Abstract
Pulmonary arterial hypertension (PAH) is a proliferative vasculopathy characterized by high circulating CD34+CD133+ proangiogenic progenitors, and endothelial cells that have pathologic expression of hypoxia-inducible factor 1 α (HIF-1α). Here, CD34+CD133+ progenitor cell numbers are shown to be higher in PAH bone marrow, blood, and pulmonary arteries than in healthy controls. The HIF-inducible myeloid-activating factors erythropoietin, stem cell factor (SCF), and hepatocyte growth factor (HGF) are also present at higher than normal levels in PAH blood, and related to disease severity. Primary endothelial cells harvested from human PAH lungs produce greater HGF and progenitor recruitment factor stromal-derived factor 1 α (SDF-1α) than control lung endothelial cells, and thus may contribute to bone marrow activation. Even though PAH patients had normal numbers of circulating blood elements, hematopoietic alterations in myeloid and erythroid lineages and reticulin fibrosis identified a subclinical myeloproliferative process. Unexpectedly, evaluation of bone marrow progenitors and reticulin in nonaffected family members of patients with familial PAH revealed similar myeloid abnormalities. Altogether, the results show that PAH is linked to myeloid abnormalities, some of which may be related to increased production of HIF-inducible factors by diseased pulmonary vasculature, but findings in nonaffected family suggest myeloid abnormalities may be intrinsic to the disease process.
Introduction
Pulmonary arterial hypertension (PAH) is a vasculopathy of the pulmonary circulation characterized by arterial obliteration secondary to unchecked pathologic angiogenic processes.1-3 An abundance of studies over the past decade provide evidence for the paradigm of lifelong interdependence between angiogenesis and hematopoiesis.4-6 The concept of a common hematopoietic-endothelial stem cell, that is, hemangioblast, with bidirectional, reversible gene transcription and persistence is well established in developmental biology.7 In postnatal life to adulthood, hemangioblasts are readily identifiable in the bone marrow by the CD133-selective expression on a small subpopulation of CD34-positive hematopoietic stem cells.8 Hemangioblasts give rise to all blood cellular components, but whether these cells give rise to endothelium during postnatal neovascularization is uncertain.9,10 In contrast, studies clearly substantiate that CD34+CD133+ progenitors are vital contributors to angiogenesis via proangiogenic effects on endothelial cells in vessels.11-18 Our and other studies identify that CD34+CD133+ progenitors are present at higher than normal levels in the circulation of PAH patients and are more proliferative than circulating progenitors of healthy controls.19,20 The relationship of numbers of circulating CD34+CD133+ cells to severity of PAH suggest that these cells may promote the angioproliferative vascular remodeling.20 However, whether the source of greater numbers of circulating CD34+CD133+ cells in PAH patients is related to a greater number of stem cells in the bone marrow and/or more mobilization of progenitors to the circulation—and/or because of higher levels of the effectors that promote either of these processes—is unknown.
In general, proliferation and mobilization of bone marrow progenitors is under the control of growth factors that are transcriptionally regulated by hypoxia-inducible factors (HIF). Classically, HIF-inducible factors that affect bone marrow progenitors include erythropoietin (Epo), hepatocyte growth factor (HGF) and stem cell factor (SCF, also known as Steel Factor), and vascular endothelial growth factor (VEGF).21-24 In addition to inductive effects on progenitors, Epo and other HIF-inducible growth factors also act on pulmonary artery endothelial cells in the vascular bed to induce a proliferative, promigratory, and antiapoptotic endothelial cell.6,25,26 Effects on the bone marrow stem cells and endothelial cells are mediated via activation of signal transducers and activators of transcription factors (STAT).25,27-33 Once mobilized from the bone marrow, circulating CD34+CD133+ cells are recruited to activated tissue sites where vascular repair or growth is needed; recruitment to specific vascular sites is regulated via local production of HIF-inducible factors, such as stromal-derived factor-1α (SDF-1α).11,16,31,32,34 Thus, bone marrow events and local vascular changes are coordinately regulated by HIF activation and lead to robust angiogenic responses. Given the recent discovery of HIF activation in PAH lung endothelial cells, we hypothesized that HIF-inducible factors may be higher than normal in PAH patients and cause both the proliferation of multipotent hemangioblasts in the bone marrow and increased mobilization of progenitors from the bone marrow. To test this, numbers of CD34+CD133+ hemangioblasts in the bone marrow and CD34+CD133+ progenitors in the circulation and within the pulmonary arteries were quantitated, and the serum levels of HIF-inducible factors were determined in individuals with PAH in comparison to healthy controls. Because the proliferation, mobilization, and recruitment of progenitors to the pulmonary vasculature contribute to proliferative vasculopathy, we also assessed the relationship of HIF-inducible factors to clinical parameters of PAH severity, such as 6-minute walk distance and cardiac function. To evaluate the mechanisms contributing to proliferation and mobilization of proangiogenic cells, numbers of progenitor cells and the activation of tyrosine kinases that are central to myeloproliferative processes, that is, Janus Kinase 2 (JAK2) and STAT3 and STAT5, were evaluated in bone marrow of patients with PAH in comparison to healthy controls. Alternatively, to evaluate whether the pulmonary vascular cells within the diseased lungs nurture the bone marrow progenitor response, pulmonary artery endothelial cells of PAH patients were compared with normal pulmonary artery endothelial cells for production of HIF-inducible factors.
Methods
Study population
Volunteers with familial PAH (FPAH), idiopathic PAH (IPAH), and associated PAH (APAH) were enrolled in the study as well as nonaffected family members. All PAH subjects underwent blood draw for quantification of circulating proangiogenic progenitors and mobilizing factors. A subgroup agreed to have bone marrow biopsy and aspirate. For those subjects, blood was sent for a complete cell count and differential, reticulocyte count, and peripheral smear evaluation by the clinical pathologist; and on the same visit, they underwent bone marrow biopsy and aspirate. Healthy controls with no known lung diseases provided bone marrow aspirates and blood. The Institutional Review Board (IRB) at the Cleveland Clinic approved this study and all subjects gave written informed consent in accordance with the Declaration of Helsinki. Explanted lungs from PAH subjects undergoing transplant and donor lungs not used in transplantation were harvested to obtain pulmonary artery endothelial cells under an IRB-approved protocol.
Flow cytometric evaluation of progenitor cells and colony-forming assays
CD34+CD133+ progenitors in peripheral blood and bone marrow were analyzed by flow cytometry as described in detail previously.20 Colony formation was performed as described by Hill et al35 using Endocult Liquid Medium (StemCell Technologies) as reported by our group.20 To analyze progenitor homing to the pulmonary artery vascular bed, the pulmonary artery was dissected to terminal branches as far as visible to the human eye from lungs explanted at transplantation or from donor lungs not used in transplantation. The blood vessels were excised longitudinally and endothelium was dispersed by collagenase type II solution (2 mg/mL in phosphate-buffered saline [PBS]; Worthington Biochemical) on the inner surface, followed by brief incubation at 37°C. Cells were collected and washed in PBS/1% bovine serum albumin (BSA)/0.02% sodium azide. After counting, 100 × 103 to 200 × 103 cells were stained with Red LIVE/DEAD dye (Invitrogen) followed by cell-surface staining for CD34 and CD133.20 Cells (50 × 103 to 100 × 103) were acquired on a FACScan (Becton Dickinson) flow cytometer and the percentage of CD34+CD133+ within the live cell fraction was quantified using CellQuest 3.3 software (Becton Dickinson).
Bone marrow biopsy/aspirate processing
Bone marrow aspirate and biopsy were performed under local anesthesia. Aspirate was collected in an EDTA tube and flow cytometric analysis was performed as described in “Flow cytometric evaluation of progeuitor cells and colony-forming assays.” Smears from the bone marrow aspirates were collected for cell counts and differential performed by experienced pathologists. Bone marrow biopsies were fixed overnight in 10% formalin followed by overnight decalcification in decalcifying solution (Fischer) and processing and embedding in paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin (H&E). Reticulin (type III collagen) staining was performed in the clinical laboratory using standard silver-impregnation methods. Reticulin was quantified by an experienced pathologist blinded to the subjects' diagnosis using the Bauermeister scale: 0, no demonstrable reticulin fibers; 1, occasional fine individual fibers and foci of a fine fiber network; 2, fine fiber network throughout most of the section, but no coarse fibers; 3, diffuse fiber network with scattered thick coarse fibers, but no mature collagen; and 4, diffuse, often coarse fiber network with areas of collagen reticulin stained.36 JAK2 V617F mutation that is commonly found in myeloproliferative disorders was evaluated in bone marrow tissues as described previously.37 Immunohistologic analysis for pSTAT3 and pSTAT5 was carried out by using the rabbit polyclonal antihuman phosphoSTAT3 (Cell Signaling), and the mouse monoclonal anti–human phosphoSTAT5a/b (AdvantexBio) after antigen retrieval with Antigen Retrieval Citra Plus (Bio Genex). Diaminobenzidine (DAB) and hydrogen peroxide (H2O2) were applied to develop color. Negative control of secondary antibody alone was performed on each section of tissue studied. All slides were counterstained with hematoxylin. The pSTAT3 and pSTAT5 was evaluated by counting numbers cells with nuclear positivity. A minimum of 5 fields was examined at ×400 magnification and at least 500 cells were counted.
ELISA for HIF-inducible factors
Epo, HGF, SCF, SDF-1α, and VEGF were measured in plasma using a quantikine enzyme-linked immunosorbent assay (ELISA; R&D Systems). Epo, HGF, and SDF-1α levels measured in the supernatant of cultured primary human pulmonary artery endothelial cells were normalized to protein concentration of cells in culture.
EpoR expression
Epo receptor (EpoR) expression was analyzed by staining of day-5 colony-forming units–endothelial cells (CFUs-ECs) with fluorescein isothiocyanate (FITC)–conjugated anti–human EpoR monoclonal antibodies (R&D Systems). Isotype-matched control antibody was used as control. Cells were preincubated with Fc-block (eBioscience) before staining to block nonspecific Fc-receptor–mediated binding.
CFU-EC:electrophoretic mobility shift assay
Whole-cell extracts (WCEs) from CFU-ECs were prepared as previously described.1 The duplex oligonucleotide (5′-AGATTTCTAGGAATTCAATCC-3′) specific for STAT5 binding was purchased from Santa Cruz Biotechnology. To specifically identify DNA-binding factor in binding complexes, rabbit polyclonal anti–STAT-5 antibody (Ab; Santa Cruz Biotechnology) was added to the binding reaction mix.
Immunohistochemical analyses
For SDF-1α and HGF immunostaining, control lungs were collected from donor lungs not used in transplantation (n = 3). IPAH lung tissues were obtained from explanted IPAH lung or postmortem tissues (n = 3). Rabbit polyclonal anti–SDF-1α Ab (Abcam) and anti-HGF Ab (MyBioSource) were used after antigen retrieval. Positive control for SDF-1α was consisted of tissue section of a lung carcinoma and mouse liver tissue section was used as positive control for HGF. Negative control of secondary Ab alone was performed on each section of tissue studied.
Pulmonary artery endothelial cells
Primary pulmonary artery endothelial cells (PAECs) were isolated from explanted PAH lungs or healthy control lungs not used for transplantation as previously described.3 Briefly, cells were cultured in endothelial cell growth medium (EGM-2; Cambrex) on plates precoated with coating media containing fibronectin and passaged at 70%-80% confluence by dissociation from plates with trypsin-EDTA (Invitrogen). Primary cultures of passages 5-8 were used in experiments. Human umbilical vein endothelial cells (HUVECs; Lonza) were cultured in EGM-2 (Cambrex).
Statistical analysis
Descriptive measures for quantitative variables consist of means with appropriately derived standard errors in the form mean ± SE. Comparisons of PAH, unaffected family members, and healthy subjects were performed using analysis of variance (ANOVA) or t test when 2 means were compared. When ANOVA was significant, Tukey was performed for pairwise comparison. Spearman correlation coefficients were used to describe relationships among pairs of quantitative variables in a manner free of the normality assumption.
Results
Study population
Patients with PAH (13 familial [FPAH: class 1.2], 24 idiopathic [IPAH: 1.1], and 15 associated [APAH: 1.3]) and healthy volunteers (N = 62) were enrolled in the study (Table 1). Because of sample limitations, not all samples were available for all assays. Nine nonaffected family members of FPAH subjects were also recruited for the study. The hematologic profiles of PAH subjects were within the normal range of values although there were significant differences among the groups (Table 2). A subgroup agreed to undergo bone marrow aspirate and biopsy (14 PAH, 7 healthy controls, 6 nonaffected family members). The differential cell count in bone marrow aspirates varied among PAH and controls (Table 3). IPAH patients had increased myelocyte numbers, and FPAH and APAH patients had higher numbers of erythroid precursors compared with controls.
Greater numbers of hematopoietic progenitors in PAH
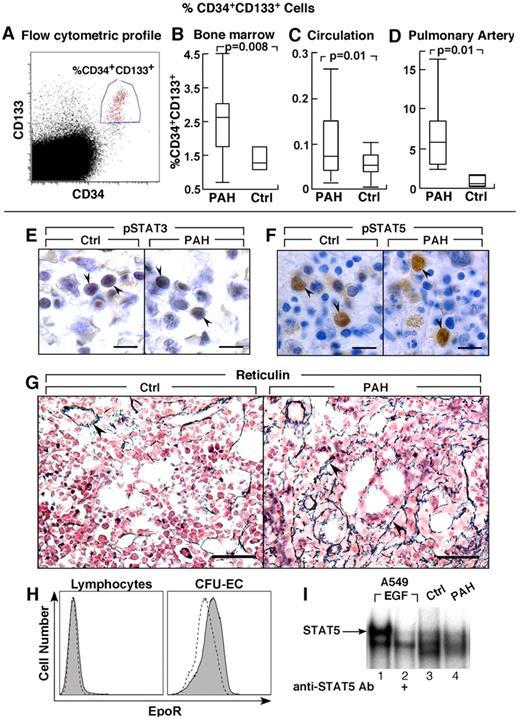
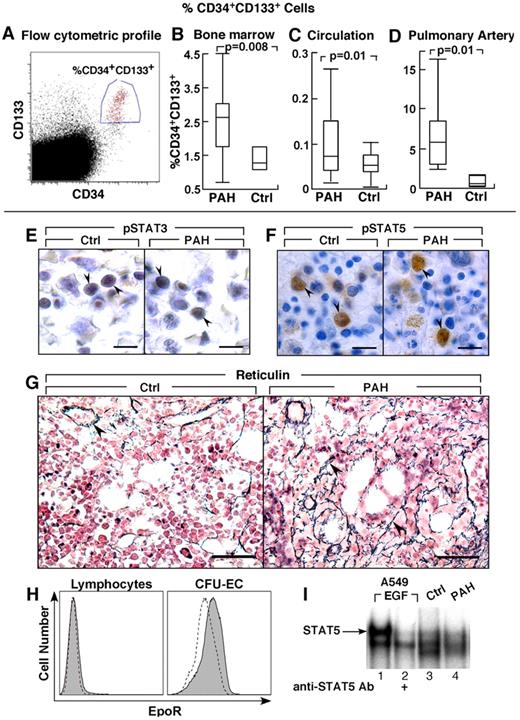
Bone marrow aspirates were evaluated for numbers of CD34+ and CD133+ progenitors. The total progenitors, including CD133+CD34−, CD133+CD34+, and CD133−CD34+ cells, were similar between PAH and controls (% total progenitors: PAH [N = 14], 6.3 ± 0.6; controls [N = 3], 5.1 ± 0.9, P = .3) and among the different PAH subgroups (% total progenitor cells: IPAH 6.2 ± 2.3; FPAH 6.0 ± 0.7; APAH 7.5 ± 0.8, ANOVA P = .7). The CD133+CD34− cells were similar between PAH and controls and among the PAH groups (% cells: PAH 2.3 ± 0.3; controls 3.1 ± 0.8, P = .4). However, numbers of the more mature CD133+CD34+ cells in bone marrow of PAH patients were higher than healthy controls (P = .008), but similar among PAH groups (% CD34+CD133+ cells: IPAH 1.9 ± 0.6; FPAH 2.9 ± 0.4; APAH 2.0 ± 0.8, ANOVA P = .3; Figure 1). Consistent with an expansion of progenitors at the CD34 differentiation level, the numbers of CD34+CD133− cells in the bone marrow were also higher in PAH compared with controls without significant difference among FPAH, IPAH, and APAH (% CD34+CD133− cells: PAH 1.53 ± 1.9; controls 0.63 ± 0.7, P < .0005). The percentage of CD34+CD133+ cells in the bone marrow was related to pulmonary vascular resistance (R = 0.7, P = .04) and tended to correlate with pulmonary artery pressures (R = 0.6, P = .06).
Greater CD34+CD133+ progenitors in PAH. (A-D) CD34+CD133+ progenitors in PAH determined by flow cytometry. Box plots indicate median values, upper and lower quartiles. There are increased hemangioblasts in PAH bone marrow, and higher levels of CD34+CD133+ progenitors in circulation and within the pulmonary artery endothelium than in controls. (E-F) STAT3 and STAT5 activation and localization in bone marrow biopsies. Cellular localization of phosphoSTAT3 (pSTAT3) by immunohistochemical staining in bone marrow biopsy (arrowheads) from PAH patient and healthy control (E). Immunohistochemical staining for phosphoSTAT5 (pSTAT5) in bone marrow biopsy (arrowheads) from PAH patient and healthy control (F). (G) Reticulin increase in bone marrow of PAH patients. Reticulin staining as a measure of myelofibrosis, was increased in PAH bone marrows. Arrowheads identify reticulin staining in bone marrow biopsies from PAH subject. The reticulin stain around a blood vessel is normally present, and is shown as internal positive in the control tissue. (H) EpoR expression by flow cytometry. Dashed lined histogram indicates background staining with isotype control antibodies, solid histogram indicates EpoR staining. CFU-EC have low levels of EpoR, while lymphocytes are shown as negative control have no EpoR. (I) STAT5 activation in CFU-EC. Electrophoretic mobility shift assay for STAT5 DNA binding activation in whole cell extract of CFU-EC from PAH patient (lane 4) and healthy control (lane 3) is shown. STAT5 DNA-binding activation is similar among PAH and control CFU-EC. A549 cells stimulated with Epidermal Growth Factor (EGF) is a positive control, and the supershift using antibody to STAT5 identifies the STAT5-DNA complex (arrow). Scale bars: 6 μm (E); 10 μm (F); 40 μm (G).
Greater CD34+CD133+ progenitors in PAH. (A-D) CD34+CD133+ progenitors in PAH determined by flow cytometry. Box plots indicate median values, upper and lower quartiles. There are increased hemangioblasts in PAH bone marrow, and higher levels of CD34+CD133+ progenitors in circulation and within the pulmonary artery endothelium than in controls. (E-F) STAT3 and STAT5 activation and localization in bone marrow biopsies. Cellular localization of phosphoSTAT3 (pSTAT3) by immunohistochemical staining in bone marrow biopsy (arrowheads) from PAH patient and healthy control (E). Immunohistochemical staining for phosphoSTAT5 (pSTAT5) in bone marrow biopsy (arrowheads) from PAH patient and healthy control (F). (G) Reticulin increase in bone marrow of PAH patients. Reticulin staining as a measure of myelofibrosis, was increased in PAH bone marrows. Arrowheads identify reticulin staining in bone marrow biopsies from PAH subject. The reticulin stain around a blood vessel is normally present, and is shown as internal positive in the control tissue. (H) EpoR expression by flow cytometry. Dashed lined histogram indicates background staining with isotype control antibodies, solid histogram indicates EpoR staining. CFU-EC have low levels of EpoR, while lymphocytes are shown as negative control have no EpoR. (I) STAT5 activation in CFU-EC. Electrophoretic mobility shift assay for STAT5 DNA binding activation in whole cell extract of CFU-EC from PAH patient (lane 4) and healthy control (lane 3) is shown. STAT5 DNA-binding activation is similar among PAH and control CFU-EC. A549 cells stimulated with Epidermal Growth Factor (EGF) is a positive control, and the supershift using antibody to STAT5 identifies the STAT5-DNA complex (arrow). Scale bars: 6 μm (E); 10 μm (F); 40 μm (G).
Greater numbers of circulating CD34+CD133+ progenitors
Similar to previous report,20 circulating CD34+CD133+ cells were higher in individuals with PAH compared with controls (P = .01; Figure 1). Here, new data are provided to show that FPAH patients have the highest numbers of circulating CD34+CD133+ cells (% CD34+CD133+ cells: FPAH 0.2 ± 0.04; IPAH 0.09 ± 0.01; APAH 0.1 ± 0.07; controls 0.06 ± 0.008, ANOVA P < .01); FPAH was higher compared with IPAH or controls (all comparisons, Tukey P < .05). The numbers of CD34+CD133− cells were also higher in PAH compared with controls, but without significant difference between FPAH, IPAH, and APAH (% CD34+CD133− cells: PAH 0.06 ± 0.00; controls 0.04 ± 0.00, P = .01). The numbers of CD133+CD34− cells were elevated in subjects with PAH compared with controls (% CD133+CD34− cells: IPAH 1.7 ± 0.3; FPAH 0.8 ± 0.1; APAH 1.3 ± 0.2; controls 0.3 ± 0.05, ANOVA P < .001) with IPAH subjects having higher numbers than FPAH or controls (all comparisons Tukey P < .05) and APAH having higher numbers than controls (Tukey P < .05). The total circulating progenitors including CD34+CD133−, CD133+CD34−, and CD133+CD34+ cells were higher in PAH compared with controls (% total progenitor cells: PAH 1.5 ± 0.2; controls 0.3 ± 0.05, P < .001) and similar among the different PAH subgroups (% total progenitor cells: IPAH 1.8 ± 0.3; FPAH 1.1 ± 0.2; APAH 1.5 ± 0.3, ANOVA P = .3).
Proliferation of CD34+CD133+ cells as determined by colony-forming assays was significantly higher in individuals with PAH compared with controls (CFU-EC colonies/106 mononuclear cells: PAH [N = 28], 187 ± 31; controls [N = 10], 48 ± 5, P < .01). Patients with IPAH had the most proliferative progenitors compared controls (Tukey P < .05) but not significantly different from FPAH or APAH (CFU-EC colonies/106 mononuclear cells: IPAH 251 ± 56; FPAH 134 ± 19; APAH 158 ± 69; controls 48 ± 5, ANOVA P = .02).
Hematopoietic CFU assays
CFU-ECs have been shown to be enriched in myeloid CFU–granulocyte monocyte (CFU-GM) colony forming progenitors.38 Here, hematopoietic CFU assays were performed using sorted bone marrow hematopoietic progenitors (CD34+CD133+ cells) from healthy controls and patients with PAH. The CD34+CD133+ cells gave rise to all hematopoietic lineages (erythroid, myeloid, monocyte/macrophage, and megakaryocytes), but the CFU-GM colony formation was increased among PAH patients (number of CFU-GM colonies: PAH 112 ± 23; controls 38 ± 8, P = .04). This suggested that the greater numbers of CFU-EC derived from PAH bone marrow and circulation may be related to alteration of progenitor lineage commitment.
Recruitment of CD34+CD133+ progenitors into the pulmonary vascular bed
Lungs of PAH patients undergoing transplantation and donor lungs not used in transplantation were harvested for the study of progenitor cell recruitment into the pulmonary vasculature. To assess whether the increased mobilization of progenitors is paralleled by greater recruitment into the pulmonary arteries, the percentage of CD34+CD133+ cells was measured in single-cell suspensions of the pulmonary artery endothelium obtained from subjects undergoing lung transplantations or in donor lungs not used in transplantation. PAH subjects had ∼ 10-fold higher CD34+CD133+ progenitors recruited into the pulmonary artery wall compared with controls (P = .01; Figure 1).
JAK/STAT activation in PAH bone marrow
The circulating progenitor cell counts are increased in patients with myeloproliferative disorders, and related to constitutive activation of the JAK-STAT signal transduction pathway, most commonly because of JAK2 1849G>T mutation (valine-to-phenylalanine change at amino acid 617 [JAK 2 V617F]).39,40 Hence, we evaluated JAK2 V617F mutation in genomic DNA derived from circulating mononuclear cells, as well as activation of pSTAT3 and pSTAT5 in bone marrow aspirates and biopsies. JAK2 mutation was not detected in samples of PAH subjects (N = 18). Immunohistochemical staining of bone marrow showed that pSTAT3 and pSTAT5 were present and localized to nuclei of erythroid and granulocytic precursor cells and megakaryocytes (Figure 1E-F). Nuclear positive staining of phosphorylated STAT5 (pSTAT5) was easily detectable in a small subpopulation of mononuclear cells in bone marrow biopsies of patients and controls (Figure 1F). In contrast, pSTAT3 immunoreactivity in bone marrow aspirates was present in more cells, but was less intense (Figure 1E). Because a prior report identified myelofibrosis in patients with PAH, we evaluated bone marrows for argyrophilic (+) reticulin as a measure of fibrosis. Among hematologically healthy individuals, reticulin is usually absent, or present at low levels, that is, Bauermeister grade 0 in 69%, grade 1 in 27%, and grade 2 in 4%.36,41 Here, reticulin in all healthy human bone marrow was grade 0 (N = 4). Reticulin was present at greater than normal levels in PAH bone marrows (PAH bone marrow: Bauermeister grade 0 [14%], 1 [72%], 2 [14%]; Figure 1G). The high numbers of hematopoietic progenitors and reticulin fibrosis is consistent with a myeloproliferative process in PAH patients. Together, these findings and the greater numbers of circulating progenitors prompted us to analyze soluble factors that may promote myeloproliferation and progenitor mobilization.
Circulating levels of HIF-regulated bone marrow–activating factors
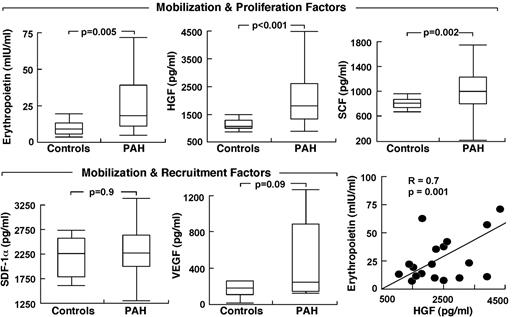
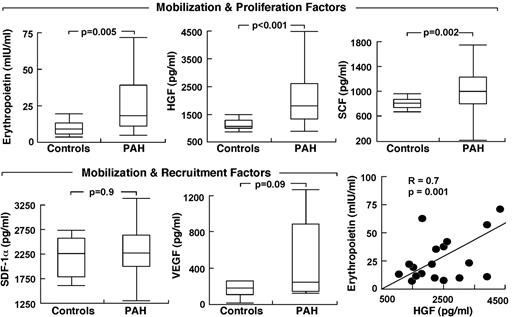
Because HIF plays a central role in the regulation of stem/progenitor cells and is activated in PAH endothelial cells, we evaluated the circulating levels of HIF-regulated factors. Epo was higher in PAH compared with controls (P = .005) with no difference among the PAH subgroups (Epo, mIU/mL: IPAH 25 ± 5; FPAH 45 ± 13; APAH 57 ± 32, ANOVA P = .4; all comparisons Tukey P > .05). Other progenitor cell mobilization and proliferation factors measured were SCF and HGF; both were higher in serum of patients with PAH compared with controls (all P < .05; Figure 2). VEGF tended to be higher in PAH (P = .09) but SDF-1α was not different between the groups (Figure 2). Factors were unrelated to circulating or bone marrow percentage of CD34+CD133+ cells, percentage of CD34+ cells, and percentage of CD133+ cells (all P > .1). However, Epo levels were related to progenitor cell colony formation (CFU-EC), an indicator of proliferation potential (R = 0.4, P = .01).
Elevated circulating HIF-inducible factors in PAH. Factors are grouped according to their biologic activity on stem cells/progenitors. Box plots indicate median values, upper and lower quartiles. Levels of erythropoietin are strongly related to HGF in PAH subjects.
Elevated circulating HIF-inducible factors in PAH. Factors are grouped according to their biologic activity on stem cells/progenitors. Box plots indicate median values, upper and lower quartiles. Levels of erythropoietin are strongly related to HGF in PAH subjects.
Epo was also related to parameters of clinical disease, including right ventricular systolic pressure (R = 0.3, P = .05), Pulmonary vascular resistance (PVR; R = 0.4, P = .06), and cardiac index (R = −0.3, P = .05) and tended to associate with brain natriuretic peptide (BNP), a sign of cardiac failure (R = 0.3, P = .08). Although patients were not anemic and had normal platelet counts, Epo was inversely related to hemoglobin (R = −0.4, P = .01) and platelets (R = −0.4, P = .01), but not to white blood cell count or creatinine (all P > .1). Interestingly, BNP was inversely related to platelets (R = −0.4, P = .03) and hemoglobin (R = −0.3, P = .06). Platelets were also related to right ventricular systolic pressure (R = −0.5, P = .001), while hemoglobin was modestly related to 6-minute walk distances (R = 0.4, P = .02). HGF, SCF, SDF-1α, and VEGF were unrelated to any clinical parameters of disease severity (all P > .1), even though Epo was strongly related to HGF (R = 0.6, P = .005) and SCF (R = 0.3, P = .03). Given the higher levels of HIF-regulated soluble factors, we questioned whether PAH PAECs that have intrinsic activation of HIF-1α might be the source of production of the factors.
Production of HIF-regulated factors by PAH PAECs
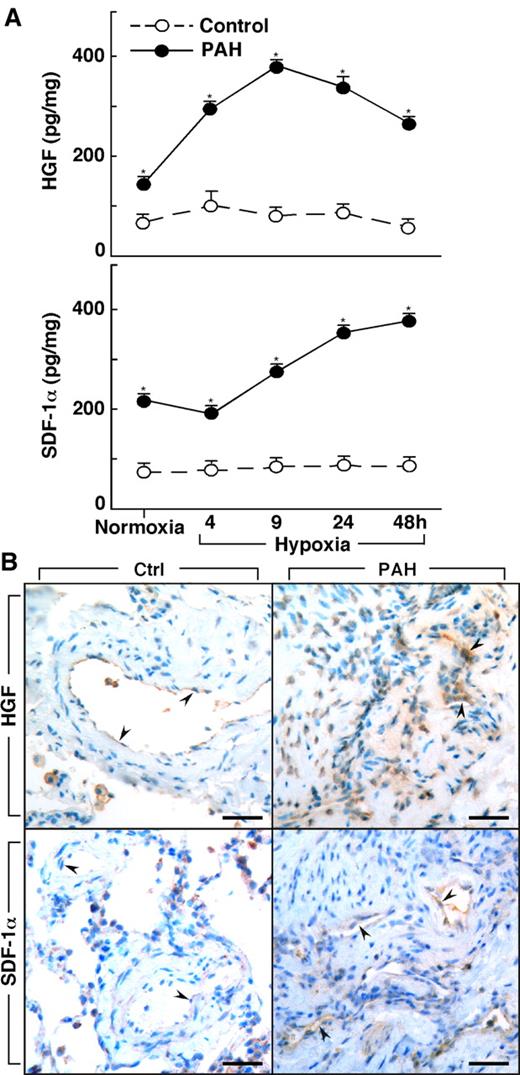
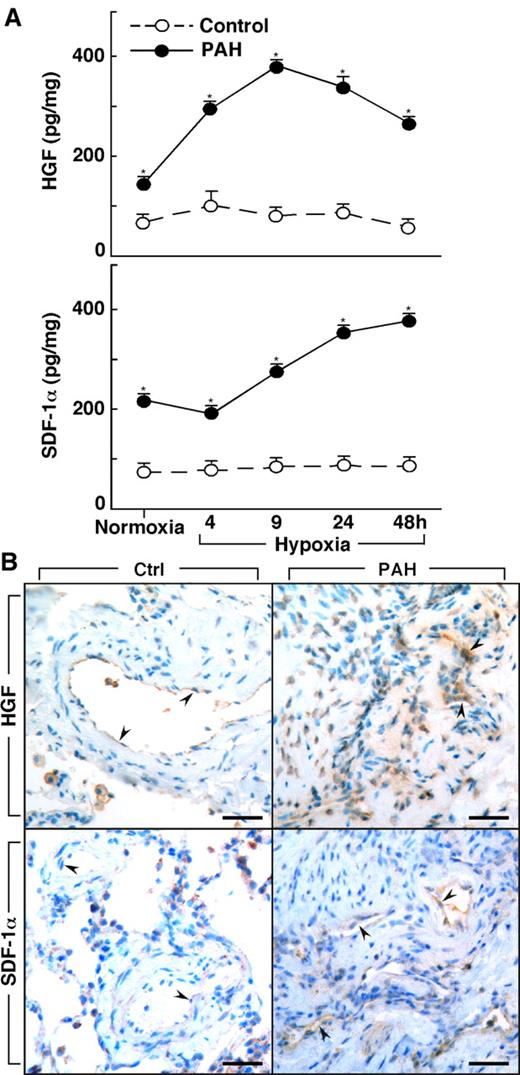
Lungs of PAH patients undergoing transplantation (n = 4) and donor lungs not used in transplantation (n = 3) were available for harvest of primary PAECs. Epo, HGF, and SDF-1α were measured in the supernatant of cultured PAECs that were obtained from PAH and healthy control donor lungs. Epo was not produced by cells in culture, whereas HGF was produced by cultured PAH PAECs at levels higher than in control cells (HGF [pg/mg protein]: controls 49 ± 6; PAH 682 ± 369, P = .03). HGF production by PAH cells was also greater than control cells when the cells were cultured under hypoxia (< 5% oxygen [all P < .05]; Figure 3). Although control cells had no significant change in HGF over time of hypoxia (ANOVA P = .6), HGF production by PAH PAECs significantly increased over time of hypoxia (ANOVA P = .0001; Figure 3). SDF-1α increased in both PAH cells (ANOVA P = .0001) and control cells under hypoxia (ANOVA P = .0007). SDF-1α production was greater in PAH cells compared with control cells at baseline culture conditions and under conditions of hypoxia (all P < .05; Figure 3).
Increased production of HGF and SDF-1α by primary human pulmonary arterial endothelial cells (PAECs) from PAH. (A) HGF and SDF-1α is secreted by PAH PAEC in culture at higher levels than by control cells under both normoxia and hypoxia conditions (*all P < .05). HGF secretion by PAH PAEC was significantly increased on exposure to hypoxia (ANOVA P = .0001). SDF-1α levels also significantly increased with hypoxia in both PAH (ANOVA P = .0001) and control PAEC (ANOVA P = .0007). (B) HGF and SDF-1α in lung tissues from controls and PAH patients. Endothelial cells in plexiform lesions of PAH lungs had strong positive immunoreactivity for HGF (arrowheads identify endothelial cells lining vascular lumens). Endothelial cells of control lung expressed HGF, but immunopositivity was much less prominent (arrowheads). SDF-1α expression was present in endothelial cells of plexiform lesions in PAH lung (arrowheads). Only mild immunopositivity for SDF-1α was found in vessel endothelium of control lung (arrowheads). Scale bars: 40 μm.
Increased production of HGF and SDF-1α by primary human pulmonary arterial endothelial cells (PAECs) from PAH. (A) HGF and SDF-1α is secreted by PAH PAEC in culture at higher levels than by control cells under both normoxia and hypoxia conditions (*all P < .05). HGF secretion by PAH PAEC was significantly increased on exposure to hypoxia (ANOVA P = .0001). SDF-1α levels also significantly increased with hypoxia in both PAH (ANOVA P = .0001) and control PAEC (ANOVA P = .0007). (B) HGF and SDF-1α in lung tissues from controls and PAH patients. Endothelial cells in plexiform lesions of PAH lungs had strong positive immunoreactivity for HGF (arrowheads identify endothelial cells lining vascular lumens). Endothelial cells of control lung expressed HGF, but immunopositivity was much less prominent (arrowheads). SDF-1α expression was present in endothelial cells of plexiform lesions in PAH lung (arrowheads). Only mild immunopositivity for SDF-1α was found in vessel endothelium of control lung (arrowheads). Scale bars: 40 μm.
To assess the in vivo production of HGF and SDF-1α by vascular endothelium, we performed immunostaining on lung tissues from controls and PAH (Figure 3B). Similar to the report by Toshner et al,19 immunostaining of lung tissues from control samples (n = 3) and patients with PAH (n = 3) revealed that SDF-1α expression was plainly detectable in the endothelium of plexiform lesions and vessels in PAH lungs, but only mild positivity was observed in vessel endothelium of control lungs. Likewise, strong HGF immunopositivity was observed in endothelial cells of plexiform lesions in PAH lungs, but HGF was less prominent or absent in endothelial cells of healthy lung. The presence of HGF and SDF-1α strong immunopositivity in endothelium of PAH lesional tissues, together with greater HGF levels in PAH blood and greater secretion of factors by PAH endothelial cells in culture, suggests that the mobilization and/or recruitment of progenitors to PAH lungs may be related to the production of these factors by a pulmonary vascular source in vivo.
Epo signaling in CFU-EC
Because CFU-EC were elevated in PAH patients in whom Epo concentrations were also elevated, Epo receptor (EpoR) and signal transduction (STAT5 activation) in CFU-EC of PAH patients were compared with controls. Flow cytometric analysis indicated similarly low expression of EpoR on CFU-EC from PAH or healthy controls (P = .5; Figure 1H). DNA-binding activation of STAT5, one of the downstream mediators of EpoR signaling that mediates hematopoiesis, was detectable under standard culture conditions for the CFU-EC, and activation was similar among PAH (n = 3) and control cells (n = 3; Figure 1I). STAT5 activation increased slightly after Epo stimulation (30 minutes) of cells in culture (data not shown). The modest STAT5 activation is likely because of low-level EpoR on the cells. These results indicate that alterations in EpoR/STAT5 activation did not account for greater CFU-EC numbers in PAH.
Nonaffected family members of patients with FPAH
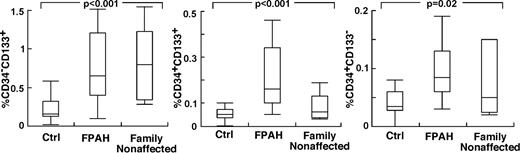
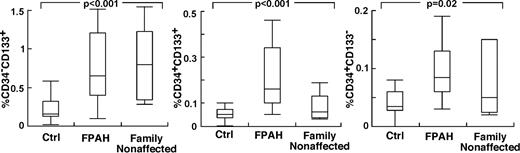
The findings of higher circulating levels of hematopoietic-active factors in PAH, and the greater production of HGF and SDF-1α by PAH endothelial cells, suggested that the hematopoietic abnormalities and greater progenitor mobilization in PAH occur, in part, in response to the primary pulmonary angioproliferative disease. On the other hand, abnormalities in hematopoietic precursors might be intrinsic to PAH. To test this latter possibility, we reasoned that if bone marrow abnormalities were intrinsic to PAH, then family members of patients with FPAH, who do not have pulmonary hypertension, might manifest hematopoietic abnormalities, for example, high numbers of circulating and/or bone marrow–resident CD34+CD133+ progenitors. Nine nonaffected family members agreed to participate (age 43 ± 4 years; female/male, 2/7) in the study. Echocardiography studies of all nonaffected family members were normal without signs of PAH Right ventricular systolic pressure (RVSP; mm Hg: 22 ± 3). Circulating blood counts were similar to FPAH subjects with the exception of higher platelet count in nonaffected family members (Table 4). Strikingly, all nonaffected family members had circulating CD133+ cells and CD34+ cell numbers similar to their afflicted relatives, and higher than healthy nonrelated controls (Tukey P < .05; Figure 4). Bone marrow–resident CD133+ cells, CD34+CD133+ cells, and CD34+CD133− cells were similar between FPAH and nonaffected family members and healthy controls (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, bone marrow of nonaffected family members had an increase of reticulin (100% Bauermeister grade 1; N = 6) compared with healthy unrelated controls (100% grade 0; N = 4). In contrast to the findings of high circulating progenitors and increased reticulin in nonaffected family members, levels of Epo, a valid indicator of enhanced HIF activation or responsiveness, were not different from controls and lower than FPAH (Epo, mIU/mL: nonaffected family members 9 ± 2; FPAH 45 ± 13; controls 11 ± 1, ANOVA P < .001 and TUKEY P > .05 for controls compared with nonaffected family members and < 0.05 for FPAH compared with controls or nonaffected family members).
Increased circulating progenitor cells in nonaffected family members of patients with FPAH. Values of circulating progenitors in nonaffected family members were comparable with their afflicted family members. Single- and double-positive CD34 and CD133 cells were measured in circulation using flow cytometry. Box plots indicate median values, upper and lower quartiles.
Increased circulating progenitor cells in nonaffected family members of patients with FPAH. Values of circulating progenitors in nonaffected family members were comparable with their afflicted family members. Single- and double-positive CD34 and CD133 cells were measured in circulation using flow cytometry. Box plots indicate median values, upper and lower quartiles.
Discussion
Increased bone marrow hemangioblast numbers, alterations in erythroid/myeloid lineages, increased reticulin, and greater mobilization of bone marrow progenitor cells define hematopoietic abnormalities as an integral part of PAH disease. Higher levels of bone marrow–activating factors are present in circulation, some of which are produced by the PAH-diseased pulmonary vascular endothelium and are likely instrumental in causing the hematopoietic effects (Figure 5). However, Epo, one of the most potent mediators of bone marrow activation, is not derived from PAH PAECs, which favors an underlying disturbance in the myeloid system.
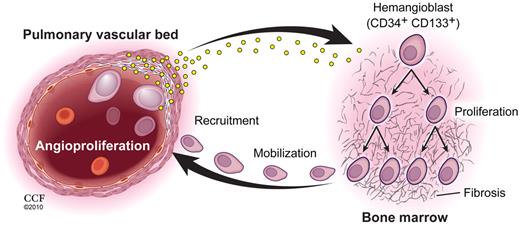
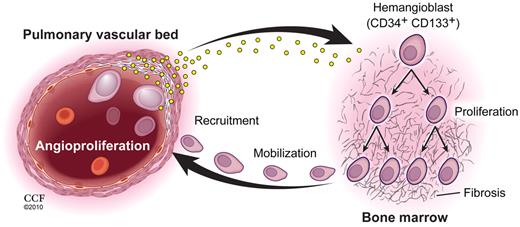
Pulmonary vascular disease and myeloid abnormalities in PAH. This study identifies hemangioblast proliferation and fibrosis as key components in the pathophysiology of PAH. Increased levels of HIF-inducible factors, some of which are produced by diseased pulmonary vascular cells, promote hemangioblast proliferation and progenitor cell mobilization. Local production of recruitment factors by pulmonary vascular endothelium attract mobilized progenitor cells into the pulmonary artery wall where these cells fuel vascular remodeling. (Illustration by David Schumick, BS, CMI. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography, 2010. All rights reserved.)
Pulmonary vascular disease and myeloid abnormalities in PAH. This study identifies hemangioblast proliferation and fibrosis as key components in the pathophysiology of PAH. Increased levels of HIF-inducible factors, some of which are produced by diseased pulmonary vascular cells, promote hemangioblast proliferation and progenitor cell mobilization. Local production of recruitment factors by pulmonary vascular endothelium attract mobilized progenitor cells into the pulmonary artery wall where these cells fuel vascular remodeling. (Illustration by David Schumick, BS, CMI. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography, 2010. All rights reserved.)
In this report, we confirm prior work that circulating progenitors are present at higher than normal levels and are more proliferative in PAH than controls. Here, patients with FPAH are shown to have the highest circulating progenitors, while patients with IPAH have the most proliferative circulating progenitors. Likewise, there are differences in bone marrow progenitors; patients with FPAH and APAH have nearly double the erythroid progenitors of IPAH patients, while patients with IPAH have greater differentiation toward myeloid progenitors. In this study, we extend observations regarding myelofibrosis that is associated with PAH. Prior study identified a high incidence of myelofibrosis in PAH patients selected for study on the basis of a hematologic indication such as anemia, low platelets or both.42 Here, PAH patients had no overt hematologic abnormality by measures of circulating blood counts, yet still manifest abnormalities typical of myeloproliferative processes. However, somatic mutations of JAK2, which are typical of primary myeloproliferative processes, were not present in PAH patients. Rather, Epo levels were higher than normal in PAH patients; Epo binding to its receptor results in phosphorylation of the receptor and activation of JAK2, which then phosphorylates/activates STAT5 and STAT3. Along with JAK2 abnormalities, altered activation of STAT pathways are typically present in myeloproliferative disorders.43 In this context, although bone marrow cells did not manifest JAK/STAT abnormalities, pulmonary artery endothelial cells in PAH lungs have been shown to have intrinsically high levels of STAT3 activation,1,3 identifying alterations in the tyrosine kinase signal transduction pathway in PAH lung endothelial cells. Together with prior studies, the findings point to an interdependent hematopoietic and angiogenic pathology in PAH. How might the myeloid abnormalities influence the disease? In this report, we provide quantitative data showing CD34+CD133+ cells recruitment into the pulmonary vascular bed using immunoquantitative FACS analyses, analogous to prior report of immunostaining of tissues.44 Recruitment to the pulmonary vasculature is likely related to intrinsic production of chemoattractants HGF and SDF-1α by the diseased endothelium. The production of these factors is consistent with prior report that PAH endothelial cells have HIF-1α and STAT3 activation, leading to a proangiogenic phenotype.3,45,46 Likewise, the greater response to hypoxia in the PAH endothelial cells confirms prior findings that intracellular hypoxia sensing is altered in PAH. Based on the increases of Epo in this report, hypoxia-sensing abnormalities may be present systemically and contribute to the myeloproliferative process. On the other hand, the greater bone marrow resident and circulating progenitors in nonaffected family members of PAH patients suggests that hematopoietic abnormalities may precede and/or predispose to the disease, and are not just a consequence of pulmonary vasculopathy. In this context, although PAH patients were not anemic, high Epo levels or hemoglobin levels in the lower range were related to more severe clinical disease. This suggests that the condition of anemia or iron deficiency, which activate HIF-inducible factors including Epo, would lead to worsening clinical status in PAH patients. In fact, iron deficiency and/or anemia influences the development and clinical course of PAH. For example, low hemoglobin is related to decreased survival in all classes of PAH,47 and the classic pulmonary hypertensive response to hypoxia is significantly attenuated by intravenous iron infusion.48 Here, we identify that a subclinical myeloproliferative process is intrinsic to PAH disease, and that the myeloid abnormalities result in processes that promote the pathologic vascular remodeling in the lung. The fact that PAH patients are highly prone to develop overt myelofibrosis or thrombocytopenia provides support for the idea that PAH is coupled to underlying alterations in early bone marrow progenitors. Likewise, patients with myeloproliferative diseases, such as primary myelofibrosis and myeloid leukemia, often develop PAH3,45,46,49,50 Reports identifying that PAH resolves with treatment of the myeloproliferative process, also support a role for bone marrow progenitors in PAH pathogenesis.51,52 Overall, the findings in this study together with other reports indicate that myeloid abnormalities exist concurrently with pulmonary vascular disease, although not manifest clinically. Myeloid abnormalities appear to predispose to PAH, and PAH patients are prone to development of myeloid diseases. This supports the interdependence of hematopoiesis and angiogenesis, not only in functional repair, but also in pathologic vascular remodeling, and indicates that treatments targeting the bone marrow myeloproliferative process may be effective in treating the proliferative angiopathic processes in PAH.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank A. Janocha, J. Hanson, L. Mavrakis, and R Steinle for excellent technical assistance; L. Vargo, Dr J. Drazba, and Dr A. J. Peterson in the Lerner Research Institute Digital Imaging Core; C. Shemo and S. O'Bryant in the Lerner Research Institute Flow Cytometry Core for technical advise and excellent assistant with instrument operation; and M. Baaklini, M. Cleggett-Mattox, and M. Koo for patient recruitment.
This work was supported by grants HL60917 and M01 RR018390 from the National Institutes of Health, the Cleveland Clinic Research Programs Council, American Thoracic Society/Pulmonary Association Research grant (PH-07-003) to K.A., and the Hematopoietic Stem Cell Core Facility of the Case Comprehensive Cancer Center (P30 CA43703).
National Institutes of Health
Authorship
Contribution: S.F. conducted the study, performed research, analyzed and interpreted data, and wrote the manuscript; K.A. performed research, analyzed and interpreted the data, and wrote the manuscript; W.X. performed research, analyzed and interpreted the data, and wrote the manuscript; J.S. conducted the study and collected data; D.G. conducted the study and recruited subjects; S.C. performed research and provided tissue and cells; M.P. conducted research and recruited subjects; W.H.W.T. analyzed data and reviewed the manuscript; J.E.L. recruited subjects and reviewed the manuscript; K.T. performed research and reviewed the manuscript; R.T. performed research; E.H. performed research and reviewed the manuscript; A.L. performed research and reviewed the manuscript; and S.C.E. designed research, analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samar Farha, MD, Cleveland Clinic, 9500 Euclid Ave, NC22, Cleveland, OH 44195; e-mail: farhas@ccf.org; or Serpil Erzurum, MD, Cleveland Clinic, 9500 Euclid Ave, NC22, Cleveland, OH 44195; e-mail: erzurus@ccf.org.