated ThCD59RBC mice given ILY140FI. (E) Pretreatment with sildenafil suppressed the RRRVSP in the ThCD59RBC mice given ILY140FI. *P < .05 vs the mice without sildenafil pretreatment. (F) RRRVSP inversely correlated with the time until the death among ThCD59RBC mice with the different treatments. (G) There were low levels of RRRVSP in the mice that survived with the different treatments.
Platelet depletion, platelet inhibition, and antithrombotic therapy rescue hemolysis-associated fatal PAH
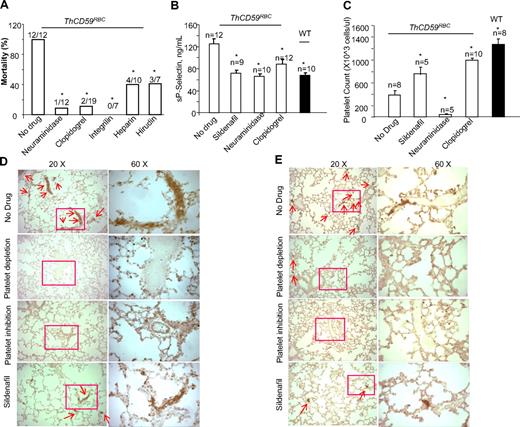
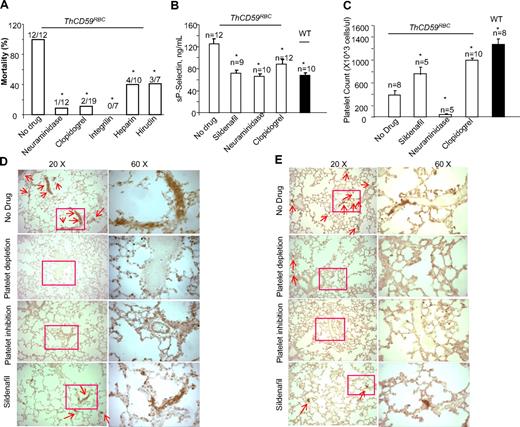
To investigate further the role of platelet activation in the pathogenesis of hemolysis-associated fatal PAH, we pretreated the ThCD59RBC mice with neuraminidase to deplete the platelet pool and prevent platelet activation or with platelet inhibitors such as clopidogrel or integrilin. Neuraminidase is a platelet depletion agent, commonly used in mice to produce thrombocytopenia.36 Clopidogrel is an antiplatelet agent that prevents adenosine diphosphate (ADP)-induced platelet aggregation by irreversibly binding to the ADP receptor P2Y12 on the platelet membrane.37 Integrilin is an antiplatelet agent that inhibits platelet aggregation by selectively blocking the platelet glycoprotein (GP) IIb/IIIa receptor.38 Both platelet depletion with neuraminidase and platelet inhibition with either clopidogrel or integrilin significantly prolonged the survival time of ThCD59RBC mice treated with ILY140FI (data not shown) and reduced their mortality rate (Figure 7A). Compared with ThCD59RBC mice without any treatment, those pretreated with neuraminidase, clopidogrel, integrilin, or sildenafil had lower sP-selectin levels, less lung integrin-αIIb and P-selectin staining, and higher platelet counts (with the exception of neuraminidase-pretreated mice, which had the lowest platelet counts due to depletion; Figure 7B-E). In summary, these results also demonstrate that platelet activation correlates with the severity of hemolysis-associated PAH. Because bleeding precluded right-ventricle catheterization to measure directly RRRVSP, in mice pretreated with neuraminidase or clopidogrel, we assessed the hemodynamic consequence of hemolysis-associated PAH indirectly by echocardiographic monitoring of right heart function. ThCD59RBC mice pretreated with either a platelet depleting or a platelet inhibiting agent before injection of ILY140FI had a significantly smaller maximal right ventricle diastolic area than those that were not pretreated (supplemental Figures 5-6; supplemental Videos 5-6). Taken together, these results support the conclusion that platelet activation is another critical factor determining the lethality and severity of PAH after acute intravascular hemolysis.
Platelet depletion, platelet inhibition, or antithrombotic therapy prevented death or prolonged survival times in the ThCD59RBC mice treated with ILY140FI. The mortality rates (A), sP-selectin levels (B), platelet counts (C), lung P-selectin (D), and integrin αIIb staining (E) in the ThCD59RBC mice pretreated with the different antithrombotic agents before ILY140FI treatment. The WT mice treated with ILY140FI were used as the control for sP-selectin and integrin αIIb staining and platelet counts. *P < .05 vs ThCD59RBC without drug pretreatment.
Platelet depletion, platelet inhibition, or antithrombotic therapy prevented death or prolonged survival times in the ThCD59RBC mice treated with ILY140FI. The mortality rates (A), sP-selectin levels (B), platelet counts (C), lung P-selectin (D), and integrin αIIb staining (E) in the ThCD59RBC mice pretreated with the different antithrombotic agents before ILY140FI treatment. The WT mice treated with ILY140FI were used as the control for sP-selectin and integrin αIIb staining and platelet counts. *P < .05 vs ThCD59RBC without drug pretreatment.
Discussion
The decreased NO bioavailability has long been suspected to participate in the pathogenesis of hemolysis-mediated PAH.1 This notion is now further supported by our experimental results showing that NO levels were dramatically reduced after ILY-induced hemolysis. Of note, we did not find that the magnitude of NO-insufficiency correlated with the severity of the PAH. This lack of correlation may result from the short-half of NO that precludes our assessing dynamic changes in NO levels in our model system soon after ILY-mediated hemolysis. Nevertheless, the fact that potentiating NO-dependent signaling by pretreatment of ThCD59RBC mice with sildenafil rescues the mice from massive hemolysis-associated death or prolongs the survival time through the suppression of RRRVSP and inhibition of platelet activation strongly support the critical role of reduced NO bioavailability in the pathogenesis of hemolysis-associated (fatal) PAH. However, there are several other factors by which platelets may be activated in our model system. For example, released ADP39 and tissue factor40 and increased free hemoglobin itself also activate platelets.41 The relative contributions of these factors or others to the development of hemolysis-associated platelet activation/aggregation will require further study.
Our results also indicate that platelet activation/aggregation plays a critical role in the pathogenesis of hemolysis-associated PAH. It is intriguing that ILY140FI but not ILY140SI results in hemolysis-associated death even though both doses eventually induced similar degree of total lysis. The possible explanation for this experimental finding is that rapid massive hemolysis triggers more abrupt reduction of NO availability by rapidly releasing free-hemoglobin into the circulation in ILY140FI than those in ILY140SI. This in turn induces massive platelet activation and in situ thrombosis in the perialveolar vessels of lungs and thereby increases pulmonary vascular resistance, leading to right ventricle failure and sudden death. In the case of ILY70FI, the less degree of induced-intravascular hemolysis led to less NO insufficiency, thereby resulting in less severe consequences for PAH than ILY140FI. The lesser degree of platelet activation in both ILY140SI and ILY70FI might not be severe enough or have sufficient time to cause significant platelet activation and PAH. This interpretation is supported by our finding that decreased platelet counts and increased sP-selectin concentration in blood and extensive integrin αIIb and P-selectin staining in the blood vessel and perialveolar microvasculature are all much more severe after ILY140FI than after ILY140SI and ILY70FI. Consistently, extensive VWF staining in the intravascular thrombi were found in the lungs of ThCD59RBC mice, but not in WT mice treated with ILY140FI (supplemental Figure 7). This extensive VWF staining in the pulmonary vasculatures could be attributed to both platelet aggregation and plasma VWF entrapped in thrombi. The VWF entrapped in the intravascular thrombi of lung may explain the slightly decreased level of circulating VWF at 15 minutes in ThCD59RBC mice after ILY injection (supplemental Figure 2A). In addition, increased sP-selectin and extensive P-selectin staining in the lungs of the hemolytic mice might result from either platelet activation and/or endothelial dysfunction because sP-selectin can be released from activated platelets and endothelium.42,43 Thus, how endothelial dysfunction contributes to the pathogenesis of hemolysis-associated complications will require further investigation.
A critical role of platelet activation in hemolysis-induced fatal PAH and death is also supported by the concordant results after pharamacologic interventions with agents such as sildenafil, which increase activity of the NO signaling pathway, neuraminidase, which depletes the circulating platelet pool, and clopidogrel or integrilin, platelet inhibitors. All these agents reduced the rate of RVSP (RRRVSP) increase and attenuated the deterioration of right ventricle function. Consistent with this conclusion is the observation that the RRRVSP after ILY injection correlates positively with the death rate and level of platelet activation, and negatively with the survival time after injection. These results highlight inhibition of platelet activation as a critical avenue for the pharmacologic treatment of hemolysis-associated PAH and death. Of note, a National Institutes of Health–funded study of sildenafil for the treatment of pulmonary hypertension in sickle cell disease was recently stopped due to increased incidence of pain crises (http://www.rhoworld.com/rho/federal-research/projects/walk-phasst). However, whether treating patients with other hemolytic disorders with sildenafil or other agents that increase NO has potential risks is unclear.
The rescuing effect conferred by antithrombotic therapy (with heparin and hirudin) further indicates that there are other prothrombotic mechanisms in acute hemolysis that, while related to platelet activation, are partly independent of it. Indeed, coagulation abnormalities are frequently reported in hemolytic anemias.40 Activation of coagulation may result from the increased production of tissue factor that is a downstream effect of low NO bioavailability40 and may be also attributed to erythrocyte-derived microparticles.44 In flowing blood, the presence of high levels of procoagulant microparticles, derived from lysed erythrocytes together with activated platelets, could be responsible for the dissemination of a prothrombotic state.45 In our study, pretreatment of ThCD59RBC mice with heparin and hirudin also rescued the mice from ILY-mediated death (Figure 7A). It is notable that the efficacy of the rescue conferred by each drug is an important and clinically relevant topic, which has not been addressed in the current studies, but will be part of future investigations. Furthermore, we demonstrated that there was enhanced activation of coagulation as evidenced by the increase in TAT level in ILY-mediated hemolysis but not in WT mice. These results also suggest that the activation of the coagulation cascade may also contribute to the development of hemolysis-associated PAH.
The vasoconstriction due to reduced NO bioavailability has a very well-established role in the pathogenesis of hemolysis-associated PAH. Surprisingly, pretreatment of ThCD59RBC mice with vasodilators such as dobutamine (2 μg/g, intraperitoneally 25 minutes before ILY injection), dopamine (1 μg/g, intraperitoneally 25 minutes before ILY injection), phentolamine (100 μg/g, intraperitoneally 25 minutes before ILY injection), isoprenaline (0.1 μg/g, intraperitoneally 1 minute before ILY injection), or oxygen (100% of O2 for 5 minutes) had no effect on RVSP (data not shown). These treatment failures could be attributed to the rapid occurrence of massive platelet activation and aggregation clogging small vessels of the lung, leading to pulmonary vascular resistance and fatal right ventricular failure. It is conceivable that the nonselective vasodilators tested could not suffice to rescue an abrupt increase in RVSP that is caused by mechanical obstruction of the pulmonary vasculature.
Another interesting finding in our studies is that thrombosis is specific to pulmonary vasculature. The underlying mechanism for this phenomenon is unclear. We reason that reduced NO bioavailability due to the scavenging effect of cell-free hemoglobin in acute hemolysis results in the following 2 consequences: (1) it will specifically increase pulmonary vascular tone, and (2) it will also lower the threshold for platelet activation, which leads to platelet thrombosis and, in turn, a further increase in pulmonary vascular resistance and pulmonary artery pressure. The animals died not simply from platelet-dependent thrombosis, but from acute right heart failure brought about by the acute increase in pulmonary vascular resistance secondary to both the increase in pulmonary vascular tone and platelet-dependent thrombosis. In an acute experiment, it is very difficult to dissociate these 2 events from one another in vivo; however, the pharmacologic data using platelet inhibitors support the view that platelet-dependent thrombosis is a major contributor to the pathophysiology. The quantitative contribution of these 2 mechanisms—an increase in pulmonary vascular tone and an increase in platelet activation/thrombosis—to the development of hemolysis-associated fatal PAH in both acute and chronic experimental settings is beyond the scope of this study.
In summary, we demonstrate that dramatically reduced NO bioavailability associated with massive hemolysis results in extensive platelet activation, coagulation pathway activation, and thrombosis, thereby leading to PAH, right ventricle failure, and death. These results indicate that the dramatic increase in platelet activation due to rapidly reduced NO in acute intravascular hemolysis plays a critical role in the hemolysis-associated (fatal) PAH. Importantly, these results strongly support the notion that the inhibition of platelet activation and enhancement NO bioavailability (with sildenafil) in acute hemolysis may be critical approaches for the treatment and prevention of hemolysis-associated PAH.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful for the assistance of Drs Wenping Song and Hongcheng Sun with the statistical analysis of the mortality rates among the groups.
This work was supported by National Institutes of Health grant RO1AI061174 (X.Q.) and by a Scientist Development grant from American Heart Association 0435483N (X.Q.); HL 61795, NO1 HV 28178, PO1 81857, U54 HL70819 (J.L.); P.R.C. National Key Technology R&D Program (2008BAI60B03; Z.P.); and the China Oversea Scholarship (2008638052; Y.T.).
National Institutes of Health
Authorship
Contribution: W.H., R.J., J.Z., T.Y., Z.P., X.G., R.T.B., J.A.H., J.L., and X.Q. contributed to the characterization of ILY-mediated hemolysis model and hemolysis-associated fatal PAH; W.H., R.J., J.Z., X.G., and X.Q. conducted the data analyses; all authors contributed to the project's planning and writing of the manuscript; and J.L. and X.Q. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xuebin Qin, Harvard Medical School, One Kendall Square, Bldg 600, 3rd Floor, Cambridge, MA 02139; e-mail: xuebin_qin@hms.harvard.edu.
References
Author notes
W.H. and R.J. contributed equally to this work.