Abstract
β-Thalassemia major results from severely reduced or absent expression of the β-chain of adult hemoglobin (α2β2;HbA). Increased levels of fetal hemoglobin (α2γ2;HbF), such as occurs with hereditary persistence of HbF, ameliorate the severity of β-thalassemia, raising the potential for genetic therapy directed at enhancing HbF. We used an in vitro model of human erythropoiesis to assay for enhanced production of HbF after gene delivery into CD34+ cells obtained from mobilized peripheral blood of normal adults or steady-state bone marrow from patients with β-thalassemia major. Lentiviral vectors encoding (1) a human γ-globin gene with or without an insulator, (2) a synthetic zinc-finger transcription factor designed to interact with the γ-globin gene promoters, or (3) a short-hairpin RNA targeting the γ-globin gene repressor, BCL11A, were tested. Erythroid progeny of normal CD34+ cells demonstrated levels of HbF up to 21% per vector copy. For β-thalassemic CD34+ cells, similar gene transfer efficiencies achieved HbF production ranging from 45% to 60%, resulting in up to a 3-fold increase in the total cellular Hb content. These observations suggest that both lentiviral-mediated γ-globin gene addition and genetic reactivation of endogenous γ-globin genes have potential to provide therapeutic HbF levels to patients with β-globin deficiency.
Introduction
The hemoglobin (Hb) disorders are highly prevalent, autosomal recessive genetic diseases in which coinheritance of 2 defective globin alleles results in severe hematologic disease. One such disorder, β-thalassemia major, results from reduced (β+) or absent (β0) expression of the β-globin chain of adult Hb (α2β2;HbA) because of a variety of deletions and mutations in the β-globin gene or its upstream regulatory elements.1 Intracellular precipitation of excess α-globin chains in bone marrow erythroid cells underlies the disease pathophysiology.2 Intramedullary apoptosis of developing erythroid cells is prominent, whereas those cells that do survive and mature to enucleated red cells have significant abnormalities and a greatly reduced life span in the periphery.3-5 The result is a life-threatening anemia that requires regular red cell transfusion therapy for survival and quality of life. The best opportunity for a cure is hematopoietic stem cell (HSC) transplantation, but this treatment is only available to persons who have human leukocyte antigen-matched donors.6 Matched-sibling allogeneic transplantation is remarkably successful for patients with early-stage β-thalassemia; however, less successful are transplantations performed in patients with late-stage disease or those performed using matched but unrelated donors.7,8 These limitations have made the development of gene therapy for β-thalassemia using autologous HSCs a highly desired goal.
Clinical evidence indicates that elevated fetal Hb (α2γ2;HbF) production mitigates the severity of β-thalassemia as well as sickle cell disease (SCD). Hereditary persistence of fetal Hb (HPFH) represents a group of conditions in which HbF continues to be expressed at significant levels in adult life because of changes both linked and unlinked to the β-globin locus. Patients with 2 β-thalassemia alleles who coinherit an HPFH allele have milder clinical presentations.9-12 Similarly, SCD patients with HPFH or those with certain β-globin locus haplotypes that are associated with increased HbF can demonstrate markedly reduced disease manifestations.13,14 These observations suggested the therapeutic potential of genetic modification of autologous HSCs to enhance HbF. Indeed, we and others have used lentiviral vectors encoding a human γ-globin gene for HSC gene transfer to successfully correct murine models of β-thalassemia intermedia and SCD through high-level HbF expression.15-17 Alternatively, other laboratories focused on using normal or mutated β-globin chains designed for antisickling activity to obtain success in similar mouse models.18-21 Using a different approach, we recently reported the successful use of an artificial transcription factor (GG1-VP64) designed to interact with proximal γ-globin gene promoters to increase production of HbF in erythroid progeny of normal human CD34+ cells.22 The recent discovery that the transcription factor BCL11A has a significant role in silencing of γ-globin expression in adult erythroid cells has led to yet another potential genetic approach to induce HbF.23
These results led us to undertake studies designed to comparatively evaluate enhancement of HbF levels through lentiviral gene transfer of (1) an erythroid-specific, γ-globin lentiviral vector (V5m3) further modified to include a 400-bp chicken HS4 insulator element (V5m3-400), (2) the GG1-VP64 zinc-finger transcription factor, and (3) a short-hairpin RNA-targeting BCL11A expression using CD34+ cells from both normal donors and patients with β-thalassemia major. Using an in vitro model of human erythropoiesis, we measured HbF levels in late-stage erythroblasts derived from normal CD34+ cells transduced with each vector. In all cases, we observed significant increases in HbF with an average vector copy of 1 or 2. Studies performed using β-thalassemic CD34+ cells resulted in similar gene transfer efficiencies with HbF production ranging from 45% to 60%, leading to a 2.5- to 3-fold increase in the total Hb content per cell. These levels would probably prove therapeutic to patients with a similar degree of β-globin deficiency.
Methods
Purification of human CD34+ cells and erythroid culture conditions
Cytokine-mobilized peripheral blood cells or steady-state bone marrow (BM) cells were obtained from normal volunteers and patients diagnosed with β-thalassemia major, based on both clinical history and transfusion dependence, according to clinical protocols approved by the Institutional Review Boards of St Jude Children's Research Hospital and the University of Athens. In each case, CD34+ cells were purified using anti-CD34+ antibodies linked to magnetic microbeads.24 Culture conditions used for expansion and differentiation of purified CD34+ cells have been previously described.22 Cell numbers and viability were determined by trypan blue exclusion. Cell morphology was assessed by Wright-Giemsa staining of cytocentrifuge preparations and images acquired with an Olympus Bx41 Upright microscope equipped with a DP70 digital camera and DP manager software v03.02 (Olympus).
Lentiviral vector design
Self-inactivating (SIN) lentiviral vectors were based on our SJ-1 HIV-based lentiviral vector system previously described25 : (1) Green fluorescent protein (GFP) control: Transcription of GFP is regulated by the internal Mp promoter consisting of a modified murine stem cell virus long terminal repeat (LTR) from which nonessential viral leader sequences have been eliminated. (2) γ-Globin: The γ-globin vector termed V5m3 was previously described.17 V5m3 encodes for γ-globin genomic sequences where the endogenous 3′-untranslated region has been replaced with its β-globin counterpart. Erythroid-specific expression is conferred by 3.1 kb of transcriptional regulatory sequences (consisting of DNAse hypersensitive sites HS4, HS3, and HS2) from the β-globin LCR and a 130-bp β-globin promoter. V5m3 was further modified to include a 400-bp core element from the chicken HS4 (cHS4) insulator in the partially deleted U3 portion of the 3′-LTR to create V5m3-400. (3) GG1-VP64/GFP: The details regarding the construction of the β-spectrin regulated bicistronic expression vector encoding for the rhesus variant of the designer zinc-finger transcription factor (GG1-VP64) and GFP have been described previously.22 (4) shBCL11A/GFP: Vectors encoding for control (pLKO.1-PGK-GFP) and U6 regulated expression of shRNA-encoding sequences specific to the γ-globin gene repressor protein BCL11A (pLKO.1-shBcl11A-PGK-Puro) were previously described.23 pLKO.1-shBCL11A-PGK-Puro was modified to replace the Puro marker with the GFP marker. For this, an 885-bp fragment that included the GFP coding sequence was excised from pLKO.1-PGK-GFP as a BamHI-Acc65I fragment and cloned between the same sites of pLKO.1-shBCL11A-PGK-Puro, resulting in pLKO.1-shBCL11A-PGK-GFP.
Lentiviral vector production and transduction
Lentiviral vector particles pseudotyped with vesicular stomatitis virus glycoprotein were prepared using a 4-plasmid system by transient transfection as previously described.25 For transduction, CD34+ cells grown for approximately 48 hours in expansion medium were transferred to RetroNectin-coated 24-well plates (50 μg/cm2; Fisher Scientific) at a concentration of 2 × 105 to 4 × 105 cells/mL in media supplemented with protamine sulfate (10 μg/mL) and incubated overnight with vector particles (multiplicity of infection [MOI], 5-20). The next day, cells were returned to the expansion medium with an aliquot (1000 cells/mL) mixed with semisolid methylcellulose medium supplemented with human cytokines (H4434, StemCell Technologies) to assess multilineage colony-forming potential and gene transfer efficiency.
Methylcellulose colony-forming unit assay
Transduced CD34+ cells were suspended in cytokine-supplemented methylcellulose and maintained for 10 to 12 days at 37°C in a humidified atmosphere of 5% CO2. Discrete colonies developed from clonogenic cells transduced with γ-globin lentiviral vectors (V5m3 and V5m3-400) were picked into 10 μL of H2O, and 2.5 μL of this cell suspension was used as template for polymerase chain reaction (PCR) amplification of vector-specific γ-globin encoding sequences using forward primer (5′-CAG AGG AGG ACA AGG CTA CT-3′) and reverse primer (5′-CAC CAT CTT CTG CCA GGA AGC-3′). The genomic β-actin gene specific forward primer (5′-GAT GAT ATC GCC GCG CTC GT-3′) and reverse primer (5′-GGT CAT CTT CTC GCG GTT GG-3′) were used as a PCR internal control in a separate reaction.
Flow cytometry analysis of gene transfer, erythroid differentiation, and apoptosis
Cells at various stages of differentiation were analyzed by flow cytometry. Live cells were identified and gated by exclusion of 7-amino-actinomycin D (BD Biosciences) and tested for expression of GFP or positivity for CD71 and CD235 using antibodies conjugated to either phycoerythrin or allophycocyanin on a FACSCalibur System (BD Biosciences) using CellQuest analysis software v5.2.1 (BD Biosciences). Apoptosis was determined by detection of double-stranded breaks in DNA using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay and analysis by flow cytometry. For this, erythroid cells (1-2 × 106) derived from β-thalassemic CD34+ cells were collected after 14 or 15 days of culture, pelleted, and fixed with 1% paraformaldehyde in phosphate-buffered saline before being washed and resuspended in 70% ethanol for storage at 20°C. Fixed cells were divided equally and washed twice with cold phosphate-buffered saline before addition of TdT buffer supplemented with digoxigenin-11-dUTP + TdT enzyme (all from Roche Applied Science). After incubating for 30 minutes at 37°C, samples were labeled with allophycocyanin-conjugated antidigoxigenin monoclonal antibody (Roche Applied Science) and incubated at room temperature for 30 minutes before being treated with RNAse (Roche Applied Science) and analyzed on a FACSAriaII (BD Biosciences) using FACSDiva analysis software v6.0 (BD Biosciences).
Determination of vector copy number by Southern blot analysis
The average vector copy number in transduced CD34+ cell populations was determined by Southern blot analysis using DNA prepared from bulk populations of erythroid cells as previously described.25
γ-Globin mRNA analysis
RNA was subjected to reverse transcription followed by quantitation of both γ-globin and α-globin transcript cDNAs by real time PCR using TaqMan One-Step RT-PCR Master Mix Reagents Kit, the γ-globin primer/probe set Hs00361131_g1 and the α-globin primer/probe set Hs00361191_g1 (all from Applied Biosystems). The relative values of γ-globin and α-globin mRNA for each sample were calculated using the ΔΔCt method using the β2-microglobulin transcript signal as an internal control. The β2-microglobulin cDNA was detected using primer/probe set 4333766F from Applied Biosystems. Final results were represented as γ/α globin mRNA ratios.
Hb analysis and quantification
Cells (10-20 million) were harvested after 7 to 9 days in the differentiative phase of erythroid culture, lysed in 40 μL of hemolysate reagent (Helena Laboratories), and refrigerated overnight before centrifuging (10 600g at 4°C for 10 minutes) to remove cellular debris. The cleared supernatants were used for characterization of Hb production by cellulose acetate Hb electrophoresis using Hb standards purchased from Helena Laboratories or high-performance liquid chromatography (HPLC) using calibrated samples for the human Hbs according to methodologies previously established in our laboratory.17
Total Hb content in the erythroid cells derived from β-thalassemic donors was estimated relative to levels obtained for erythroid cells derived from normal CD34+ cells cultured under identical conditions. To make this calculation, the combined area under the curve of HPLC peaks for acetylated HbF, HbF, and HbA was used. Alternatively, densitometry of appropriate bands on cellulose acetate gels was performed to provide relative quantitation.
Western blot analysis for BCL11A
Proteins were extracted from the various samples using m-PER (Thermo Scientific). Proteins were separated using 10% NuPAGE 10% Bis-Tris gels (Invitrogen) and were transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% milk in Tris-buffered saline with 0.5% Tween-20. BCL11A was detected using a 1:1000 dilution of mouse monoclonal antibody to Ctip1 (clone 14B5 from Abcam), followed by goat antimouse IgG1-horseradish peroxidase conjugate (Santa Cruz Biotechnology). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected using a 1:35 000 dilution of monoclonal anti-GAPDH peroxidase antibody (clone GAPDH 71.1, Sigma-Aldrich).
Statistical analysis
Microsoft Excel was used to determine descriptive statistics (mean ± SD) and significant differences between mean values using Student t test. Paired and unpaired comparisons were used as appropriate.
Results
Lentiviral vector-mediated transfer of the Aγ-globin gene into normal human adult CD34+ cells results in high levels of HbF in erythroid progeny
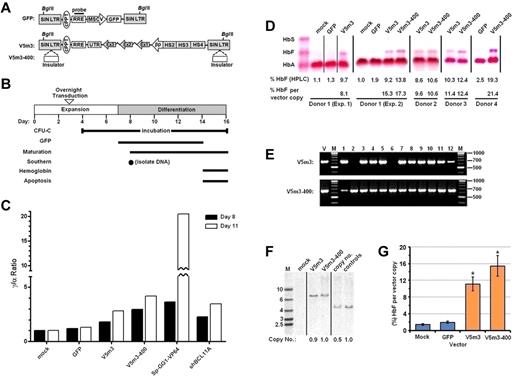
Cytokine-mobilized peripheral blood (PB) CD34+ cells from 4 different normal adult donors were transduced with the SIN V5m3 γ-globin lentiviral vector (MOI = 20) or a GFP control vector (MOI = 5; Figure 1A). We also tested a modified version of V5m3 (termed V5m3-400; MOI of 20), which contains, in the integrated proviral LTRs, a 400-bp insulator element from the chicken β-globin locus (Figure 1A). This element, or a portion thereof, has been reported to diminish position effects on expression and also reduce the risk of insertional gene activation.26-28 To assay for increased production of HbF provided by these vectors, we used a 2-phase erythroid culture model, initiated with the CD34+ cells, which reproduces the adult pattern of Hb production with levels of HbF 3% or less.22 The experimental schema is shown in Figure 1B. In an initial experiment, we measured γ-globin mRNA levels on days 8 and 11 in erythroid cells derived from mock-, GFP-, and V5m3- and V5m3-400–transduced CD34+ cells (Figure 1C). γ-Globin mRNA levels were increased 3- to 4-fold on day 11 by the V5m3 and V5m3-400 vectors, respectively, relative to control cells (mock set to relative value of 1).
Increased HbF levels in erythroid cells derived from normal CD34+ cells transduced with γ-globin lentiviral vectors. (A) Top: Schematic of control SIN lentiviral vector encoding GFP expressed from a modified murine stem cell virus LTR promoter. Bottom: Schematic of SIN, erythroid-specific γ-globin lentiviral vectors, using as transcriptional control elements 3.1 kb of sequences from the β-globin LCR and a 130-bp β-globin promoter. This vector also contains 3′-untranslated sequences (UTR) from the native β-globin coding sequence in the presence or absence of a core 400-bp insulator (open rectangle) element inserted into the deleted U3-region of the 3′-LTR referred to as V5m3 or V5m3-400, respectively. Both vector backbones contain the central polypurine track (cPPT) and the rev responsive element (RRE), as indicated, and have an SIN design in which the promoter and enhancer of the HIV U3 region are deleted. (B) Experimental schema with time course of expansion and differentiation phases as indicated. Time points or intervals are indicated for the specific determinations shown at left. (C) γ-Globin mRNA levels (represented as γ/α ratios) are shown as measured by reverse-transcriptase PCR in the various cell populations on days 8 and 11. (D) Hb electrophoresis demonstrating various production of HbF in erythroblasts derived from CD34+ cell populations from 4 independent normal donors transduced using mock conditions or with a lentiviral vector encoding GFP (MOI = 5), or human γ-globin encoding vectors V5m3 or V5m3-400 (MOI = 20), respectively. The percentage of HbF as quantified by HPLC analysis and normalized to average vector copy number in bulk cell populations as determined by Southern blot and densitometry analysis is indicated below each lane. (E) Representative PCR analysis for the presence of vector-encoded sequences in genomic DNA isolated from individual colonies derived from CD34+ cells transduced with the indicated γ-globin vectors. Size markers (M) shown at right. V indicates positive control DNA for the vector. Numbers above lanes indicate individual samples. (F) Southern blot analysis of genomic DNA, cut with BglII, from bulk cell populations transduced with the indicated lentiviral vectors. Average vector copy number was determined by densitometry relative to a K562 clone that contains a single copy of an integrated GFP-encoding lentiviral vector. (G) The percentage of HbF, relative to total cellular Hb and normalized to vector copy, in the indicated erythroblast populations (± SEM). *P < .004 compared with the mock control (paired t test); n = 3. Vertical lines have been inserted to represent repositioned lanes on the gel images.
Increased HbF levels in erythroid cells derived from normal CD34+ cells transduced with γ-globin lentiviral vectors. (A) Top: Schematic of control SIN lentiviral vector encoding GFP expressed from a modified murine stem cell virus LTR promoter. Bottom: Schematic of SIN, erythroid-specific γ-globin lentiviral vectors, using as transcriptional control elements 3.1 kb of sequences from the β-globin LCR and a 130-bp β-globin promoter. This vector also contains 3′-untranslated sequences (UTR) from the native β-globin coding sequence in the presence or absence of a core 400-bp insulator (open rectangle) element inserted into the deleted U3-region of the 3′-LTR referred to as V5m3 or V5m3-400, respectively. Both vector backbones contain the central polypurine track (cPPT) and the rev responsive element (RRE), as indicated, and have an SIN design in which the promoter and enhancer of the HIV U3 region are deleted. (B) Experimental schema with time course of expansion and differentiation phases as indicated. Time points or intervals are indicated for the specific determinations shown at left. (C) γ-Globin mRNA levels (represented as γ/α ratios) are shown as measured by reverse-transcriptase PCR in the various cell populations on days 8 and 11. (D) Hb electrophoresis demonstrating various production of HbF in erythroblasts derived from CD34+ cell populations from 4 independent normal donors transduced using mock conditions or with a lentiviral vector encoding GFP (MOI = 5), or human γ-globin encoding vectors V5m3 or V5m3-400 (MOI = 20), respectively. The percentage of HbF as quantified by HPLC analysis and normalized to average vector copy number in bulk cell populations as determined by Southern blot and densitometry analysis is indicated below each lane. (E) Representative PCR analysis for the presence of vector-encoded sequences in genomic DNA isolated from individual colonies derived from CD34+ cells transduced with the indicated γ-globin vectors. Size markers (M) shown at right. V indicates positive control DNA for the vector. Numbers above lanes indicate individual samples. (F) Southern blot analysis of genomic DNA, cut with BglII, from bulk cell populations transduced with the indicated lentiviral vectors. Average vector copy number was determined by densitometry relative to a K562 clone that contains a single copy of an integrated GFP-encoding lentiviral vector. (G) The percentage of HbF, relative to total cellular Hb and normalized to vector copy, in the indicated erythroblast populations (± SEM). *P < .004 compared with the mock control (paired t test); n = 3. Vertical lines have been inserted to represent repositioned lanes on the gel images.
As previously observed,22 lentiviral vector transduction had negligible effects on cell growth and differentiation, as gauged by acquisition of the erythroid marker glycophorin A (CD235) and cell morphology consistent with orthochromic erythroblasts (Table 1; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The GFP vector achieved an average transduction rate of 87% plus or minus 6% with no effect on production of HbF (1.9% ± 0.5% by HPLC), compared with mock-transduced cultures (Table 1; Figure 1D; supplemental Figure 2). For the globin vectors, gene transfer efficiency averaged 77% and 91%, respectively, for V5m3 and V5m-400, as determined by the presence of vector sequence in genomic DNA of progenitor cells capable of forming colonies (CFU) in methylcellulose supplemented with hematopoietic cytokines (Table 1; Figure 1E). Southern blot analysis of genomic DNA was used to confirm transmission of intact proviral genomes and determine average vector copy number (Table 1; Figure 1F). Vector copy number for both globin vectors averaged approximately one copy per cell. Erythroid cells derived from these transduced progenitors demonstrated mean levels of HbF per vector copy of 11.1% plus or minus 3.1% for V5m3 and 15.4% plus or minus 4.9% for V5m3-400, compared with background HbF levels of 1.4% and 1.9% for mock and GFP-transduced cells, respectively (Table 1; Figure 1C-G; supplemental Figure 2). The levels of HbF derived from the insulated V5m3-400 vector trended higher than the uninsulated V5m3 compared in a pair-wise fashion (P = .057; n = 3 paired experiments).
High levels of HbF through endogenous γ-globin gene activation in adult erythroid progeny after lentiviral vector-mediated expression of the GG1-VP64 transcriptional activator or shRNA-mediated knockdown of BCL11A
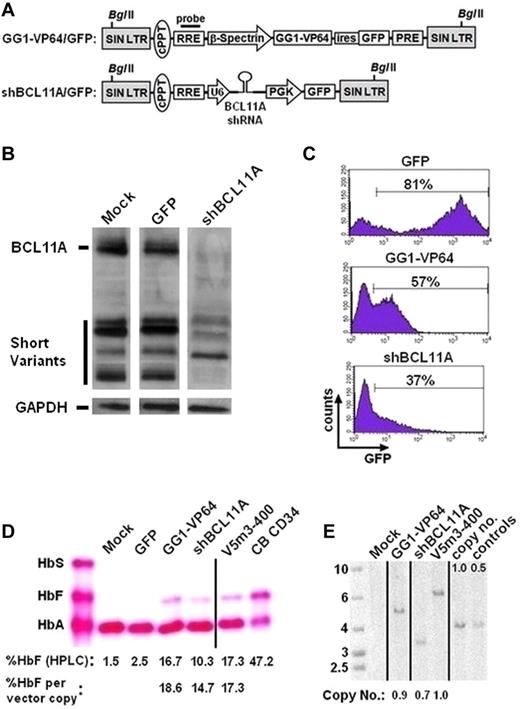
A lentiviral vector containing both an effective shRNA targeting BCL11A driven by the RNA polymerase III U6 promoter and a human phosphoglycerate kinase (PGK) promoter-driven puromycin resistance gene23 was modified to replace the drug selectable marker with the GFP marker to permit tracking of unselected transduced cells during the culture period (Figure 2A). We sought to compare outcomes with this vector after transduction of normal PB CD34+ cells with those obtained with the insulated γ-globin vector (V5m3-400) and our β-Spectrin-GG1-VP64/GFP vector (Figure 2A).22 In an initial experiment, we assessed the degree of BCL11A knockdown by Western blot analysis of erythroid cells derived from normal CD34+ cells transduced with the BCL11A shRNA vector, as well as relative γ-globin mRNA levels for all 3 vectors. As shown in Figure 2B, BCL11A protein levels were quite effectively reduced by the BCL11A shRNA vector, compared with mock-transduced and GFP-vector transduced cells. γ-Globin mRNA levels were increased 3-fold in the BCL11A shRNA vector-transduced cells on day 11 (Figure 1C), similar to that previously observed.23 Strikingly, γ-globin mRNA levels were increased approximately 20-fold in GG1-VP64 vector-transduced cells.
Increased HbF levels in erythroid cells derived from normal CD34+ cells after lentiviral vector-mediated expression of the GG1-VP64 transcriptional activator or a BCL11A shRNA. (A) Top: Schematic showing bicistronic SIN lentiviral vector encoding for GG1-VP64 and GFP transcriptionally regulated by the erythroid-specific β-spectrin promoter. The woodchuck hepatitis virus posttranscriptional regulatory element (PRE) is present as indicated. Bottom: SIN lentiviral vector containing a U6 promoter-regulated shRNA targeting BCL11A along with a second cassette that encodes GFP driven by the human PGK promoter. (B) Western blot analysis for BCL11A protein in the indicated cell populations. A total of 0.9 to 1.5 μg of protein was loaded per lane, as volume allowed. GAPDH signal, shown below each lane, was used as a protein loading control. The relevant gel lanes were repositioned and are separated by the white interface between each lane. (C) Flow cytometric analysis for GFP marker expression in erythroblasts transduced with GFP control (MOI = 5), GG1-VP64/GFP (MOI = 10), or shBCL11A/GFP (MOI = 10) lentiviral vectors. The percentages indicate the proportion of cells considered GFP+ at the end of culture. (D) Cellulose acetate Hb electrophoresis of cell lysates from erythroblasts at the end of culture. The percentage of HbF, as quantified by HPLC analysis and normalized to vector copy, is indicated below each lane. (E) Southern blot analysis of genomic DNA from transduced bulk cell populations demonstrating average vector copy number as determined by densitometry analysis, as done in Figure 1E. Vertical lines have been inserted to represent repositioned lanes on the gel images.
Increased HbF levels in erythroid cells derived from normal CD34+ cells after lentiviral vector-mediated expression of the GG1-VP64 transcriptional activator or a BCL11A shRNA. (A) Top: Schematic showing bicistronic SIN lentiviral vector encoding for GG1-VP64 and GFP transcriptionally regulated by the erythroid-specific β-spectrin promoter. The woodchuck hepatitis virus posttranscriptional regulatory element (PRE) is present as indicated. Bottom: SIN lentiviral vector containing a U6 promoter-regulated shRNA targeting BCL11A along with a second cassette that encodes GFP driven by the human PGK promoter. (B) Western blot analysis for BCL11A protein in the indicated cell populations. A total of 0.9 to 1.5 μg of protein was loaded per lane, as volume allowed. GAPDH signal, shown below each lane, was used as a protein loading control. The relevant gel lanes were repositioned and are separated by the white interface between each lane. (C) Flow cytometric analysis for GFP marker expression in erythroblasts transduced with GFP control (MOI = 5), GG1-VP64/GFP (MOI = 10), or shBCL11A/GFP (MOI = 10) lentiviral vectors. The percentages indicate the proportion of cells considered GFP+ at the end of culture. (D) Cellulose acetate Hb electrophoresis of cell lysates from erythroblasts at the end of culture. The percentage of HbF, as quantified by HPLC analysis and normalized to vector copy, is indicated below each lane. (E) Southern blot analysis of genomic DNA from transduced bulk cell populations demonstrating average vector copy number as determined by densitometry analysis, as done in Figure 1E. Vertical lines have been inserted to represent repositioned lanes on the gel images.
In a second experiment, we observed that 37% of the erythroid cells derived from the CD34+ cells transduced with the BCL11A shRNA-encoding vector expressed GFP compared with 57% for the GG1-VP64/GFP vector and 81% for the GFP control (Figure 2C). For the γ-globin vector (V5m3-400), gene transfer efficiency in CFU grown in cytokine supplemented methylcellulose was more than 80% (data not shown). Under all conditions, we observed a similar capacity of cells transduced with the 3 different vectors for erythroid differentiation as evidenced by cell morphology and expression of CD71 and glycophorin A on most cells (data not shown). Erythroblasts differentiated from mock-transduced or GFP-control virus-transduced cells demonstrated an adult pattern of Hb production with low levels of HbF in the 1% to 3% range (Figure 2D). In contrast, knockdown of BCL11A or expression of GG1-VP64 resulted in production of HbF to levels of 10% and 17%, respectively, compared with 17% for the γ-globin vector (Figure 2D). Thus, although there was a general trend in concordance, there was not a strict quantitative correlation of HbF protein with the previously measured γ-globin mRNA levels. When results were normalized to HbF output per vector copy (using Southern blot analysis for copy number determination, Figure 2E), the 3 approaches to HbF enhancement were similar, ranging from 15% to 19% HbF (Figure 2D).
Despite the significant induction of HbF by the GG1-VP64 and BCL11A shRNA vectors, we observed a modest reduction in the accumulated cell number in these cultures (supplemental Figure 3). In the case of GG1-VP64, this was consistent with our previous observations.22 Perhaps more noteworthy were results with the BCL11A shRNA vector where we also observed a loss of GFP+ cells over time: 62% GFP+ vs 37% GFP+ from day 7 to day 14 (supplemental Figure 3B). In contrast, the percentage of GFP+ cells remained stable during the culture for the GFP control and GG1-VP64 vectors (supplemental Figure 3B).
Therapeutic production of HbF in erythroblasts derived from CD34+ bone marrow cells of β-thalassemia major patients transduced with the V5m3-400 γ-globin, BCL11A shRNA, or GG1-VP64 lentiviral vectors
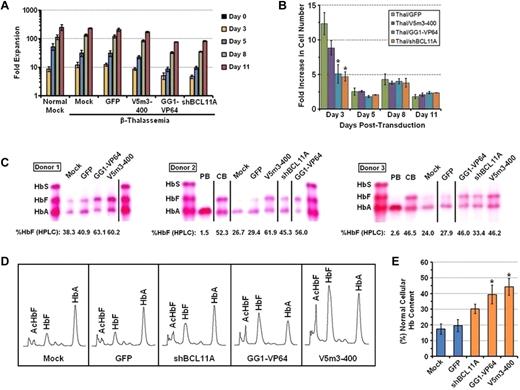
We next sought to determine whether our HbF-enhancing vectors could ameliorate the chain imbalance in β-thalassemia using CD34+ cells derived from steady-state bone marrow of 3 patients with β-thalassemia major. Cells were transduced with control GFP, V5m3-400, GG1-VP64, or BCL11A shRNA lentiviral vectors using conditions identical to those described for transduction and culture of normal CD34+ cells. Similar to the results using normal PB CD34+ cells, overall cellular expansion was somewhat diminished over the 11 days after transduction with the shBCL11A and GG1-VP64 vectors, relative to the GFP control or V5m3-400 vector (Figure 3A; Table 2; supplemental Figure 1B). Interestingly, this decrease reproducibly occurred during the first 3 days of culture after transduction and was not apparent during later time points in culture (Figure 3B). This pattern is consistent with toxicity in a proportion of high-expressing cells. Similar to what was observed using normal donor cells, thalassemic cells transduced with the BCL11A shRNA vector again demonstrated a moderate decline in GFP+ cells over time (supplemental Figure 4). However, erythroid differentiation was not affected as all cultures showed similar levels of CD235 positivity and morphology consistent with late-stage erythroblasts (Table 2; supplemental Figure 1B).
Therapeutic production of HbF in erythroblasts derived from CD34+ bone marrow cells of β-thalassemia major patients transduced with the V5m3-400 γ-globin, BCL11A shRNA, or GG1-VP64 lentiviral vectors. (A) Cell accumulation at the indicated time points during the 2-phase culture is shown. Data represent the mean ± SEM of the cumulative fold expansion of both normal and β-thalassemic viable cells either mock-transduced or transduced with the indicated lentiviral vectors. (B) Fold increase in cell number for the indicated groups as measured from the prior time point. (C) Cellulose acetate Hb electrophoresis of lysates from erythroblasts derived from CD34+ BM cells of 3 independent β-thalassemic donors that were transduced either under mock conditions or with the indicated vectors. The percentage of HbF as determined by HPLC analysis is indicated below each sample lane. Control samples include the following: PB indicates erythroblasts derived from cytokine-mobilized peripheral blood CD34+ cells of normal donors; and CB, erythroblasts derived from CD34+ cord blood cells. Vertical lines have been inserted to represent repositioned lanes on the gel images. (D) Representative Hb HPLC traces from erythroblasts derived from β-thalassemic either mock-transduced or transduced with the indicated vectors. (E) The percentage of total Hb content, relative to that of erythroblasts derived from normal PB CD34+ cells, of β-thalassemic erythroblasts derived from the indicated transduced cell populations (± SEM). *P < .05 compared with mock control (paired t test).
Therapeutic production of HbF in erythroblasts derived from CD34+ bone marrow cells of β-thalassemia major patients transduced with the V5m3-400 γ-globin, BCL11A shRNA, or GG1-VP64 lentiviral vectors. (A) Cell accumulation at the indicated time points during the 2-phase culture is shown. Data represent the mean ± SEM of the cumulative fold expansion of both normal and β-thalassemic viable cells either mock-transduced or transduced with the indicated lentiviral vectors. (B) Fold increase in cell number for the indicated groups as measured from the prior time point. (C) Cellulose acetate Hb electrophoresis of lysates from erythroblasts derived from CD34+ BM cells of 3 independent β-thalassemic donors that were transduced either under mock conditions or with the indicated vectors. The percentage of HbF as determined by HPLC analysis is indicated below each sample lane. Control samples include the following: PB indicates erythroblasts derived from cytokine-mobilized peripheral blood CD34+ cells of normal donors; and CB, erythroblasts derived from CD34+ cord blood cells. Vertical lines have been inserted to represent repositioned lanes on the gel images. (D) Representative Hb HPLC traces from erythroblasts derived from β-thalassemic either mock-transduced or transduced with the indicated vectors. (E) The percentage of total Hb content, relative to that of erythroblasts derived from normal PB CD34+ cells, of β-thalassemic erythroblasts derived from the indicated transduced cell populations (± SEM). *P < .05 compared with mock control (paired t test).
Both mock-transduced cells and cells transduced with the GFP control vector demonstrated production of a small amount of HbA (15%-20% that of HbA content of normal erythroblasts) consistent with the patients having a β+/β0 or β+/β+ thalassemic genotype, (Figure 3C-D). Importantly, erythroid cells derived from β-thalassemic CD34+ cells transduced with the V5m3-400, GG1-VP64, or BCL11A shRNA vectors demonstrated significant increases in the amount of HbF, compared with control cells (Figure 3C-D; Table 2). This resulted in corresponding substantial increases in the total cellular Hb content by all 3 vectors (Figure 3E), with the V5m3-400 and GG1-VP64 vectors approaching an Hb content of nearly 50% of normal. We also assessed terminal-stage cultures of β-thalassemic cells transduced with the various lentiviral vectors by flow cytometry for TdT-mediated dUTP nicked end labeling assay, as a measure of apoptosis, a feature reported to be characteristic of the ineffective erythropoiesis in patients with β-thalassemia major.29 Under the culture conditions and specific donor cells used, we found relatively low levels of apoptotic cells in mock-transduced cultures (7%-14%) in all experiments with a trend for a reduction only for the V5m3-400 γ-globin vector (supplemental Figure 5).
Further augmentation of HbF levels in β-thalassemic erythroid cells transduced with both GG1-VP64/GFP and V5m3-400 vectors
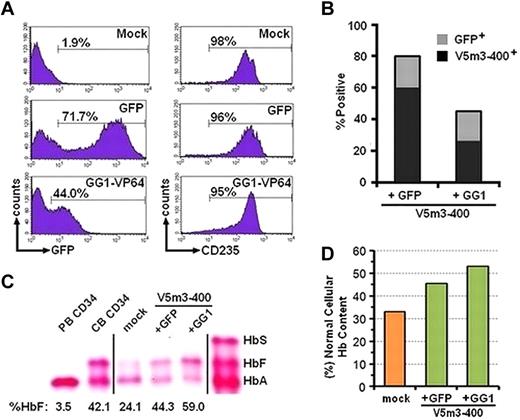
We reasoned that combining γ-globin transgene addition with increased endogenous γ-globin gene expression might further increase the amount of HbF that could be achieved with either approach alone. We therefore simultaneously exposed cells to a mixture of V5m3-400 globin and GG1-VP64/GFP vector particles. We reasoned that a substantial proportion of cells would be transduced with both vectors. As a control, cells were transduced with the V5m3-400 plus the GFP control vector. Approximately 72% of bulk cells and 80% of CFU derived from cells cotransduced with GFP control plus V5m3 vectors were GFP+, and most of these also contained the V5m3 vector as assessed by DNA PCR (Figure 4A-B). For the V5m3-400 plus GG1-VP64/GFP combination, approximately 44% of bulk cells and 40% of CFU contained the GG1-VP64/GFP vector as judged by GFP positivity, with approximately 60% of these also containing the V5m3 vector (Figure 4B). Compared with the globin (V5m3-400) plus GFP control vector, erythroid cells derived from the GG1-VP64/GFP plus V5m3-400 cotransduced CD34+ cells had greater levels of HbF (Figure 4C), despite that overall approximately 25% of the cells (60% of 40%) contained both vectors (Figure 4B). Total Hb content was also further increased with the combined approach (Figure 4D), to a level greater than 50% of that contained in normal cells.
Augmentation of HbF and total Hb content in β-thalassemic erythroblasts derived from CD34+ cotransduced with both the V5m3-400 γ-globin and GG1-VP64/GFP lentiviral vectors. (A) Histograms of flow cytometric analysis of erythroblasts derived from CD34+ cells treated as indicated. Analysis for both GFP and CD235 at the end of the culture. (B) The percentages of CFU transduced with the GFP control or GG1-VP64/GFP vectors, as judged by GFP positivity by fluorescence microscopy, are indicated by the top of the bar (gray). The percentage of GFP+ CFU that contain the V5m3-400 vector by DNA PCR is indicated by the black portion of the bar. (C) Cellulose acetate Hb electrophoresis of lysates from erythroblasts derived from CD34+ BM cells of a β-thalassemic donor cotransduced with the indicated vectors. Percentages below each lane represent the amount of HbF, relative to total Hb, as determined by HPLC. Vertical lines have been inserted to represent repositioned lanes on the gel images. (D) The percentage of total Hb content, relative to that of erythroblasts derived from normal PB CD34+ cells, of β-thalassemic erythroblasts derived from the indicated transduced cell populations. PB CD34 indicates erythroblasts derived from cytokine-mobilized peripheral blood CD34+ cells of normal donors; and CB CD34, erythroblasts derived from CD34+ cord blood cells.
Augmentation of HbF and total Hb content in β-thalassemic erythroblasts derived from CD34+ cotransduced with both the V5m3-400 γ-globin and GG1-VP64/GFP lentiviral vectors. (A) Histograms of flow cytometric analysis of erythroblasts derived from CD34+ cells treated as indicated. Analysis for both GFP and CD235 at the end of the culture. (B) The percentages of CFU transduced with the GFP control or GG1-VP64/GFP vectors, as judged by GFP positivity by fluorescence microscopy, are indicated by the top of the bar (gray). The percentage of GFP+ CFU that contain the V5m3-400 vector by DNA PCR is indicated by the black portion of the bar. (C) Cellulose acetate Hb electrophoresis of lysates from erythroblasts derived from CD34+ BM cells of a β-thalassemic donor cotransduced with the indicated vectors. Percentages below each lane represent the amount of HbF, relative to total Hb, as determined by HPLC. Vertical lines have been inserted to represent repositioned lanes on the gel images. (D) The percentage of total Hb content, relative to that of erythroblasts derived from normal PB CD34+ cells, of β-thalassemic erythroblasts derived from the indicated transduced cell populations. PB CD34 indicates erythroblasts derived from cytokine-mobilized peripheral blood CD34+ cells of normal donors; and CB CD34, erythroblasts derived from CD34+ cord blood cells.
Discussion
Clinical observations strongly suggest that increased HbF levels correlate with diminished disease severity in patients with β-thalassemia and SCD.5-10 Previously, we proved this experimentally by demonstrating that high levels of HbF provided by a γ-globin lentiviral vector using hematopoietic stem cell gene transfer and transplantation-corrected murine models of β-thalassemia15,16 and SCD.17 Our current studies now demonstrate that our γ-globin vector, further optimized by addition of an insulator element, can provide therapeutic levels of additional Hb in the context of human β-thalassemia. Using a different approach that relies on activation of the endogenous γ-globin genes, we also recently established that significant HbF production could be achieved in normal erythroid cells through expression of the GG1-VP64 artificial zinc-finger transcription factor.22 Similarly, the discovery that the transcription factor BCL11A has a significant role in the developmental silencing of γ-globin expression suggested yet another means for HbF induction through genetic knockdown of this repressor.23 Here, we show that all 3 strategies can be used successfully to result in clinically relevant levels of HbF in erythroid cells derived from both transduced normal and β-thalassemic CD34+ cells.
Previously, Imren et al, using a lentiviral vector encoding an antisickling β-globin to transduce normal cord blood hematopoietic cells, obtained β-chain transgene levels of approximately 10% per vector copy in erythroid progeny derived from cord blood progenitors.30 Ours are the first data to examine the levels of the antisickling γ-globin chain and HbF that can be obtained by lentiviral gene transfer in normal adult erythroblasts. We show here that HbF levels averaging 15% per vector copy, compared with levels of 1% to 3% in control cells, can be achieved with the V5m3-400 γ-globin lentiviral vector in the context of normal globin chain balance. These data are relevant to potential therapy in the context of SCD, where γ-globin chains would compete with a full complement of βS chains. The degree of HbF enhancement we achieved would probably have a positive therapeutic effect.31
In addition, the V5m3-400 vector provided a level of HbF that resulted in a nearly 3-fold increase in total cellular Hb in β-thalassemic erythroid cells (Figure 3E). Interestingly, erythroid cells derived from all 3 β-thalassemia major patients in our study demonstrated basal HbA production of approximately 15% to 20% of normal. Our results differ from those previously reported by others3,29 in that we did not observe evidence of arrested erythroid differentiation or poor growth of β-thalassemia major erythroid cells. Instead, we observed that cultures derived from the β-thalassemic donor cells expanded only modestly less well than those from normal donors (compare Tables 1 and 2), with most cells at the end of culture period being morphologically consistent with late-stage erythroblasts. The differences between these studies are probably reflective of both the dissimilar culture conditions used and the different inherent baseline Hb production characteristics of the donors (β+ vs β0). Puthenveetil et al found absent or minimal endogenous β-globin production in erythroid cells from the donor CD34+ cells they used,29 whereas all 3 of our donors demonstrated low but measurable production of HbA (Figure 3C-D). The conditions we used consisted of 2 distinct culture phases: the first predominantly an expansion phase for 7 to 8 days, which included low concentrations of stem cell factor, interleukin-3, and erythropoietin; and the second phase of 8 to 10 days, with only erythropoietin present, promoted differentiation to orthochromic erythroblasts. In contrast, Puthenveetil et al used a single-phase culture of 2 to 3 weeks containing low concentrations of interleukin-3 and GM-CSF but a much higher concentration (5-fold) of erythropoietin.3,29 Our conditions and observations are more similar and consistent with those reported by Amoyal et al32 and Miggliaccio et al.33 These laboratories also found that erythroid cultures derived from β-thalassemia major patients demonstrated significant expansion and differentiation to the orthochromatic erythroblast stage. Consistent with these observations, we did not find high levels of apoptotic cells using our culture conditions (supplemental Figure 5). In contrast, Puthenveetil et al reported high levels of apoptotic cells under their culture conditions, which were rescued by transfer and expression of a normal β-globin gene.29 This is an important point because our results were not probably influenced by significant selection for globin transgene-expressing cells. Thus, we might expect even higher levels of HbF and cellular Hb content in vivo given the selection which has been shown to occur in the BM and in the periphery in murine models.34,35
Our studies also demonstrate that activation of endogenous γ-globin genes can result in significant and potentially therapeutic levels of HbF in both normal and β-thalassemic erythroid cells derived from lentiviral vector-transduced CD34+ cells (Figures 2C, 3C-E). Previous studies used the same BCL11A shRNA as used here, except that drug selection was used to enrich for vector-transduced cells.23,36 In those experiments, differentiated erythroblasts derived from normal adult donors showed HbF levels of 30% to 35%. Here we show that significant HbF levels can also be obtained in the absence of selecting transduced cells, with HbF levels of approximately 10% in erythroid cells derived from normal donors and from 33% to 45% in β-thalassemic erythroid cells. The increased HbF in the β-thalassemic cells resulted in at least a 30% increase in the total cellular Hb content (Figure 3E). Consistent with previous studies,23,36 BCL11A knockdown did not result in altered erythroid differentiation in either normal or β-thalassemic cells, as judged both by acquisition of CD235 expression and cellular morphology (supplemental Figure 1B). However, we did observe a reduction in total cell accumulation using both types of donors after gene transfer with the BCL11 shRNA vector (supplemental Figures 1B, 3A-B). Further investigation showed that this was primarily attributable to a marked reduction in cell growth specifically during the first 3 days after vector transduction (Figure 3A-B). Correspondingly, we observed a loss of transduced cells, as judged by GFP positivity, during the first 8 days of culture (supplemental Figures 3B, 4). Similar observations regarding shRNA-induced cellular toxicity in the initial posttransduction period with loss of transduced cells have been previously reported.37 It has been speculated that this may be the result of excessively high levels of RNAi sequences attributable to use of polymerase III promoters (in our case, the U6 promoter), leading to interference with the endogenous microRNA (miRNA) processing pathways. Alternatively, off-target silencing of important mRNAs, as well as stimulation of cellular responses to double-stranded RNA, may play a role in the initial toxicity. To address this issue, future experiments will evaluate whether using artificial miRNAs as siRNA shuttles, as shown by several laboratories,37-39 can alleviate the toxicity. Use of weaker erythroid-specific, RNA polymerase II promoters might result in alleviation of the toxicity while simultaneously providing for lineage-specific knockdown of BCL11A. This would avoid potential effects of BCL11 knockdown on lymphoid development which has been shown to occur in murine models.40,41
The current studies extend our previous observations that the artificial zinc finger transcriptional activator, GG1-VP64, designed to interact with the γ-globin gene promoters, can enhance HbF production not only in erythroblasts derived from normal donor cells but also in erythroid cells derived from patients with β-thalassemia. Indeed, GG1-VP64, driven by the erythroid-specific β-spectrin promoter, was almost as effective as the V5m3-400 γ-globin lentiviral vector in the amount of HbF produced in both normal and β-thalassemic erythroid cells. Consistent with our prior observations and similar to the results with BCL11A knockdown in the current studies, we found a modest reduction in cell accumulation in GG1-VP64-transduced cultures (supplemental Figures 1B, 3A). Like BCL11A knockdown, the effects on cell accumulation occurred predominately during the initial period after transduction (Figure 3A-B). This might be the result of excessively high levels of GG1-VP64 expression occurring early after transduction, possibly resulting from many copies of unintegrated, episomal vector cDNA, which are ultimately lost after several rounds of cell division. In this regard, we are evaluating other erythroid-specific promoters that might provide lower levels of expression and toxicity while still effectively inducing HbF. In addition, removal from the vector of the woodchuck posttranscriptional regulatory element (Figure 2A), which augments gene expression, may reduce or eliminate toxicity because of high levels of GG1-VP64.
Based on the current work and our previous preclinical studies,15-17,42,43 we plan to use the V5m3-400 γ-globin vector in an initial clinical gene transfer trial for patients with β-thalassemia. Based on this work and the results of an initial β-thalassemic patient in a clinical trial using a β-globin vector,44,45 we think that clinical efficacy is feasible for certain β-thalassemic patients (β+) who are transfusion dependent but have a low, but measurable, baseline level of Hb production. However, a combinatorial approach to achieve even higher levels of HbF merits further consideration in the future. In this regard, we show in this current work that combining γ-globin gene addition with GG1-VP64 can result in further augmentation of HbF and improvement in total cellular Hb of β-thalassemic erythroid cells. Future studies will focus on the development and testing of multifunctional vectors capable of delivering both the γ-globin expression cassette along with either a GG1-VP64 cassette or a miRNA-embedded BCL11A shRNA cassette driven by an appropriate erythroid-specific promoter.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the flow cytometry laboratory of Anna Travelstead and Richard Ashmun for expertise in flow cytometry studies and FACS analysis, patients and normal donors for providing the samples used in these experiments, and M. Rieman and Dr S. Graphakos for help in providing the patients' samples.
This work was supported by the National Heart, Lung, and Blood Institute (PO1HL053749, A.W.N., D.A.P.; R01 HL32259 and P01 HL32262, S.H.O.), the National Heart, Lung, and Blood Institute Basic and Translational Research Program (Sickle Cell Disease grant U54HL070590) (D.A.P.), the Cooley's Anemia Foundation (D.A.P.), the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities. S.H.O. is an Investigator of the Howard Hughes Medical Institute. N.P.A. was supported by the European Commission's 6th Framework Programme (contract LSHB-CT-2004-005242-CONSERT).
National Institutes of Health
Authorship
Contribution: A.W. designed and performed research, analyzed data, and wrote the manuscript; P.W.H., Y.-S.K., and J.M.R. performed the research and reviewed the manuscript; V.G.S., S.H.O., E.P., M.G., and N.P.A. provided critical reagents, designed research, and reviewed the manuscript; A.W.N. designed the research, analyzed the data, and edited the paper; and D.A.P. provided overall organization of the research effort, designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Derek A. Persons, Department of Hematology, St Jude Children's Research Hospital, 262 Danny Thomas Pl, MS#341, Memphis, TN 38105; e-mail: derek.persons@stjude.org.