Abstract
CD28 costimulation is required for the generation of naturally derived regulatory T cells (nTregs) in the thymus through lymphocyte-specific protein tyrosine kinase (Lck) signaling. However, it is not clear how CD28 costimulation regulates the generation of induced Tregs (iTregs) from naive CD4 T-cell precursors in the periphery. To address this question, we induced iTregs (CD25+Foxp3+) from naive CD4 T cells (CD25−Foxp3−) by T-cell receptor stimulation with additional transforming growth factorβ (TGFβ) in vitro, and found that the generation of iTregs was inversely related to the level of CD28 costimulation independently of IL-2. Using a series of transgenic mice on a CD28-deficient background that bears wild-type or mutated CD28 in its cytosolic tail that is incapable of binding to Lck, phosphoinositide 3-kinase (PI3K), or IL-2–inducible T-cell kinase (Itk), we found that CD28-mediated Lck signaling plays an essential role in the suppression of iTreg generation under strong CD28 costimulation. Furthermore, we demonstrate that T cells with the CD28 receptor incapable of activating Lck were prone to iTreg induction in vivo, which contributed to their reduced ability to cause graft-versus-host disease. These findings reveal a novel mechanistic insight into how CD28 costimulation negatively regulates the generation of iTregs, and provide a rationale for promoting T-cell immunity or tolerance by regulating Tregs through targeting CD28 signaling.
Introduction
Regulatory T cells (Tregs) play an essential role in the maintenance of immunologic tolerance to prevent autoimmune disease. The development of Tregs in the thymus requires Foxp3, a member of the group of transcription factors characterized by their winged helix–forkhead DNA-binding domain.1 Although it is widely accepted that natural Tregs (nTregs) develop in the thymus, compelling evidence indicates that Tregs with an identical phenotype can be induced in the periphery from CD4+ non-Treg precursors under certain conditions. For example, all CD4+ cells from RAG−/− T-cell receptor (TCR)–transgenic (Tg) mice are CD25−, but a small proportion of these cells convert to a CD25+ Treg phenotype after adoptive transfer into antigen-bearing mice or mice that have been administered a tolerizing dose of peptide antigen.2,3 Furthermore, de novo generation of CD4+CD25+ Tregs from CD4+CD25− cells can also occur in thymectomized mice.4 Such Tregs that are induced in the periphery are called induced Tregs (iTregs). Although our understanding of the microenvironment for iTreg development in vivo is still limited, it is clear that TCR stimulation, transforming growth factorβ (TGFβ), and interleukin-2 (IL-2) are required for their development.5-8
A crucial regulator of Tregs is the CD28 receptor, a dominant costimulatory molecule for T-cell activation. The first clue to the critical role of the CD28 family in nTreg function was the observation that prevention of CD28 ligation with CTLA4-Ig exacerbated autoimmune disease in nonobese diabetic (NOD) mice.9 Mice deficient for CD28 or its ligands (B7, CD80, and CD86) have a substantially reduced number of nTregs.9,10 As a consequence, NOD mice lacking CD28 develop more rapid and severe autoimmune diabetes compared with wild-type (WT) mice. Recent studies have indicated that CD28 is essential for nTreg development in the thymus and for nTreg survival and homeostasis in the periphery.10,11 Thus, whereas these mice are lacking potent costimulation (CD28) for T-effector cells (Teffs), they are also lacking nTregs, the most effective mediators of self-tolerance, yielding a balanced deficit that results in the preservation of antigen-mediated activation.12
A potential role of CD28 in the generation of iTregs has not been rigorously investigated. Conversion of conventional CD4+CD25− T cells into Tregs occurs in thymectomized mice and requires B7 costimulation.4 Furthermore, CD28 costimulation is required for the generation of iTregs from naive CD4+CD25− T cells through the production of IL-2.13 However, there is also scattered evidence suggesting that high levels of CD28 costimulation reduce Foxp3 expression and limit iTreg generation through an undefined mechanism(s).14,15 In this study, we clearly demonstrate that the high levels of CD28 costimulation suppress generation of iTregs from naive CD4 T cells while promoting the expansion of Teffs. Using a series of Tg mice on a CD28-deficient background that bears either WT or mutated CD28 in its cytosolic tail incapable of binding to lymphocyte-specific protein tyrosine kinase (Lck), phosphoinositide 3-kinase (PI3K), or IL-2–inducible T-cell kinase (Itk), we found that strong CD28 costimulation suppresses iTreg induction through Lck signaling, but that this is independent of IL-2 production.
Methods
Mice
C57BL/6 (B6) and BALB/c mice were purchased from the National Cancer Institute (Bethesda, MD). Ly5.1 and Thy1.1 mice congenic on a B6 background were purchased from The Jackson Laboratory. The Foxp3gfp reporter strain was obtained from the laboratory of A. Y. Rudensky (University of Washington, Seattle, WA).16,17 Founders of CD28 WT and mutation Tg mice on a B6 background, including CD28-WT, CD28-Lck, CD28-PI3K, and CD28-Itk Tg strains, were provided by Drs X. Tai and A. Singer at the National Cancer Institute.10 Each of these 4 Tg strains was bred into CD28 knockout mice so that Tg CD28 molecules were the only CD28 receptors expressed by the Tg T cells. We screened CD28 Tg mice by fluorescence-activated cell sorting (FACS) and, among different strains of mice, selected those that expressed CD28 on CD4+ cells comparably. These strains of mice were bred and all mice used in this study were housed at the H. Lee Moffitt Cancer Center & Research Institute (Tampa, FL). Experimental procedures were reviewed and approved by the institutional animal care and use committee.
Reagents and antibodies
Recombinant mouse IL-2 and human TGF-β1 were purchased from R&D Systems. Anti-mouse CD3 (clone 145.2C11) and CD28 (clone 37.51) monoclonal antibodies (mAbs) were produced and purified in our laboratory. Purified, phycoerythrin-conjugated anti–mouse/rat Foxp3 (clone FJK-16s) was purchased from eBioscience. Other fluorochrome-conjugated mAbs were purchased from eBioscience or Becton Dickinson.
T-cell purification and iTreg generation
CD4+CD25− T cells were purified through negative selection as described in our previous work.13 The purity of CD4+CD25− cells ranged from 85% to 95%, but that for CD4+CD25+ cells was always less than 1% among total CD4+ cells. CD4+CD25− T cells were seeded at 2.5 × 105/well in 48-well plates and stimulated with 0.5-1.0 μg/mL of anti-CD3 mAb in the presence of 1.25 × 106 irradiated syngeneic T cell–depleted (TCD) splenocytes as antigen-presenting cells (APCs) with or without TGF-β1. Exogenous IL-2 at a 2 ng/mL concentration was added in some culture conditions. Alternatively, CD4+CD25− T cells were stimulated by plate-bound anti-CD3 mAb at a 5 or 10 μg/mL concentration without or with plate-bound anti-CD28 mAb at various concentrations in the absence of APCs. In some cases, allogeneic B cells or bone marrow (BM)–derived dendritic cells were used to stimulate CD4+CD25− T cells with TGFβ in generating iTregs. After stimulation for various times, cells were harvested for the measurement of Foxp3 expression or green fluorescent protein (GFP) expression if T cells from Foxp3gfp reporter mice were used.
CFSE labeling and flow cytometric analysis
For measurement of proliferative response in vitro and in vivo, T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) as described previously,18 stimulated, and the CFSE dilution in T cells was analyzed by flow cytometry. Multicolor flow cytometry was performed to measure the expression of surface molecules and intracellular Foxp3 according to the manufacturer's instructions (eBiosciences). Analysis was performed using a FACSCalibur or LSR II flow cytometer and CellQuest Pro Version 5.9.1 (BD Biosciences) or FlowJo Version 8.5.3 software (TreeStar).
Bone marrow transplantation
BALB/c mice were exposed to 800-900 cGy of total body irradiation. TCD-BM cells alone or in combination with purified donor CD25− T cells were injected into recipients via the tail vein within 24 hours after irradiation. Recipient mice were monitored every other day for clinical signs of graft-versus-host disease (GVHD), such as ruffled fur, hunched back, lethargy or diarrhea, and mortality. Animals judged to be moribund were killed and counted as GVHD lethalities, as described in our previous work.19,20 In separate experiments, cell expansion and iTreg generation of donor T cells were measured in the recipient spleens at different times after bone marrow transplantation (BMT).
Statistical analysis
The log-rank test was used to detect statistical differences in recipient survival in GVHD experiments. Student t test was used to compare percentages or numbers of donor T cells.
Results
Strong CD28 costimulation suppresses the generation of iTregs
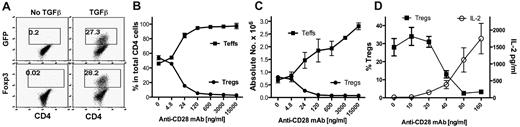
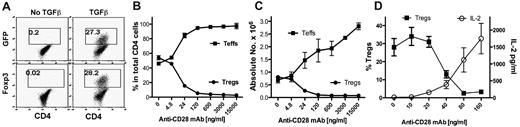
CD28 is required for the full development of nTregs in the thymus and for homeostasis of nTregs in the periphery. In addition to developing in the thymus, Tregs can also be generated or differentiated from naive CD4 T cells in the periphery, and these Tregs are termed iTregs. How CD28 costimulation modulates the generation of iTregs is unclear. We investigated the effect of CD28 costimulation on iTregs generated from naive CD4+CD25−Foxp3− cells in vitro during TCR stimulation in the presence of TGFβ. CD4+CD25−GFP− cells were purified from Foxp3gfp reporter mice and were stimulated with anti-CD3 mAb plus various concentrations of agonistic anti-CD28 mAb. After culturing for 4 days, iTregs (CD4+GFP+) were generated in the presence of TGFβ (Figure 1A). Anti-CD28 mAb inhibited iTreg generation in a dose-dependent manner, both in the percentage of cells (Figure 1B) and in the absolute cell number (Figure 1C). In contrast, anti-CD28 stimulation increased both the numbers of Teffs (CD4+GFP−; Figure 1C) and IL-2 production (Figure 1D) in a dose-dependent manner, which is consistent with the established concept that agonistic anti-CD28 mAb provides T-cell costimulation.
Soluble anti-CD28 mAb reduces iTreg generation in vitro. CD4+GFP− cells from Foxp3gfp reporter mice were purified by FACS and stimulated with anti-CD3 mAb plus irradiated TCD splenocytes as APCs in the absence or presence of TGFβ. Soluble anti-CD28 mAb was added at different concentrations into the culture. Four days after stimulation, cultured cells were harvested and measured for CD4, GFP, and intracellular Foxp3 expression. (A) Data showing the percentage of GFP+ (top panel) or Foxp3+ (bottom panel) cells on gated CD4+ cells. (B) Data presented as the mean of percentage of Teffs (GFP−) and Tregs (GFP+) cells in total CD4+ cells in triplicate wells. (C) Data presented as the mean ± SD of absolute numbers of Teffs (CD4+GFP−) and Tregs (CD4+GFP+) cells in triplicate wells. (D) In separate experiments, cultured cells were harvested and measured for CD4 and GFP expression and the culture supernatant was measured for IL-2 production. The data show the percentage of GFP+ on gated CD4+ cells (left y-axis), and IL-2 production (right y-axis). The data are presented as the mean ± SD in triplicate wells, and represent 1 of 3 replicate experiments.
Soluble anti-CD28 mAb reduces iTreg generation in vitro. CD4+GFP− cells from Foxp3gfp reporter mice were purified by FACS and stimulated with anti-CD3 mAb plus irradiated TCD splenocytes as APCs in the absence or presence of TGFβ. Soluble anti-CD28 mAb was added at different concentrations into the culture. Four days after stimulation, cultured cells were harvested and measured for CD4, GFP, and intracellular Foxp3 expression. (A) Data showing the percentage of GFP+ (top panel) or Foxp3+ (bottom panel) cells on gated CD4+ cells. (B) Data presented as the mean of percentage of Teffs (GFP−) and Tregs (GFP+) cells in total CD4+ cells in triplicate wells. (C) Data presented as the mean ± SD of absolute numbers of Teffs (CD4+GFP−) and Tregs (CD4+GFP+) cells in triplicate wells. (D) In separate experiments, cultured cells were harvested and measured for CD4 and GFP expression and the culture supernatant was measured for IL-2 production. The data show the percentage of GFP+ on gated CD4+ cells (left y-axis), and IL-2 production (right y-axis). The data are presented as the mean ± SD in triplicate wells, and represent 1 of 3 replicate experiments.
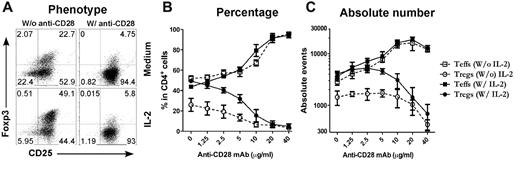
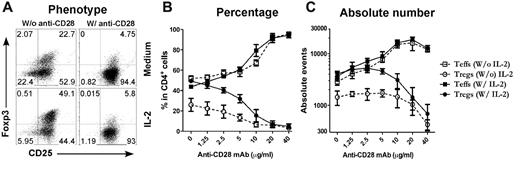
Because T cells receive other costimulatory signals provided by APCs in the culture, it is possible that signals other than CD28 were also required for the inhibition of iTreg generation. To address this possibility, purified CD4+CD25− T cells were stimulated with cross-linking anti-CD3/anti-CD28 plus TGFβ in the absence of APCs (Figure 2A). Under this culture condition, we also observed that anti-CD28 inhibited iTreg generation in a dose-dependent manner and increased expansion of Teffs (Figure 2C), indicating that other signals from APCs may not have a significant effect in this phenomenon. Because CD28 costimulation promotes IL-2 production, it is possible that strong CD28 costimulation may suppress iTreg generation through high levels of IL-2. However, when a high concentration of exogenous IL-2 at a 2 ng/mL concentration was added to the culture, it did not suppress iTreg generation or affect the suppression of iTreg generation by strong CD28 costimulation (Figure 2). We therefore conclude that strong CD28 costimulation suppresses the generation of iTregs independently of IL-2 production. However, because there was a difference in both the percentage and the absolute number of the Tregs generated without and with IL-2 in the presence of low but not high levels of anti-CD28 (Figure 2), it is possible that IL-2 can circumvent inhibition mediated by lower levels of CD28 costimulation.
Plate-bound anti-CD28 mAb reduces iTreg generation in vitro. CD4+CD25− cells were purified from spleen and lymph node cells from normal B6 mice and stimulated with plate-bound anti-CD3 mAb plus anti-CD28 mAb in the absence of APCs. Exogenous TGFβ was also included in the culture with or without additional IL-2. Four days after stimulation, cultured cells were harvested and measured for CD4, CD25, and intracellular Foxp3. (A) Expression of CD25 and Foxp3 on gated CD4+ cells. (B) Data presented as the mean of percentage of Teffs (CD25+Foxp3−) and Tregs (CD25+Foxp3+) in total CD4+ cells in triplicate wells. (C) Data presented as the mean ± SD of absolute numbers of Teffs (CD4+CD25+Foxp3−) and Tregs (CD4+CD25+Foxp3+) in triplicate wells. The data represent 1 of 4 replicate experiments.
Plate-bound anti-CD28 mAb reduces iTreg generation in vitro. CD4+CD25− cells were purified from spleen and lymph node cells from normal B6 mice and stimulated with plate-bound anti-CD3 mAb plus anti-CD28 mAb in the absence of APCs. Exogenous TGFβ was also included in the culture with or without additional IL-2. Four days after stimulation, cultured cells were harvested and measured for CD4, CD25, and intracellular Foxp3. (A) Expression of CD25 and Foxp3 on gated CD4+ cells. (B) Data presented as the mean of percentage of Teffs (CD25+Foxp3−) and Tregs (CD25+Foxp3+) in total CD4+ cells in triplicate wells. (C) Data presented as the mean ± SD of absolute numbers of Teffs (CD4+CD25+Foxp3−) and Tregs (CD4+CD25+Foxp3+) in triplicate wells. The data represent 1 of 4 replicate experiments.
Lck signal is critical for CD28-mediated suppression of iTreg generation
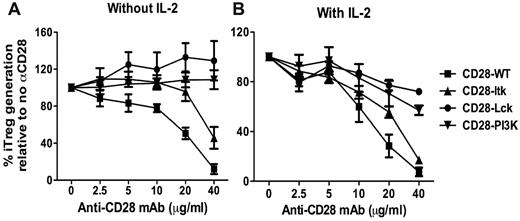
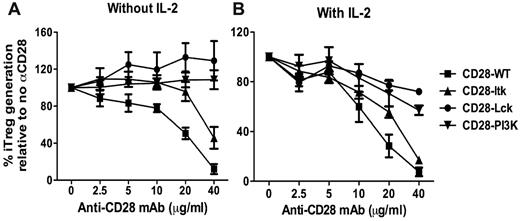
Through its cytoplasmic motifs, CD28 can recruit and activate several kinases including Lck, PI3K, and Itk.21 To explore the question of which CD28-mediated signal(s) is responsible for suppressing iTreg generation, we used a set of CD28 Tg mice that express CD28 receptors with different mutations in the CD28 cytosolic tail: CD28 with an unmutated cytosolic tail (hereafter CD28-WT); CD28 with mutations in P187A and P190A that abrogate Lck binding (CD28-Lck); CD28 with a mutation in Y170F that abrogates PI3K binding (CD28-PI3K); and CD28 with mutations in P175A and P178A that abrogate Itk binding (CD28-Itk).10 To identify the CD28 kinase–activating domains (cytoplasmic motifs) required for suppressing iTreg generation, purified CD4+CD25− T cells from CD28-WT, CD28-Lck, CD28-PI3K, or CD28-Itk Tg mice were stimulated with cross-linking anti-CD3/anti-CD28 plus TGFβ without APCs. In the absence of exogenous IL-2, additional anti-CD28 inhibited iTreg generation from CD28-WT naive CD4 T cells in a dose-dependent manner (Figure 3A). iTreg generation from CD28-Itk cells was also inhibited by anti-CD28. These data showed that strong CD28 costimulation suppressed iTreg generation and that Itk activation mediated by CD28 did not play a significant role in this process. In contrast to CD28-WT or CD28-Itk cells, anti-CD28 did not inhibit iTreg generation from CD28-PI3K cells and even increased iTreg generation from CD28-Lck cells (Figure 3A), indicating that CD28-mediated PI3K and Lck signals are required for the suppression of iTreg generation.
CD28-mediated Lck and PI3K signals contribute to the suppression of iTreg generation. CD4+CD25− cells were purified from a series of Tg mice on a CD28-deficient background that bears WT CD28 or unmutated CD28 in its cytosolic tail incapable of binding to Lck (CD28-Lck), PI3K (CD28-PI3K), or Itk (CD28-Itk). Purified CD4+CD25− cells were then stimulated with plate-bound anti-CD3 and the indicated concentrations of anti-CD28 in the presence of TGFβ without (A) or with (B) additional IL-2 at a 2-ng/mL concentration. Four days after stimulation, cultured cells were harvested and measured for expression of surface CD4, CD25, and intercellular Foxp3, and CD4+CD25+Foxp3+ cells were considered to be iTregs. The data show the percentage relevance of iTreg generation with anti-CD28 to that without anti-CD28. The numbers are presented as the mean ± SD of percent relevance of pooled data from triplicate experiments.
CD28-mediated Lck and PI3K signals contribute to the suppression of iTreg generation. CD4+CD25− cells were purified from a series of Tg mice on a CD28-deficient background that bears WT CD28 or unmutated CD28 in its cytosolic tail incapable of binding to Lck (CD28-Lck), PI3K (CD28-PI3K), or Itk (CD28-Itk). Purified CD4+CD25− cells were then stimulated with plate-bound anti-CD3 and the indicated concentrations of anti-CD28 in the presence of TGFβ without (A) or with (B) additional IL-2 at a 2-ng/mL concentration. Four days after stimulation, cultured cells were harvested and measured for expression of surface CD4, CD25, and intercellular Foxp3, and CD4+CD25+Foxp3+ cells were considered to be iTregs. The data show the percentage relevance of iTreg generation with anti-CD28 to that without anti-CD28. The numbers are presented as the mean ± SD of percent relevance of pooled data from triplicate experiments.
Because CD28-mediated Lck activation is critical for IL-2 production,10,22,23 and because CD28-mediated PI3K activation also contributes to IL-2 production,24-26 it was possible that IL-2 produced by CD28-WT or CD28 Itk T cells stimulated by high concentrations of anti-CD28 was responsible for the suppression of iTreg generation, whereas CD28-PI3K or CD28 Lck T cells were unable to produce high levels of IL-2. In the presence of exogenous IL-2, anti-CD28 suppressed iTreg generation similarly from CD28-WT or CD28-Itk T cells in a dose-dependent manner, but suppression was less on CD28-PI3K T cells and was minimal on CD28-Lck T cells (Figure 3B). These results demonstrate that strong CD28 costimulation suppresses the generation of iTregs through Lck and PI3K signaling independently of IL-2 production. Because CD28-mediated Lck signaling plays the most important role in this process, we focused on the Lck pathway in further studies.
CD28- and TCR-mediated Lck signaling contribute to iTreg generation differently
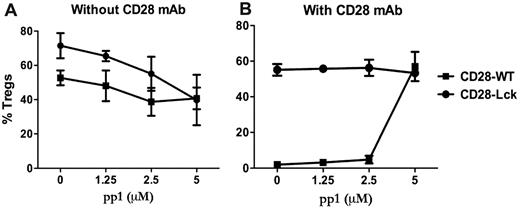
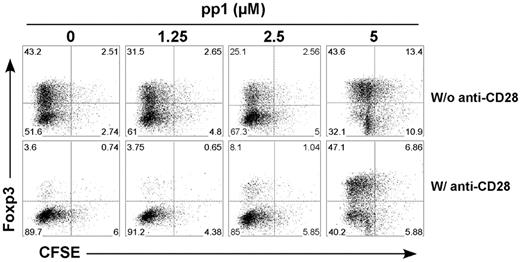
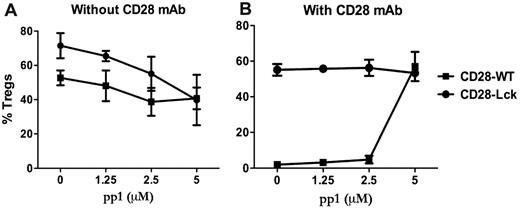
Because Lck activation can be induced by TCR or CD28 engagement (among others), we investigated the contribution of CD28- and TCR-mediated signaling in the generation of iTregs. This was addressed using an Src-specific inhibitor (pp1) in cultures of CD28-WT or CD28-Lck T cells stimulated by anti-TCR with or without anti-CD28 mAb in the absence of APCs. Lck signaling comes from the TCR in CD28-Lck T cells, whereas Lck signaling comes from both CD28 and TCR in CD28-WT T cells. In the absence of anti-CD28, pp1 reduced the generation of iTregs from CD28-WT or CD28-Lck T cells to a similar extent (Figure 4A), indicating that TCR-mediated Lck signaling promotes the generation of iTregs in the absence of CD28 costimulation. On the other hand, in the presence of anti-CD28, pp1 restored the generation of iTregs from CD28-WT cells, thereby neutralizing the anti-CD28 suppressive effect, whereas the inhibitor had no effect on the generation of iTregs with CD28-Lck cells (Figure 4B). It was interesting that pp1 diminished iTreg generation from CD28-Lck T cells in the absence (Figure 4A) but not in the presence of anti-CD28 mAb (Figure 4B). The reason for this is not clear, but other signals (ie, PI3K) derived from CD28 ligation through anti-CD28 might overcome the inhibition through Lck blockade, as the data shown in Figure 3 suggest.
Lck activation through TCR and CD28 regulates iTreg generation. CD4+CD25− cells isolated from CD28-WT and CD28-Lck Tg mice were stimulated with plate-bound anti-CD3 in the presence of TGFβ, IL-2, and the indicated concentrations of pp1. Cells were also stimulated without (A) or with (B) 40 μg/mL of plate-bound anti-CD28 mAb. Four days after stimulation, cultured cells were harvested and measured for the expression of surface CD4, CD25, and intracellular Foxp3. The data show mean ± SD of percent Tregs (CD25+Foxp3+ among gated CD4+ cells) in triplicate wells, and represent 1 of 3 replicate experiments.
Lck activation through TCR and CD28 regulates iTreg generation. CD4+CD25− cells isolated from CD28-WT and CD28-Lck Tg mice were stimulated with plate-bound anti-CD3 in the presence of TGFβ, IL-2, and the indicated concentrations of pp1. Cells were also stimulated without (A) or with (B) 40 μg/mL of plate-bound anti-CD28 mAb. Four days after stimulation, cultured cells were harvested and measured for the expression of surface CD4, CD25, and intracellular Foxp3. The data show mean ± SD of percent Tregs (CD25+Foxp3+ among gated CD4+ cells) in triplicate wells, and represent 1 of 3 replicate experiments.
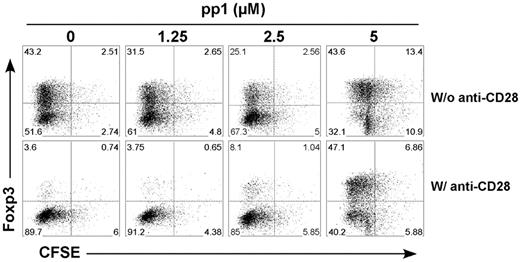
Inhibition of Lck signaling might affect T-cell division and therefore interfere with iTreg generation. To address this issue, we labeled T cells with CFSE and tested how pp1 affected TCR-driven cell division. Under the culture condition with exogenous IL-2, we did not observe a significant effect on T-cell division with pp1 at the concentrations tested (Figure 5). These data indicate that CD28-mediated Lck signaling primarily contributes to the suppression of iTreg generation, in which TCR-mediated Lck signaling plays a very small role.
Lck activation regulates T-cell division and iTreg generation in vitro. CFSE-labeled CD4+CD25− T cells from CD28-WT mice were stimulated with plate-bound anti-CD3 mAb in the presence of TGFβ, IL-2, and the indicated concentrations of pp1. Cells were also stimulated without (top panel) or with (bottom panel) plate-bound anti-CD28 mAb. Four days after stimulation, cultured cells were harvested and measured for CD4 and Foxp3 expression and CFSE profile. The data show Foxp3 expression and CFSE profile on gated CD4+ cells, and represent 1 of 2 replicate experiments.
Lck activation regulates T-cell division and iTreg generation in vitro. CFSE-labeled CD4+CD25− T cells from CD28-WT mice were stimulated with plate-bound anti-CD3 mAb in the presence of TGFβ, IL-2, and the indicated concentrations of pp1. Cells were also stimulated without (top panel) or with (bottom panel) plate-bound anti-CD28 mAb. Four days after stimulation, cultured cells were harvested and measured for CD4 and Foxp3 expression and CFSE profile. The data show Foxp3 expression and CFSE profile on gated CD4+ cells, and represent 1 of 2 replicate experiments.
Strong CD28 signal derived from B7 suppresses iTreg generation
Because strong CD28 costimulation with an agonistic anti-CD28 mAb could suppress iTreg generation, we further investigated whether strong CD28 costimulation elicited by its natural ligands B7 (CD80 and CD86) would also suppress the generation of iTregs in vitro. CD4+CD25− T cells were purified from Foxp3gfp reporter mice and stimulated with naive or lipopolysaccharide (LPS)–activated allogeneic B cells from BALB/c mice. As expected, LPS-activated B cells expressed much higher levels of CD86 than did naive B cells (Figure 6A). Under the culture condition with TGFβ and IL-2, ∼ 70% of iTregs (GFP+CD25+) were generated from naive CD4 T cells stimulated by allogeneic naive B cells, but a much lower percentage of iTregs was generated when stimulated with allogeneic, LPS-activated B cells (Figure 6B). Moreover, the addition of CTLA4-Ig to block B7:CD28 interactions increased iTreg generation in a dose-dependent manner (Figure 6C). These results confirm that CD28 costimulation elicited by high expression of B7 on activated B cells was responsible for the suppression of iTreg generation in vitro. We then sought to determine whether iTregs induced after stimulation with B cells were suppressive and whether their activities were different when generated with resting versus activated B cells. To this end, polyclonal nTregs and iTregs generated with allogeneic B cells were isolated by FACS and used as Tregs to suppress the allogeneic response of naive B6 T cells to BALB/c APCs. As expected, nTregs suppressed the alloresponse in high ratios of Treg:Teff up to 1:16. The iTregs generated with allogeneic B cells suppressed the alloresponse, even in low ratios up to 1:64 and regardless of whether the B cells were resting or activated (Figure 6D), confirming the function and specificity of iTregs generated in vitro.
Strong CD28 signal derived from B7 suppresses iTreg generation. (A) CD86 expression is shown on resting and LPS-activated B cells (B220+). (B) CD4+CD25− cells isolated from Foxp3gfp reporter mice on a B6 background were stimulated with resting or activated allogeneic B cells from BALB/c mice in the presence of TGFβ and IL-2. Six days after stimulation, cultured cells were harvested and measured for expression of CD4, CD25, and GFP. The data show mean ± SD of percent Tregs (%CD25+GFP+ on gated CD4+ cells) in triplicate wells. (C) The culture condition was the same as in panel B, except that LPS-activated B cells were used and CTLA4-Ig at various concentrations was added into the culture. The data present the fold of increase in the generation of iTregs (CD25+GFP+ cells among gated CD4+ cells) in the culture with CTLA4-Ig relevant to that without CTLA4-Ig. The numbers were presented as the mean ± SD of the fold increase in triplicate wells. The data presented in each panel were reproduced in at least 3 replicate experiments. (D) CD4+CD25+GFP+ nTregs from Foxp3gfp reporter B6 mice and iTregs generated as in panel B were purified by FACS and used as suppressor cells. Purified T cells from normal B6 mice (50 × 103/well) were stimulated with irradiated TCD splenocytes from BALB/c mice (250 × 103/well) without or with various numbers of suppressor cells to achieve the ratios of Treg:Teff as indicated. T-cell proliferation was measured after stimulation for 5 days with a [3H]TdR incorporation assay. Data are presented as percent inhibition compared with no Tregs, and error bars indicate 1 SD in triplicate wells. (E) CD4+CD25− cells were purified from Ly5.1+ B6 Foxp3gfp reporter mice and transferred into sublethally irradiated WT or B7.1/7.2 double-knockout syngeneic recipients. PBS or LPS at 50 μg/mouse was injected subcutaneously on day 0 and 7. Two weeks after cell transfer, recipient splenocytes were isolated and stained for expression of CD4, Ly5.1, CD25, and GFP. Percentages of CD25+GFP+ cells are shown among gated CD4+Ly5.1+ donor cells, and the data present 6-7 mice per group from 2 pooled experiments.
Strong CD28 signal derived from B7 suppresses iTreg generation. (A) CD86 expression is shown on resting and LPS-activated B cells (B220+). (B) CD4+CD25− cells isolated from Foxp3gfp reporter mice on a B6 background were stimulated with resting or activated allogeneic B cells from BALB/c mice in the presence of TGFβ and IL-2. Six days after stimulation, cultured cells were harvested and measured for expression of CD4, CD25, and GFP. The data show mean ± SD of percent Tregs (%CD25+GFP+ on gated CD4+ cells) in triplicate wells. (C) The culture condition was the same as in panel B, except that LPS-activated B cells were used and CTLA4-Ig at various concentrations was added into the culture. The data present the fold of increase in the generation of iTregs (CD25+GFP+ cells among gated CD4+ cells) in the culture with CTLA4-Ig relevant to that without CTLA4-Ig. The numbers were presented as the mean ± SD of the fold increase in triplicate wells. The data presented in each panel were reproduced in at least 3 replicate experiments. (D) CD4+CD25+GFP+ nTregs from Foxp3gfp reporter B6 mice and iTregs generated as in panel B were purified by FACS and used as suppressor cells. Purified T cells from normal B6 mice (50 × 103/well) were stimulated with irradiated TCD splenocytes from BALB/c mice (250 × 103/well) without or with various numbers of suppressor cells to achieve the ratios of Treg:Teff as indicated. T-cell proliferation was measured after stimulation for 5 days with a [3H]TdR incorporation assay. Data are presented as percent inhibition compared with no Tregs, and error bars indicate 1 SD in triplicate wells. (E) CD4+CD25− cells were purified from Ly5.1+ B6 Foxp3gfp reporter mice and transferred into sublethally irradiated WT or B7.1/7.2 double-knockout syngeneic recipients. PBS or LPS at 50 μg/mouse was injected subcutaneously on day 0 and 7. Two weeks after cell transfer, recipient splenocytes were isolated and stained for expression of CD4, Ly5.1, CD25, and GFP. Percentages of CD25+GFP+ cells are shown among gated CD4+Ly5.1+ donor cells, and the data present 6-7 mice per group from 2 pooled experiments.
In separate experiments, we tested whether iTreg generation would be reduced through high levels of CD28 costimulation in vivo using an adoptive cell-transfer model that was established previously in our laboratory.13 Purified CD4+CD25− T cells from Ly5.1+ B6 Foxp3gfp reporter mice were transferred into sublethally irradiated syngeneic WT B6 or B7.1/7.2 double-deficient recipients. The percentage of iTregs generated in WT recipients was significantly higher than in B7-deficient recipients (P < .001), confirming our previous observation that a CD28 signal at base levels is required for optimal generation of iTregs in vivo. Under this situation, iTreg generation was significantly reduced with LPS stimulation in WT but not in B7-deficient recipients (Figure 6E). These results suggest that inflammation caused by LPS limited iTreg generation from naive CD4 T cells in vivo, and the B7/CD28 interaction was largely attributed to such a reduced iTreg generation. These data extended our in vitro observations that LPS stimulation increased B7 expression by B cells (Figure 6B-C) or dendritic cells (data not shown), and that those activated APCs induced iTreg generation at much lower levels compared with nonactivated APCs. We concluded that strong CD28 costimulation signals derived from the B7:CD28 ligation suppress iTreg generation both in vitro and in vivo.
Suppression of iTreg generation through CD28-mediated Lck signal contributes to T-cell pathogenicity in vivo
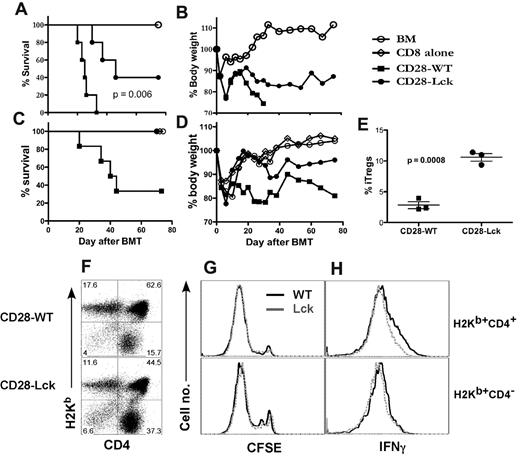
To determine whether suppression of iTreg generation mediated by CD28 costimulation would be relevant to T-cell responses in vivo, we compared the ability of CD4 and CD8 T cells isolated from CD28-WT and CD28-Lck mice to induce acute GVHD. Because the development of nTregs is severely impaired in CD28-Lck mice, nTregs (CD4+CD25+) were removed from the donor T-cell graft for a fair comparison. We observed that all of the recipients of CD28-WT T cells died within 5 weeks after BMT, but the recipients of CD28-Lck T cells died significantly later and 40% survived long-term (P = .005) (Figure 7A-B). We hypothesized that increased iTreg generation from CD28-Lck CD4 T cells might be accounted for by the reduced GVHD mediated by CD28-Lck T cells. To test this hypothesis, we determined the ability of CD28-WT or CD28-Lck CD4 T cells to induce GVHD in a combination of WT CD8 T cells, in which the only difference was CD4 cells. In this situation, CD28-WT but not CD28-Lck CD4 T cells were capable of causing severe GVHD (Figure 7C-D). To directly test the role of the CD28-mediated Lck signal in iTreg generation in vivo, purified CD4+CD25− T cells from CD28-WT and CD28-Lck mice were transferred to irradiated BALB/c mice. After 2 weeks, significantly higher percentages of iTregs were generated from CD28-Lck T cells than from CD28-WT T cells in vivo (Figure 7E). The absolute number of iTregs generated from CD28-Lck T cells was also substantially higher than that from CD28 WT T cells (2.5 ± 0.1 × 105 vs 0.6 ± 0.5 × 105 per spleen). Thus, elevated iTreg generation was associated with diminished GVHD development. An additional or alternative mechanism by which CD28-Lck T cells induced significantly less GVHD could be the impaired activation and expansion of Teffs without CD28-mediated Lck signaling. Using CFSE labeling, we observed that both CD28-WT and CD28-Lck T cells divided rapidly in irradiated allogeneic BALB/c recipients (Figure 7G). As expected, a subset of donor T cells produced IFNγ in the allogeneic recipients, but CD28-WT T cells had a slightly higher percentage of IFNγ-producing cells than CD28-Lck T cells on CD4+ but not CD8+ cells (Figure 7H). These data support the hypothesis that enhanced iTreg generation with CD28-Lck T cells contributes to the decreased ability of these T cells to induce acute GVHD.
CD28-mediated Lck signaling regulates GVHD development and iTreg generation in vivo. BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 of TCD-BM cells alone or plus 1 × 106 T cells (CD4+ or CD8+ CD25−) from CD28-WT or CD28-Lck mice. Recipient mice were monitored throughout the experimental period for survival (A) and weight change (B). Using the same BMT setting as in panels A and B, 0.5 × 106 CD8+ cells from normal B6 donors alone or plus 0.5 × 106 CD4+ cells from CD28-WT or CD28-Lck mice were transplanted. Data show recipient survival (C) and weight changes (D) at different days after BMT. (E) BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 TCD-BM cells alone or plus 1 × 106 T cells (CD4+CD25−) from CD28-WT and CD28-Lck mice. Two weeks after transplantation, recipient spleens were harvested and measured for expression of surface CD4, CD25, and intercellular Foxp3. Percentage of Tregs (CD25+Foxp3+) on gated donor T cells (H2Kb+CD4+) is shown in each mouse for a total of 3 mice per group, and the data represent 1 of 3 replicate experiments. In separate experiments, CFSE-labeled T cells (CD4+ or CD8+ CD25−) isolated from CD28-WT and CD28-Lck mice were transplanted into lethally irradiated (800 cGy) BALB/c mice at 2 × 106 per mouse. Four days after transplantation, recipient spleens were harvested and measured for CFSE profile, expression of surface CD4 and H2Kb, and intracellular IFNγ. Expression of CD4 and H2Kb was shown on live spleen cells (F). CFSE profile (G) and intracellular IFNγ expression (H) were shown on gated donor CD4 (H2Kb+CD4+) or CD8 (H2Kb+CD4−) T cells. The data represent 1 of 2 replicate experiments.
CD28-mediated Lck signaling regulates GVHD development and iTreg generation in vivo. BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 of TCD-BM cells alone or plus 1 × 106 T cells (CD4+ or CD8+ CD25−) from CD28-WT or CD28-Lck mice. Recipient mice were monitored throughout the experimental period for survival (A) and weight change (B). Using the same BMT setting as in panels A and B, 0.5 × 106 CD8+ cells from normal B6 donors alone or plus 0.5 × 106 CD4+ cells from CD28-WT or CD28-Lck mice were transplanted. Data show recipient survival (C) and weight changes (D) at different days after BMT. (E) BALB/c mice were lethally irradiated (800 cGy) and transplanted with 5 × 106 TCD-BM cells alone or plus 1 × 106 T cells (CD4+CD25−) from CD28-WT and CD28-Lck mice. Two weeks after transplantation, recipient spleens were harvested and measured for expression of surface CD4, CD25, and intercellular Foxp3. Percentage of Tregs (CD25+Foxp3+) on gated donor T cells (H2Kb+CD4+) is shown in each mouse for a total of 3 mice per group, and the data represent 1 of 3 replicate experiments. In separate experiments, CFSE-labeled T cells (CD4+ or CD8+ CD25−) isolated from CD28-WT and CD28-Lck mice were transplanted into lethally irradiated (800 cGy) BALB/c mice at 2 × 106 per mouse. Four days after transplantation, recipient spleens were harvested and measured for CFSE profile, expression of surface CD4 and H2Kb, and intracellular IFNγ. Expression of CD4 and H2Kb was shown on live spleen cells (F). CFSE profile (G) and intracellular IFNγ expression (H) were shown on gated donor CD4 (H2Kb+CD4+) or CD8 (H2Kb+CD4−) T cells. The data represent 1 of 2 replicate experiments.
Discussion
The data presented here indicate that strong CD28 costimulation suppresses the generation of iTregs from naive CD4 T cells, primarily through Lck signaling. It is well established that CD28 costimulation positively regulates the T-cell response by promoting IL-2 production, cell-cycle entrance, and the activation and survival of T cells.21,27 On the other hand, CD28 costimulation plays a critical role in the development of nTregs in thymus and in the maintenance of nTregs in the periphery, to which CD28 contributes by maintaining self-tolerance.28 In addition, our previous work indicates that a low or base level of CD28 costimulation is required for iTreg generation through IL-2 production primarily mediated by CD28-mediated Lck.13 The current finding adds a new facet to the regulatory function of CD28 costimulation, which at high levels can promote the T-cell response by shutting down iTreg generation from naive CD4 T cells. This new concept fits well with the overall mission of T-cell responses. Under acute infection, high levels of CD28 costimulation not only promote the activation and expansion of effector T cells, but also suppress the generation of iTregs that permits effective immune responses against infectious agents. In contrast, under quiescent situations or in cancer, low levels of CD28 costimulation not only limit the activation and expansion of effector T cells, but also promote homeostasis of nTregs and the generation of iTregs, permitting immune tolerance to self- or tumor antigens.
Efficient development of nTregs in the thymus relies on CD28 costimulation through its cytosolic Lck-binding motif and c-Rel, leading to activation of nuclear factor-κB.29-32 Although the same motif is required with CD28 costimulation for IL-2 production, CD28-mediated nTreg development is independent of IL-2 production.10 Ironically, our current work demonstrates that the same Lck-binding motif is again required for the suppression of iTreg generation mediated by strong CD28 costimulation independently of IL-2 production. Thus, the same CD28-mediated Lck signal is responsible, independently of IL-2 production, for the efficient development of nTregs in the thymus, but also for the control of iTreg generation in the periphery. We propose that, through an Lck- and c-Rel–dependent signaling pathway, CD28 costimulation at the base level is required for nTreg development in the thymus and iTreg generation in quiescent situations, whereas CD28 costimulation at high levels limits iTreg generation during active immune responses. How the CD28-mediated Lck signal regulates these 2 distinct processes is currently unclear, although apparently T cells are in different stages (immature vs mature) and CD28 costimulation is at different levels (base vs elevated).
Interestingly, although TCR ligation also activates Lck, the TCR-mediated Lck signal promoted rather than suppressed the generation of iTregs (Figure 4). Under the culture conditions specified in this report, high levels of TCR stimulation (ie, high concentrations of anti-CD3 mAb) without CD28 costimulation were not suppressive to iTreg generation (data not shown). The different outcomes may have resulted from the distinguished patterns of Lck activation mediated by TCR and CD28, respectively. Upon TCR engagement, Lck is recruited and remains only transiently in the immunologic synapse. Subsequently, the SH3 domain of Lck interacts with the C-terminal proline motif of CD28, which disrupts the formation of potentially inhibitory intramolecular Lck interactions between the Lck SH3 domain and its kinase domain that interfere with Lck activity.33 In this way, CD28 costimulation greatly increases both the intensity and duration of Lck activity at the immunologic synapse of TCR-engaged T cells, which may be critically important for suppressing the generation of iTregs.
A recent study by Gottschalk et al elegantly demonstrated that TCR ligand density and potency determine the induction of iTregs in the periphery, and the authors concluded that a low density of a strong TCR agonist is optimal to induce a persistent generation of iTregs in vivo.34 That study focused on T-cell responses under noninflammatory conditions in which CD28 costimulation was at a low or base level. Because CD28 costimulation reduces the extent of TCR ligation required for effective T-cell responses, likely by lowering the threshold of TCR signal transduction for T-cell activation and promoting the formation of an immunologic synapse,35,36 CD28 regulation of iTreg induction could be through modifying TCR signaling. However, we propose that the combined signals through TCR and CD28 determine the proper levels of iTreg induction and Teff generation from naive CD4 T cells.
Using a GVHD model, we showed that CD28-Lck donor T cells caused significantly less GVHD than WT donor T cells (Figure 7A-B). Furthermore, in combination with WT CD8 T cells, WT CD4 T cells induced severe GVHD, whereas CD28-Lck CD4 T cells failed to do so (Figure 7C-D). Because significantly higher rate of iTregs were generated from CD28-Lck than from CD28-WT CD4 T cells (Figure 7E), we conclude that the enhanced generation of iTregs contributed to the decreased GVHD induced by CD28-Lck donor T cells, although lower effector function might still be a part of the mechanism. Our data disagree with the observation that CD28 costimulation is paradoxically inhibitory in major histocompatibility complex class II–mismatched murine cardiac graft rejection and in the development of autoimmune diabetes in NOD mice.9,37 The outcomes in these 2 studies reflect the important role of CD28 costimulation at base levels in the generation and maintenance of Tregs under noninflammatory conditions. In contrast, inflammation is created under myeloablative allogeneic BMT, in which CD28 costimulation, presumably at high levels, would limit the generation of iTregs and facilitate the development of GVHD.
In summary, our study investigating how CD28 costimulation promotes a productive T-cell response revealed a novel mechanism: that CD28 costimulation promotes immunity by suppressing the generation of iTregs in the periphery. CD28 costimulation executes this negative regulation on iTregs through its Lck-binding motif independently of IL-2 production. CD28 provides a predominant costimulation and Tregs are one of the most critical regulatory components in many if not all aspects of immunology. Therefore, the current findings may provide a rationale for promoting T-cell immunity or tolerance by regulating iTreg induction through targeting CD28 signaling in many immunologic responses related to autoimmunity, transplantation, and cancer development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs A. Singer and X. Tai (National Cancer Institute, Bethesda, MD) for providing CD28 Tg and A. Rudensky (University of Washington, Seattle) for providing Foxp3gfp reporter mice. We also thank Drs Esteban Celis and Amer Beg for their critical comments. We are grateful for the technical assistance provided by the Flow Cytometry and Mouse Core Facility at the H. Lee Moffitt Cancer Center and Research Institute.
This research was supported in part by National Institutes of Health grants AI 51693 (to C.A.) and CA 143812 and CA 118116 (to X.-Z.Y.).
National Institutes of Health
Authorship
Contribution: K.S. participated in the experimental design, performed research, collected, analyzed, and interpreted data, performed statistical analysis, and revised the manuscript; A.N. and Y.Y. performed research, collected and analyzed data, and edited the manuscript; C.A. and H.W. participated in the experimental design, interpreted data, and revised the manuscript; and X.-Z.Y. designed research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xue-Zhong Yu, MD, H. Lee Moffitt Cancer Center & Research Institute, SRB-2, 12902 Magnolia Dr, Tampa, FL 33612-9497; e-mail: Xue.Yu@moffitt.org.

![Figure 6. Strong CD28 signal derived from B7 suppresses iTreg generation. (A) CD86 expression is shown on resting and LPS-activated B cells (B220+). (B) CD4+CD25− cells isolated from Foxp3gfp reporter mice on a B6 background were stimulated with resting or activated allogeneic B cells from BALB/c mice in the presence of TGFβ and IL-2. Six days after stimulation, cultured cells were harvested and measured for expression of CD4, CD25, and GFP. The data show mean ± SD of percent Tregs (%CD25+GFP+ on gated CD4+ cells) in triplicate wells. (C) The culture condition was the same as in panel B, except that LPS-activated B cells were used and CTLA4-Ig at various concentrations was added into the culture. The data present the fold of increase in the generation of iTregs (CD25+GFP+ cells among gated CD4+ cells) in the culture with CTLA4-Ig relevant to that without CTLA4-Ig. The numbers were presented as the mean ± SD of the fold increase in triplicate wells. The data presented in each panel were reproduced in at least 3 replicate experiments. (D) CD4+CD25+GFP+ nTregs from Foxp3gfp reporter B6 mice and iTregs generated as in panel B were purified by FACS and used as suppressor cells. Purified T cells from normal B6 mice (50 × 103/well) were stimulated with irradiated TCD splenocytes from BALB/c mice (250 × 103/well) without or with various numbers of suppressor cells to achieve the ratios of Treg:Teff as indicated. T-cell proliferation was measured after stimulation for 5 days with a [3H]TdR incorporation assay. Data are presented as percent inhibition compared with no Tregs, and error bars indicate 1 SD in triplicate wells. (E) CD4+CD25− cells were purified from Ly5.1+ B6 Foxp3gfp reporter mice and transferred into sublethally irradiated WT or B7.1/7.2 double-knockout syngeneic recipients. PBS or LPS at 50 μg/mouse was injected subcutaneously on day 0 and 7. Two weeks after cell transfer, recipient splenocytes were isolated and stained for expression of CD4, Ly5.1, CD25, and GFP. Percentages of CD25+GFP+ cells are shown among gated CD4+Ly5.1+ donor cells, and the data present 6-7 mice per group from 2 pooled experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/11/10.1182_blood-2010-08-301275/4/m_zh89991167990006.jpeg?Expires=1769246636&Signature=xTbxxScDvb656jwXVlSVz0dTAhi5MgrYPG1u8a8T4tYhizBq-OtmU60Blm5E8UkcP7WzfbJomhWJqpWfs6h-VxnxrYEvryBbUBq3wzeqeWfKw~EfMCHlz99QRZXbH15zxtpj9Ak3k5tEnurBw9gL8KdtdBS6-U12eMP7RRhzfs4UREkT-qCXMjF3wysxyMK2gJj4yel2f4Y98ARulYdbRNTBA-bY-6T1nEEVnHqkoO2ZCNAu0UK8FeVArThvCxkQx1R5NNxOP~P1bV33XnqaHFkItFTHiKZgz0OajbTlokl6IozSWS1rQ7EnDLIekDUnGTz7kz7ty1cuSSYp-VXf4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Strong CD28 signal derived from B7 suppresses iTreg generation. (A) CD86 expression is shown on resting and LPS-activated B cells (B220+). (B) CD4+CD25− cells isolated from Foxp3gfp reporter mice on a B6 background were stimulated with resting or activated allogeneic B cells from BALB/c mice in the presence of TGFβ and IL-2. Six days after stimulation, cultured cells were harvested and measured for expression of CD4, CD25, and GFP. The data show mean ± SD of percent Tregs (%CD25+GFP+ on gated CD4+ cells) in triplicate wells. (C) The culture condition was the same as in panel B, except that LPS-activated B cells were used and CTLA4-Ig at various concentrations was added into the culture. The data present the fold of increase in the generation of iTregs (CD25+GFP+ cells among gated CD4+ cells) in the culture with CTLA4-Ig relevant to that without CTLA4-Ig. The numbers were presented as the mean ± SD of the fold increase in triplicate wells. The data presented in each panel were reproduced in at least 3 replicate experiments. (D) CD4+CD25+GFP+ nTregs from Foxp3gfp reporter B6 mice and iTregs generated as in panel B were purified by FACS and used as suppressor cells. Purified T cells from normal B6 mice (50 × 103/well) were stimulated with irradiated TCD splenocytes from BALB/c mice (250 × 103/well) without or with various numbers of suppressor cells to achieve the ratios of Treg:Teff as indicated. T-cell proliferation was measured after stimulation for 5 days with a [3H]TdR incorporation assay. Data are presented as percent inhibition compared with no Tregs, and error bars indicate 1 SD in triplicate wells. (E) CD4+CD25− cells were purified from Ly5.1+ B6 Foxp3gfp reporter mice and transferred into sublethally irradiated WT or B7.1/7.2 double-knockout syngeneic recipients. PBS or LPS at 50 μg/mouse was injected subcutaneously on day 0 and 7. Two weeks after cell transfer, recipient splenocytes were isolated and stained for expression of CD4, Ly5.1, CD25, and GFP. Percentages of CD25+GFP+ cells are shown among gated CD4+Ly5.1+ donor cells, and the data present 6-7 mice per group from 2 pooled experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/11/10.1182_blood-2010-08-301275/4/m_zh89991167990006.jpeg?Expires=1769246637&Signature=O-sAXTB5tqg~73gaa1M4PNz0wAcwmiDnLRov3EBdgZXtyxOUfSA7sUP2By01kwDMrhYIZOaV4wztt1e8iBirjqLcMgcnmF02BFqdVYbLtxDuTfRRDUmWA24~BHtxGb~1HzhtMuTJ9kqB28P0iVvioUtl79bnVZbUNwXP9WWryJjFu0l9aCryJtTrCIaKlyh~pszL7Egf3I5ThripBfiV~wMA9FsHOd2GlxKk2bHHaxYJaSOYUw9WU8uqkYnYjLheSqAWfvxXpawzmEf3NBOb8YfF6jg-NZBNb6B3noSj0ePf8qLowmanKnpWwyI1Hx5~2PyvpRlj89u3A2h5iMDdIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)