Total body irradiation (TBI) has been an integral component of allogeneic hematopoietic stem cell transplantation since this treatment modality was pioneered by Dr E. Donald Thomas in the 1970s.1 Although higher TBI doses are associated with better disease control in patients with myeloid leukemias, the increased risk of TBI-related toxicities negate any potential survival advantage.2-4
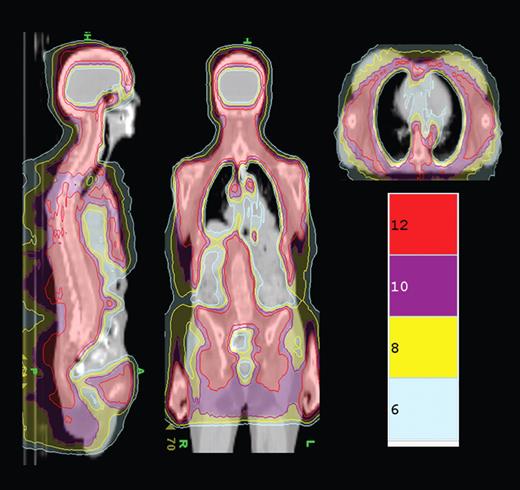
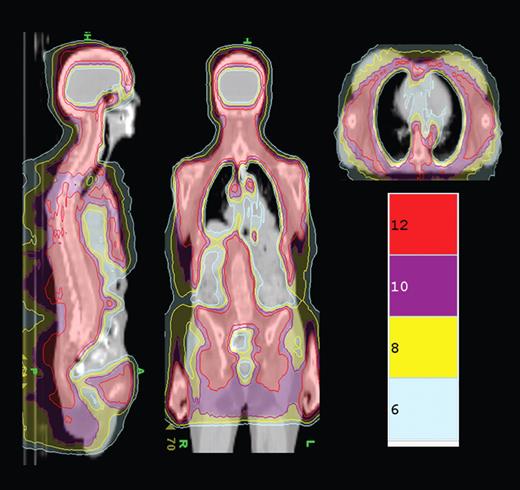
In this issue of Blood, Rosenthal et al report on a novel method of delivering TBI using the Helical Tomotherapy Hi-Art System (Tomotherapy Inc) which combines spiral computed tomography and intensity-modulated radiation therapy technology.5 This technology allows for precise delivery of radiation doses to complex shaped organ targets while sparing normal tissue. The authors hypothesized that this technology could be used to deliver high doses of radiation to marrow and lymphoid tissue in patients receiving a reduced-intensity conditioning regimen with fludarabine and melphalan. In this initial experience of 33 patients with a variety of malignant hematologic disorders, a total of 12 Gy were delivered to the marrow and lymphoid tissues with a median dose that was significantly less in normal tissues (ie, 5.8 Gy to lungs vs the expected 8-9 Gy with TBI; see figure). The combination of total marrow and lymph node irradiation (TMLI) with fludarabine and melphalan was associated with severe, grade 3 or 4 mucositis in 91% of patients and a 100-day nonrelapse mortality rate of 15%. With a median follow-up of 14.7 months, 2-year overall survival and event-free survival (EFS) were estimated at 46% (95% confidence interval [CI] 23%-66%) and 40% (95% CI 19%-61%), respectively.
TMLI isodose distribution shows the colorwash isodose distribution of a patient receiving 12 Gy TMLI. Representative sagittal, coronal, and axial planes are displayed. Isodose distributions for 12, 10, 8, and 6 Gy are depicted. See the complete figure in the article beginning on page 309.
TMLI isodose distribution shows the colorwash isodose distribution of a patient receiving 12 Gy TMLI. Representative sagittal, coronal, and axial planes are displayed. Isodose distributions for 12, 10, 8, and 6 Gy are depicted. See the complete figure in the article beginning on page 309.
Thus, we now have a novel way of delivering radiotherapy to marrow and lymphoid tissue that results in lower exposure of normal tissues; however, significant organ toxicities were seen (90% mucositis rate) and relapses still occurred in 7 patients. What remains to be determined is whether this strategy actually improves outcomes over other less-intensive regimens such as fludarabine melphalan or even fully ablative but well-tolerated ablative regimens such as fludarabine and busulfan.6,7 The 1-year EFS of 72% for high-risk patients is encouraging and suggests that further study is warranted in phase 2 trials of single diseases in these patients. However, the results in low-risk patients (50% EFS at 1 year) seem to be inferior to other less toxic regimens and suggest that other strategies should be explored in this patient group. We look forward to seeing further work with this method of delivering radiation therapy in this as well as other potential transplant indications such as autografting for myeloma.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■