Abstract
Respiratory syncytial virus (RSV) is a common cause of seasonal respiratory viral infection in patients who have undergone hematopoietic stem cell transplantation. RSV usually presents as an upper respiratory tract infection in this patient population but may progress rapidly to lower respiratory tract infection. Available therapies that have been used for the treatment of RSV infections are limited to ribavirin, intravenous immunoglobulin, and palivizumab. The use of aerosolized ribavirin, alone or in combination with either palivizumab or intravenous immunoglobulin, remains controversial. In this comprehensive review, we present and discuss the available literature on management of RSV infections in adult hematopoietic stem cell transplantation recipients with a focus on therapeutic modalities and outcomes.
Introduction
Respiratory syncytial virus (RSV) is a common cause of seasonal respiratory viral infection in patients who have undergone hematopoietic stem cell transplantation (HSCT). In the United States, RSV infections occur in the fall, winter, and spring, with an attack rate up to 10% during winter time.1,2 This paramyxovirus has been reported to affect approximately 2% to 17% of HSCT recipients.3-7 Several factors have been identified that increase the risk of acquiring RSV infection after HSCT, such as male sex, type of transplant (ie, allogeneic), cytomegalovirus seropositivity, and pre-engraftment status.1,7,8 In addition, allogeneic HSCT recipients are more likely to experience RSV pneumonia if they received mismatched/unrelated transplant, myeloablative regimen, and have advanced age.6,7 Patients who remain lymphopenic and acquire RSV infection are at high risk of progressing to pneumonia during the first 3 months after HSCT.9,10
Detection of RSV in clinical specimens, such as nasal washes, nasopharyngeal swabs, and/or bronchoalveolar lavages can be made by various diagnostic methods, including viral culture (conventional or shell vial technique), detection of viral antigens, and detection of viral RNA (molecular methods). Viral isolation by conventional culture is the “gold standard” for diagnosis of RSV infection; however, it may take up to one week to finalize the culture and it has a low sensitivity.11,12 Rapid diagnosis of RSV infection can be made by direct antigen testing on clinical specimens (ie, direct immunofluorescence staining), shell vial culture technique, which provides results within 48 hours, with a sensitivity of 93% and a specificity of 97%,13 and by real-time polymerase chain reaction assays for detection of RSV RNA with a higher sensitivity and specificity.11,12 HSCT recipients with influenza-like symptoms (ie, runny nose, fever, nasal congestion, sore throat, and cough) during the cold season should be tested for RSV and other respiratory viruses. At our institution, we only screen symptomatic patients for respiratory viruses by obtaining nasal washes that are submitted for direct immunofluorescence antigen detection for RSV and influenza virus on the clinical specimen in addition to shell vial cultures. Other institutions rely on molecular assays for the diagnosis of respiratory viruses.
RSV usually presents as an upper respiratory tract infection (URI) in this patient population but may progress rapidly to lower respiratory tract infection (LRI). These infections are associated with significant morbidity and mortality in the HSCT population.3,6,14-17 Patients with RSV infection often have delayed engraftment or graft failure,18,19 and the infection often progresses to respiratory failure, necessitating admission to an intensive care unit and mechanical ventilation.15,18,20-22 As reported in a few studies, approximately 17% to 84% of patients with RSV infection develop an LRI,3,8-10,23,24 and this is a strong predictor of mortality in HSCT patients1,19 as are transplants from unrelated donors, cord blood transplants, and a need for supplemental oxygen at the time of RSV diagnosis.15,19 RSV-associated mortality has been reported to range from 7% to 83% in patients whose infection progresses to LRI.19,21,25,26 The wide ranges in rates of incidence of RSV infection, progression to LRI, and mortality could be in part explained by the increased awareness of this viral infection in HSCT recipients over the years and subsequent diagnosis of milder cases, and the improvement in supportive care, which may have accounted for better outcomes in recent published studies.
HSCT recipients have impaired T-cell immunity, which not only increases their risk of acquiring respiratory viral infections but may also reduce their ability to clear the virus, which may subsequently increase disease severity and duration.27 Graft-versus-host disease (GVHD) in HSCT recipients and its therapy prolong T-cell immunodeficiency and may allow ongoing viral replication and progression to LRI with subsequent inflammatory lung damage.28 Besides GVHD, pre-engraftment or early posttransplant status (< 1 month), lymphopenia, greater age, and transplant from a mismatched, unrelated donor have been identified by many studies as significant risk factors for progression to LRI.1,3,6,10,29
Prevention of RSV transmission in a hospital setting, especially where immunocompromised patients are housed, is considered a basic standard of care in many hospitals. Strict infection control precautions considered critical to preventing spread within a hospital ward include respiratory isolation of infected patients, handwashing before and after contact with patients, and educational efforts targeting healthcare workers and family members. Chemoprophylaxis in susceptible patients may be considered, especially in outbreak situations when horizontal transmission is occurring.30 RSV infection and progression to serious LRI can be prevented by use of polyclonal or monoclonal immunoglobulins. This has only been proven in studies involving young children and not in immunocompromised hosts of any age. Intravenous RSV immunoglobulin (RSV-IVIG) was approved by the U.S. Food and Drug Administration (FDA) in February 1996 for the prophylaxis of RSV infection in preterm infants and in children with bronchopulmonary dysplasia.31,32 However, the drug is no longer available for use. Palivizumab (PVZ) is the only commercially available drug that has been licensed (June 1998) for prevention of RSV infection in high-risk children.33,34 A newer drug, motavizumab, has been studied in high-risk children and appears to be noninferior to PVZ.35 However, this drug is not yet approved by the FDA for prophylaxis in high-risk children. Currently, there is no commercially available vaccine that can prevent RSV infection.
Management of RSV infection should target (1) viral replication, (2) virus-induced lung inflammation, (3) coinfections or superimposed infections, and (4) respiratory dysfunction.28 Available therapies that have been used for treatment of RSV infections are limited to ribavirin, IVIG, and PVZ. RSV infection at the URI or LRI stage in HSCT recipients has been treated with systemic (oral or intravenous) or aerosolized ribavirin (AR), alone or in combination with either PVZ or IVIG, but this remains controversial. The lack of well-designed randomized controlled trials leaves clinicians with a few studies, mostly retrospective and from single centers, as the only available clinical data; those data and “expert opinions” are the only published guidance on therapy and outcome of this serious and sometimes fatal viral infection in HSCT recipients.
In this comprehensive review, we present and discuss the available literature on management of RSV infections in adult HSCT recipients (hereafter simply “HSCT recipients”), with a focus on therapeutic modalities and outcomes.
Methods
An electronic literature search was conducted using PubMed and the Cochrane Central Register of Controlled Trials for the period from 1980 to 2010. The following Medical Subject Heading terms were used: respiratory syncytial virus, respiratory viruses, HSCT, cancer, ribavirin, PVZ, and RSV-immunoglobulin.
The full texts of the articles selected were reviewed by both authors. The references in all of the selected studies were also reviewed to identify additional articles that did not appear in the initial search. These selected studies were independently reviewed by both authors as well.
Inclusion and exclusion criteria for identified studies
Inclusion and exclusion criteria were defined a priori. The following inclusion criteria were required:
Involvement of adult patients who had undergone HSCT and had been infected with RSV,
Study designs composing retrospective and prospective observational studies and randomized controlled trials, if any, and
Focus on 2 outcome measures of RSV infection: (a) progression of RSV URI to LRI and (b) RSV-related mortality.
The following were exclusion criteria:
Outcomes of RSV infection not included,
Involvement of cancer patients who had not undergone HSCT,
Involvement of cancer patients who had undergone solid-organ transplant,
Data on therapy and/or primary outcome measures were not abstractable,
Review papers or meta-analytic studies,
Case reports, and
Articles not in English.
Types of interventions and outcome measures
Antiviral therapy was considered an intervention measure. Antiviral therapy included ribavirin (aerosolized, intravenous, or oral) alone or in combination with an immunomodulator (PVZ, IVIG, or RSV-IVIG). We defined outcome measures as (1) progression of RSV URI to LRI and (2) RSV-related mortality.
Data abstraction
One reviewer (J.N.S.) independently extracted the data, and the second reviewer (R.F.C.) validated the extracted data by reviewing the selected articles.
Rates of RSV infection, of progression to LRI, and of RSV-related mortality in treated and untreated HSCT recipients were calculated independently.
Statistical considerations
The reported data represent combinations of extracted data. Outcomes (ie, progression to LRI or not, and death or not) are descriptively summarized as percentages. Outcomes of treated and nontreated groups were compared by χ2 or Fisher exact tests, as appropriate. Odds ratios were calculated with 95% confidence intervals. All statistical analyses were performed using SAS Version 9.1 (SAS Institute).
Results
Available modalities for treatment of RSV infection
Ribavirin.
Ribavirin is a guanosine analog that is active against RNA and DNA viruses. It is available in aerosol, intravenous, and oral forms. The aerosolized form was approved by the FDA in 1986 for treatment of RSV LRI in hospitalized high-risk infants and young children, and it is still the only drug approved for this indication.36
PVZ.
The immunomodulator PVZ is an RSV-specific monoclonal antibody derived from murine antibodies and directed against the F glycoprotein of RSV. PVZ has been shown to reduce viral titers in pulmonary tissues and viral replication in animal models,37,38 with no effect on cytokine response in young and aged cotton rats.39 The serum half-life of PVZ is approximately 20 days in healthy adults40 and approximately 11 days in HSCT patients with RSV infection.23 The difference in half-lives may be attributable to reduced concentrations secondary to binding of PVZ to RSV and/or to gut leakage secondary to GVHD. PVZ is well tolerated in HSCT patients.41 The efficacy of PVZ in HSCT patients has not been evaluated in randomized clinical trials, but the randomized Impact-RSV clinical trial in very young high-risk children (age < 2 years) demonstrated significant reduction in hospitalization rates when PVZ was used prophylactically.34 PVZ was approved by the FDA for this indication in June 1998. It is an expensive drug, especially for adult patients; the cost per dose was reported to be at least $10 000.15 PVZ is administered at a dose of 15 mg/kg as a single intramuscular or intravenous injection42 and is sometimes used in combination with ribavirin in HSCT patients with RSV LRI. Repeat dosing may be required in severe infections.
IVIG and RSV-IVIG.
Polyclonal immunoglobulins have been used often in patients with severe RSV infection. Both standard and RSV-specific IVIG with high anti-RSV neutralization titers have been used. RSV-IVIG contains 5 times the RSV neutralizing titers found in standard IVIG. Not only is it potentially more efficient than standard IVIG, it requires single dosing, achieves rapid peak plasma levels, and contains less fluid volume than IVIG.43 However, RSV-IVIG (RespiGam) was withdrawn by the manufacturers in March 2004 for reasons not related to safety concerns (http://www.empireblue.com/provider/noapplication/f2/s5/t9/pw_ad084861.pdf).44
Studies in immunocompetent and immunocompromised animal models have shown that IVIG and RSV-IVIG prevent RSV replication in lung tissues, antibody response, and lymphocyte infiltration in the lungs, reduce viral loads in pulmonary tissues, and prevent subsequent development of illness.45,46 IVIG and RSV-IVIG have been shown to mediate the respiratory tract inflammation induced by RSV through cytokines; however, the role of chemokines in the action of PVZ requires further elucidation.39,45,47-52 Combination therapy with ribavirin and IVIG or RSV-IVIG has been shown to reduce pulmonary viral load to a greater degree than ribavirin alone in an animal model of RSV infection49 and improves clinical outcome when initiated before the need for mechanical ventilation in HSCT patients.53
Therapeutic outcomes
Available data on the use of ribavirin in any of its forms, alone or in combination with of any of these immunomodulators, are summarized here and in Tables 1,Table 2–3. The initial search yielded a total of 37 studies. Eight of the studies did not meet all the inclusion criteria.5,8,15,18,53,61-63 In 3 other studies,19,64,65 more than one form of ribavirin was used, but data on outcome measures for each form were not available separately. The data from these 3 studies were not looked at individually but were included and analyzed only as part of the total number of patients treated versus untreated (Table 4). A total of 26 studies met all inclusion criteria. These studies were mainly reported from centers from the United States (n = 14) and Europe (n = 9). The studies from the United States were predominantly from 3 large cancer centers. The studies from Europe were mainly from the United Kingdom (n = 3), Sweden (n = 2), and one each from France, Germany, Spain, and Switzerland. One study from Australia and one from Brazil are also included. Few studies were multicenter in design.
AR.
AR has been the most frequently used treatment for RSV in the United States. It is administered on a continuous or intermittent schedule by a small-particle aerosol generator unit via a face mask; because of concerns from animal studies about teratogenicity, it is administered inside a scavenging tent to prevent environmental contamination.66 The continuous regimen constitutes administration of 6 g of AR over 18 hours, and the intermittent regimen requires administration of 2 g of AR over 2 to 3 hours every 8 hours. Duration of treatment is usually 5 to 7 days, but treatment is prolonged in severe infections, mainly in patients with LRI. Claustrophobia, cough, dyspnea, bronchospasm, nausea, conjunctival irritation, rash, and deterioration in pulmonary function are often reported by patients. The usage of AR is often complicated by development of bronchoconstriction and may not be ideal for use in patients with asthma or chronic obstructive pulmonary disease.67-69 Furthermore, patients with severe RSV infection requiring mechanical ventilation and receiving AR should be monitored for mechanical ventilator dysfunction and associated increased pulmonary pressures.70 In addition, precipitation of the particles of the drug in the artificial airway may lead to formation of mucous plugs and impaired ventilation.67-69 AR has been associated with significant expenditure, estimated in one study to add £3614 per patient for an average of 11 days of treatment.19 At our institution, a 10-day course of AR can cost more than $50 000.
Table 1 summarizes the available data on the usage of AR alone in HSCT recipients with RSV infection. Usage of AR alone was reported in 11 studies6,7,10,16,21,22,24,54-57 : 4 were prospective studies, 6 were retrospective, and only 1 was a randomized controlled trial. Information on 273 patients was reported; 128 (47%) cases progressed to LRI and 59 patients (46%) died of RSV infection. Forty-four patients were treated at URI stage and in 11 (25%; range, 0%-32%) the infection progressed to LRI. A higher proportion of cases progressed to LRI (54, 47%; range, 27%-100%) among the 116 patients who were not treated at URI stage (P = .01). Patients whose infection progressed to LRI and were not treated had a higher mortality rate than those whose infection was treated (deaths among treated: 28 of 56 [50%]; range, 33%-88%; deaths among untreated: 8 of 9 [89%]; range, 50%-100%, P = .04).
AR and an immunomodulator.
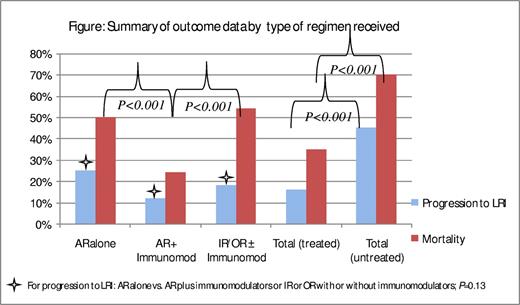
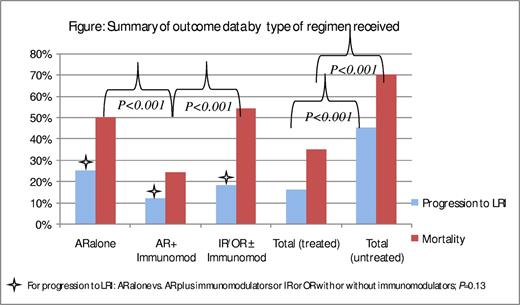
Thirteen studies reported treatment with AR and an immunomodulator in combination (mainly IVIG and/or RSV-IVIG, 10 studies); these studies are summarized in Table 2.1,3,7,9,10,14,17,23,24,26,29,58,59 Five studies were prospective in design, 7 were retrospective, and only one study was a randomized controlled trial. A total of 407 patients were included in these studies. Rates of progression to LRI and RSV-related mortality averaged 48% (196) and 29% (56), respectively. Among 95 patients whose infection was treated at URI stage, the infection progressed to LRI in 11 (12%; range, 0%-29%). On another hand, among the 174 patients whose infection was not treated at URI stage, the infection progressed to LRI in 78 (45%; range, 0%-100%). Among the 136 patients who were treated after the infection progressed to LRI, 33 died (24%; range, 0%-67%); the mortality rate was higher (77%; range, 25%-100%) among the 22 patients whose infection was not treated. It is interesting to note that the rate of progression to LRI was higher in patients who were treated with AR alone than in those treated with a combination of AR and an immunomodulator (25% vs 12%; P = .13), as was the RSV-related mortality rate (50% vs 24%, P < .001; Figure 1).
Summary of outcome data by type of regimen received. For progression to LRI: AR alone versus AR plus immunomodulators or IR or OR with or without immunomodulators. P = .13. AR indicates aerosolized ribavarin; IR, intravenous ribavarin; and OR, oral ribavarin.
Summary of outcome data by type of regimen received. For progression to LRI: AR alone versus AR plus immunomodulators or IR or OR with or without immunomodulators. P = .13. AR indicates aerosolized ribavarin; IR, intravenous ribavarin; and OR, oral ribavarin.
Intravenous or oral ribavirin with or without an immunomodulator.
Because of the paucity of published studies using either the intravenous or oral form of ribavirin, separately or in combination with an immunomodulator(s), we combined the studies for each form into one table (Table 3). The use of intravenous or oral ribavirin with or without immunomodulator(s) has been reported from few centers, mainly in Europe.4,10,19,20,25,57,60,64,65 Intravenous ribavirin may be a good therapeutic option when lung consolidation may prevent penetration or absorption of the aerosolized drug. Intravenous ribavirin has been shown to be well tolerated in HSCT patients.60 Reported side effects include hemolysis, leukopenia, and hyperbilirubinemia. On another hand, oral ribavirin has been shown be well tolerated in HSCT patients with RSV infection.4,65 It is well absorbed, with 50% bioavailability through first-pass metabolism. It is less costly than the intravenous and the aerosolized forms and does not require hospitalization of the patient. However, the use of oral ribavirin has often been associated with the development of anemia, which is usually reversible with no delay in engraftment, and with nausea.20,71 Hemolysis with subsequent hyperbilirubinemia and leukopenia were reported with the use of IV ribavirin.1,4,25,60 Moreover, absorption of oral ribavirin in patients with GVHD may not be optimal and was not studied. This may account for worse outcomes in the studies involving oral ribavirin.
Six studies reported the outcome of RSV infection in HSCT patients who were treated with intravenous and/or oral ribavirin,4,10,20,25,60,69 including 2 studies4,10 in which patients also received an immunomodulator(s). Four of the studies were retrospective in design and 2 were prospective. Overall, information on 210 patients was reported, with average rates of progression to LRI of 46% (96 patients) and mortality of 52% (50 patients). The RSV was treated at URI stage in a small proportion of patients22 and progressed to LRI in 4 (18%; range, 0%-50%); among the 52 untreated patients, it progressed to LRI in 18 (35%; range, 0%-100%). When the infection was treated at LRI stage, 26 of 48 (54%; range, 33%-80%) died, whereas 3 of the 4 patients (75%; range, 50%-100%) whose infection was untreated despite progression to LRI died.
In summary, the rate of progression to LRI in these studies was higher in patients whose infection was treated with AR alone than in those whose infection was treated with a combination of AR and an immunomodulator or intravenous and/or oral ribavirin with or without an immunomodulator (25% vs 12% vs 18%, respectively, P = .13). Among patients whose infection progressed to LRI, those treated with AR and an immunomodulator had a lower mortality rate than those treated with AR alone or with intravenous or oral ribavirin with or without an immunomodulator (24% vs 50% vs 54%, respectively; P < .001; Figure 1). It is evident from the data that mortality rates in more recent studies were lower than in older studies across all 3 groups. The contribution of better supportive care, such as early identification of infection, early institution of treatment, better management of concomitant infections, and improvement in ventilatory support, should be considered.
Discussion
We attempted in this review to condense all published data on therapy and outcomes of RSV infection (treated or untreated) in adult HSCT recipients by following a systematic approach to extract information from identified studies on therapeutic modalities and their impact on progression to LRI and mortality. Before we examine what we learned from this thorough review, we need to discuss its many limitations.
All published studies involving RSV infection in adult HSCT patients have been plagued by limitations. Small sample sizes and lack of randomized controlled studies often render the results inevaluable in terms of the efficacy of a treatment modality. In many of the studies, treatment may have been administered only to sicker patients with more severe disease, thus introducing a selection bias. This is more evident in studies involving immunomodulators, which often are not started until after the infection progresses to the lower respiratory tract. On the other hand, patients with mild symptoms might not be tested for RSV and therefore treated or hospitalized, thus introducing a publication bias. Another important limitation of almost every study is the lack of standardized guidelines on therapeutic regimens and duration of treatment for RSV infection. The variability in diagnostic criteria for this infection and in definition of progression to LRI or even RSV-related death makes comparison of these data even more difficult. Physicians and stem cell transplantation centers follow their own guidelines for starting and stopping therapy, and again this lack of standardization may render the data incomparable. The retrospective nature of the data available to us is another major limitation. Slow accrual of patients has precluded the conductance of randomized controlled trials, and hence retrospective or prospective observational data are the only source of information on outcome of this infection. Finally, the complexity of different types of infections that may occur in the HSCT population with regard to diagnosis and management as well as the likelihood that more than one infection is present may leave clinicians with mainly subjective tools to evaluate outcome(s).
To our knowledge, this is the first attempt to systematically review published data on management of RSV infection in adult HSCT recipients and to determine the impact of different forms of ribavirin and immunomodulators on 2 major outcomes: progression to LRI and RSV-related mortality.
From this review, we learned first that, regardless of therapy, LRI was the final site of infection in almost half of the patients studied and that, on average, almost one-third to one-half of patients with RSV LRI had RSV-related or attributable mortality. We can assume that RSV infection has a substantial impact on our HSCT recipients by causing significant morbidity and mortality, especially when contracted in the first few months after HSCT.
Second, for patients treated with ribavirin, regardless of the form or duration of therapy or the addition of an immunomodulator, rate of progression to LRI was much lower than in patients who did not receive any form of RSV therapy. This was also true for mortality rate, which was lower in patients who were treated at the LRI stage.
Third, by looking separately at the different regimens reported in these studies, we found a trend toward a better outcome in regard to progression to LRI and death among patients treated with AR and an immunomodulator than in those treated with AR alone. Delivery of the ribavirin at the site of infection in a rather fast way and probably at a high concentration and the impact of IVIG and RSV-IVIG on lowering the immune response (mainly in the lungs), at least as demonstrated in many animal studies, may explain in part the better outcome seen in many centers that use this regimen. On the other hand, data on the use of oral ribavirin are very limited, but Khanna et al,4 in the largest published study to date on the use of oral ribavirin in HSCT recipients for RSV infection, reported a rather similar outcome from a single center.
On the horizon: newer therapeutic modalities
The many drugs that are undergoing testing for therapy or prevention of RSV infection may be many years away from licensing.
Motavizumab (MEDI-524, MedImmune Inc) is a new monoclonal antibody that has 75-fold higher affinity for binding with RSV fusion protein than PVZ and a 20-fold improvement in neutralization of RSV in vitro. It has been shown to reduce pulmonary RSV titers as much as 100-fold lower than PVZ at equivalent concentrations in animal models.72 Phase 3 trials have shown that it is noninferior to PVZ for prevention of RSV-related hospitalizations in high-risk children and has a comparable adverse effect profile35 ; however, it was not approved by the FDA in a recent filing for licensure.
A new investigational drug, RI-001 (ADMA Biologics Inc), an intravenous high-titer RSV immune globulin that is isolated from healthy adults with high RSV titers, is undergoing phase 2/3 trials for evaluation of safety and efficacy in preventing the progression of RSV infection to LRI stage in immunocompromised patients. It is similar to the withdrawn RSV-IVIG (http://clinicaltrials.gov/ct2/show/study/NCT00632463).73 Administration of RI-001 to 3 immunocompromised adults with RSV LRI showed an increase in serum-neutralizing antibodies and a 10-fold drop in viral RNA in the sputum.74
Fusion inhibitors preclude the lipid viral membrane of RSV from fusing with the lipid membrane of the host, which inhibits infectivity. Although development of these compounds has been plagued with a high failure rate, a few still may be tested in clinical trials.2
A few antisense anti-RSV agents are under development, including one promising compound, ALN-RSV01 (Alnylam Pharmaceuticals). This compound inhibits replication of RSV by interrupting synthesis of the viral nucleocapsid protein (N-protein). Phase 2 trials are underway with the inhaled formulation of this drug.2
Perspectives and final recommendations
Ribavirin is one of the few antiviral modalities available for management of RSV infections in the vulnerable HSCT population. Combination therapy with immunomodulators, such as IVIG, RSV-IVIG, or PVZ, has shown variable success. We think that prompt diagnosis of infection should be coupled with an aggressive approach to management in HSCT patients who have at least one identifiable risk factor for progression to LRI, such as absolute lymphocyte count less than 300/μL or GVHD, with early initiation of ribavirin therapy in either aerosolized or oral form before development of RSV LRI. The oral form of this drug should be explored further, as it is less expensive and probably has fewer and milder side effects than the aerosolized formulation. Moreover, in patients with established RSV LRI, we think that initiation of combination therapy (IVIG or PVZ) before the onset of respiratory failure and need for mechanical ventilation may reduce mortality. Although the cost of a course of AR with either IVIG or PVZ may be comparable, IVIG should be considered because of its anti-inflammatory properties in animal models of RSV infection. Finally, in HSCT patients with multiple risk factors, such as pre-engraftment status, a mismatched donor, and advanced grade GVHD, management of RSV should be considered with combination therapy. These recommendations are based mainly on data from retrospective or prospective observational studies from different centers in the United States and Europe; until completion of randomized clinical trials, physicians are compelled to rely on this kind of data for guidance on management of this potentially fatal infection. Randomized controlled trials are needed in this area; however, the lack of adequate number of cases and the seasonality of this infection, lack of standardized primary endpoints, and guidelines for management of these patients across centers may continue to challenge investigators. In addition, a placebo arm in a randomized trial for therapy of RSV infection in high-risk HSCT recipients would be considered as “unethical” and against “standard of care,” at least at our institution. These challenges must be borne in mind while designing future clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Kathryn Hale for editorial support.
Authorship
Contribution: J.N.S. and R.F.C. designed the study, reviewed the articles, wrote the manuscript, and gave final approval for the manuscript.
Conflict-of-interest disclosure: R.F.C. was supported by research grants from Valeant Pharmaceuticals and ADMA Biologics. The remaining author declares no competing financial interests.
The current affiliation of J.N.S. is Department of Neurology, Baylor College of Medicine, Houston, TX.
Correspondence: Roy F. Chemaly, Department of Infectious Diseases, Infection Control and Employee Health, Unit 402, University of Texas M. D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: rfchemaly@mdanderson.org.