Abstract
It is currently assumed that myelofibrosis (MF) originates from acquired mutations that target the hematopoietic stem cell and induce dysregulation of kinase signaling, clonal myeloproliferation, and abnormal cytokine expression. These pathogenetic processes are interdependent and also individually contributory to disease phenotype–bone marrow stromal changes, extramedullary hematopoiesis, ineffective erythropoiesis, and constitutional symptoms. Molecular pathogenesis of MF is poorly understood despite a growing list of resident somatic mutations that are either functionally linked to Janus kinase (JAK)–signal transducer and activator of transcription hyperactivation (eg JAK2, MPL, and LNK mutations) or possibly involved in epigenetic dysregulation of transcription (TET2, ASXL1, or EZH2 mutations). Current prognostication in primary MF is based on the Dynamic International Prognostic Scoring System-plus model, which uses 8 independent predictors of inferior survival to classify patients into low, intermediate 1, intermediate 2, and high-risk disease groups; corresponding median survivals are estimated at 15.4, 6.5, 2.9, and 1.3 years. Such information is used to plan a risk-adapted treatment strategy for the individual patient, which might include observation alone, conventional or investigational (eg, JAK inhibitors, pomalidomide) drug therapy, allogenic stem cell transplantation with reduced- or conventional-intensity conditioning, splenectomy, or radiotherapy. I discuss these treatment approaches in the context of who should get what and when.
Terminology: What do I mean by myelofibrosis?
Bone marrow fibrosis, usually shown by silver (reticulin fibrosis) or trichrome (collagen fibrosis) stains, can accompany a number of hematologic and nonhematologic conditions, including myeloid or lymphoid neoplasms, metastatic cancer, autoimmune diseases, hyperparathyroidism, vitamin D deficiency, pulmonary hypertension, gray platelet syndrome, treatment with growth factors (eg, thrombopoietin agonists), hereditary thrombocytosis (eg, germline MPLS505N mutation), infections (eg, AIDS, leishmaniasis), and exposure to toxic substances (eg, thorium dioxide).1 When describing such occurrences, I prefer to use the phrase “with bone marrow fibrosis” instead of “with myelofibrosis”: for example, “myelodysplastic syndromes (MDSs) with bone marrow fibrosis” rather than “MDS with myelofibrosis.” I use the term myelofibrosis (MF) exclusively in reference to the myeloproliferative neoplasm (MPN) that is classified by the World Health Organization (WHO) system as primary myelofibrosis (PMF),2 or the phenotypically similar condition that develops in the setting of either polycythemia vera (post-PV MF) or essential thrombocythemia (post-ET MF).3 In addition, given our current understanding about the clonal nature of MF,4 it is inaccurate to continue using alternative terms such as “agnogenic myeloid metaplasia” or “chronic idiopathic myelofibrosis.”5
Classification: WHO all the way
The WHO classification system for hematopoietic tumors recognizes 5 categories of myeloid malignancies, including acute myeloid leukemia (AML), MDS, MPN, MDS/MPN overlap, and PDGFR/FGFR1-rearranged myeloid/lymphoid neoplasms with eosinophilia (Table 1).2 The WHO MPN category includes 8 subcategories: PV, ET, PMF, chronic myelogenous leukemia (CML), chronic neutrophilic leukemia, chronic eosinophilic leukemia not otherwise specified, mastocytosis, and MPN unclassifiable. The first 4 were originally described by William Dameshek as “myeloproliferative disorders” and are therefore currently referred to as “classic” MPN (Table 1).6 “BCR-ABL1–negative MPN” is an operational term that is used in reference to PV, ET, and PMF.7 Post-PV MF and post-ET MF are classified and diagnosed according to consensus criteria established by the International Working Group for MPN Research and Treatment (IWG-MRT).3 The IWG-MRT also recommends the use of the term “blast phase PMF” when describing leukemic transformation.3,5
Pathogenesis: What do abnormal clones, mutations, and cytokines mean?
MPN are stem cell–derived monoclonal (or possibly oligoclonal) hemopathies.8,9 Family studies and JAK2 single nucleotide polymorphism/haplotype analyses suggest genetic predisposition to MPN.10-12 Although the disease-initiating mutation in MF is not known, the majority of the patients harbors JAK2V617F and a minority harbors MPL, LNK, CBL, TET2, ASXL1, IDH, IKZF1, or EZH2 mutations (Table 2).13,14 These mutations are neither disease- specific nor mutually exclusive13 and probably constitute secondary events with unpredictable clonal hierarchy.15 Activating JAK2 and MPL mutations and LNK loss-of-function result in constitutive Janus kinase–signal transducer and activator of transcription (JAK-STAT) activation and induce MPN-like disease in mice.16-19 Therefore, it was appropriate to pursue the therapeutic value of anti-JAK adenosine triphosphate (ATP) mimetics.20,21 TET2, ASXL1, and EZH2 mutations are suspected of playing a role in epigenetic dysregulation of transcription.22-24 IDH mutations result in the formation of oncoproteins that might promote disease progression.25 The individual frequency of the aforementioned mutations in MF (Table 2), save for JAK2V617F, is too low to be overexcited about their utility as drug targets.
Clonal myeloproliferation in MF is accompanied by a secondary inflammatory state characterized by bone marrow stromal changes and abnormal cytokine expression.4 Myeloid cell–derived transforming growth factor-β, platelet-derived growth factor, fibroblast growth factor-b, and vascular endothelial growth factor have all been implicated as mediators of bone marrow fibrosis, osteosclerosis, and angiogenesis in MF.4 The abnormal release of cytokines, chemokines, and extracellular matrix metalloproteinases (or their inhibitors) might be enhanced by a pathologic cell-cell interaction that involves megakaryocytes, monocytes, and neutrophils26 and contributes to MF-associated abnormal peripheralization of CD34+ myeloid progenitors27 and endothelial cells.28 Plasma levels of proinflammatory cytokines are elevated in MF and might be pathogenetically linked to disease-associated constitutional symptoms and cachexia.21 In addition, a recent study suggested significant associations between increased interleukin-8 (IL-8), IL-10, IL-15, or IL-2 receptor (IL-2R) and inferior overall survival (OS) and leukemia-free survival in PMF.29 These observations lend to the possibility that inflammatory mediators might affect survival in MF by either accelerating death from comorbid conditions or promoting clonal evolution.
Diagnosis: How do I diagnose PMF, post-PV/ET MF, and prefibrotic PMF?
Current diagnosis of PMF is based on WHO criteria and includes clinical, morphologic, cytogenetic, and molecular assessments (Table 3).30 The diagnosis of post-PV or post-ET MF is according to IWG-MRT criteria (Table 3).3 In all 3 MF variants, typical laboratory features include anemia (microcytic in ∼ 28% of PMF cases),31 peripheral blood leukoerythroblastosis, dacryocytosis, leukocytosis/thrombocytosis, increased lactate dehydrogenase (LDH), excess circulating blasts or CD34+ cells, and bone marrow fibrosis, osteosclerosis, and angiogenesis. Occasionally, overt bone marrow fibrosis might be absent (ie, prefibrotic PMF) and, in the presence of thrombocytosis, a spurious diagnosis of ET is made. The possibility of prefibrotic PMF, as opposed to ET, should be considered in the presence of persistently increased serum LDH, anemia, leukoerythroblastosis, increased circulating CD34+ cell count, and marked splenomegaly.32 It is underscored that the distinction between ET and prefibrotic PMF is clinically relevant because both OS and leukemia-free survival are significantly inferior in the latter.32 However, there is currently no evidence to suggest a different treatment approach for patients with prefibrotic PMF and thrombocytosis; I manage them the same as if they had ET.
The differential diagnosis of PMF should also include bone marrow fibrosis associated with nonneoplastic or other neoplastic conditions, including metastatic cancer, lymphoid neoplasm, or another myeloid malignancy, especially CML, MDS, chronic myelomonocytic leukemia (CMML), or AML. The presence of JAK2 or MPL mutation, with a combined mutational frequency of ∼ 70%, reliably excludes reactive bone marrow fibrosis or a nonmyeloid malignancy.33 The absence of BCR-ABL1 excludes the possibility of CML. MDS or CMML should be considered in the presence of dyserythropoiesis/dysgranulopoiesis or peripheral blood monocytosis (> 1 × 109/L), respectively.34 In this regard, the presence of +9 or 13q− cytogenetic abnormality favors a PMF diagnosis.35 In contrast, JAK2V617F can occur in both MDS and CMML, although infrequently,36 and is therefore not very useful in distinguishing one myeloid malignancy from another. “Acute myelofibrosis,” “acute panmyelosis with myelofibrosis,” and “acute megakaryoblastic leukemia” are terms used to describe a biologically aggressive myeloid malignancy that presents acutely with bone marrow fibrosis, pancytopenia, and usually no splenomegaly.37 Such cases should be managed as AML regardless of which term one uses to describe them.
Risk stratification: prognosis dictates treatment
The International Prognostic Scoring System (IPSS)38 uses the following 5 risk factors for estimating survival from time of diagnosis: age > 65 years, hemoglobin level < 10 g/dL, leukocyte count > 25 × 109/L, circulating blasts ≥ 1%, and presence of constitutional symptoms.38 The presence of 0, 1, 2, and ≥ 3 adverse factors define low, intermediate 1, intermediate 2, and high-risk disease with median survivals of 11.3, 7.9, 4, and 2.3 years, respectively.38 With the use of the same prognostic variables, IPSS was later modified to Dynamic IPSS (DIPSS) for use at any time during the disease course.39 Most recently, DIPSS was upgraded to DIPSS-plus (Figure 1) by the incorporation of 3 additional IPSS/DIPSS independent risk factors, including red cell transfusion need, platelet count < 100 × 109/L, and unfavorable karyotype40 ; the latter includes complex karyotype or sole or 2 abnormalities that include +8, −7/7q−, i(17q), inv(3), −5/5q−, 12p−, or 11q23 rearrangement.41 The 8 DIPSS-plus risk factors are used to define low (no risk factors), intermediate 1 (1 risk factor), intermediate 2 (2 or 3 risk factors), and high (≥ 4 risk factors) risk groups with respective median survivals of 15.4, 6.5, 2.9, and 1.3 years (Figure 1).40 Leukemic transformation was predicted by the presence of unfavorable karyotype or platelet count < 100 × 109/L.40
The Dynamic International Prognostic Scoring System (DIPSS) plus prognostic model for primary myelofibrosis (PMF). The DIPSS and prognostic model for PMF uses 8 risk factors for inferior survival: age > 65 years, hemoglobin level < 10 g/dL, leukocyte count > 25 × 109/L, circulating blasts ≥ 1%, presence of constitutional symptoms, presence of unfavorable karyotype, platelet count < 100 × 109/L, and the presence of red cell transfusion need.40 *Please note that a transfusion-dependent patient automatically has 2 risk factors because of transfusion need (1 risk point) and hemoglobin level < 10 g/dL (1 risk point). **Constitutional symptoms constitute weight loss > 10% of baseline value in the year preceding diagnosis, unexplained fever, or excessive sweats persisting for > 1 month.38 ***Unfavorable karyotype constitutes complex karyotype or sole or 2 abnormalities that include +8, −7/7q−, i(17q), inv(3), −5/5q− 12p−, or 11q23 rearrangement.41
The Dynamic International Prognostic Scoring System (DIPSS) plus prognostic model for primary myelofibrosis (PMF). The DIPSS and prognostic model for PMF uses 8 risk factors for inferior survival: age > 65 years, hemoglobin level < 10 g/dL, leukocyte count > 25 × 109/L, circulating blasts ≥ 1%, presence of constitutional symptoms, presence of unfavorable karyotype, platelet count < 100 × 109/L, and the presence of red cell transfusion need.40 *Please note that a transfusion-dependent patient automatically has 2 risk factors because of transfusion need (1 risk point) and hemoglobin level < 10 g/dL (1 risk point). **Constitutional symptoms constitute weight loss > 10% of baseline value in the year preceding diagnosis, unexplained fever, or excessive sweats persisting for > 1 month.38 ***Unfavorable karyotype constitutes complex karyotype or sole or 2 abnormalities that include +8, −7/7q−, i(17q), inv(3), −5/5q− 12p−, or 11q23 rearrangement.41
The presence or absence of JAK2,42,43 Ten-Eleven Translocation-2 (TET2),44 or isocitrate dehydrogenase (IDH)45 mutations has not been shown to affect either survival or leukemic transformation in PMF. Instead, nullizygosity for JAK2 46/1 haplotype12,45 and low JAK2V617F allele burden.42,43 have been shown to be detrimental for survival. Similarly, increased plasma levels of IL-8, IL-10, IL-15, or IL-2R have recently been associated with poor survival that was not accounted for by conventional risk categorization.29 In other words, our current ability to accurately estimate prognosis in the individual patient with PMF is better than ever and will probably continue to improve with time.
Risk-adapted therapy: Who gets what?
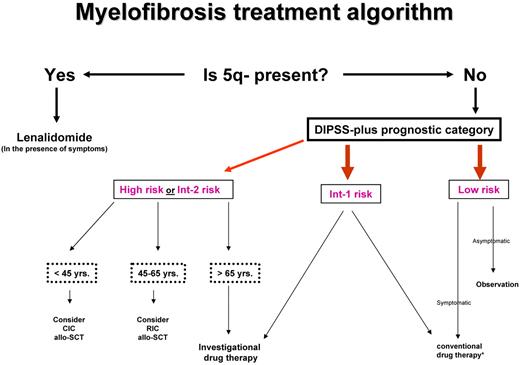
Current drug therapy in PMF is neither curative nor essential for survival. Similarly, it is not clear if the application of allogeneic stem cell transplantation (allo-SCT), with its attendant risk of death or chronic morbidity from graft-versus-host disease (GVHD), has had a favorable or unfavorable net effect. Therefore, one must first determine whether a particular patient needs any form of therapy at all and, if so, carefully select the treatment strategy with the best chance of inducing disease control without compromising life expectancy (Figure 2).
Risk-adapted therapy in primary myelofibrosis. DIPSS-plus indicates Dynamic International Prognostic Scoring System (DIPSS)-plus prognostic model for primary myelofibrosis40 ; Int, intermediate; yrs, years; CIC, conventional-intensity conditioning; RIC, reduced-intensity conditioning; allo-SCT, allogeneic stem cell transplantation. *Conventional drug therapy includes erythropoiesis-stimulating agents, androgens, danazol, corticosteroids, thalidomide, lenalidomide, hydroxyurea, and cladribine. Please see text about which agent is used when.
Risk-adapted therapy in primary myelofibrosis. DIPSS-plus indicates Dynamic International Prognostic Scoring System (DIPSS)-plus prognostic model for primary myelofibrosis40 ; Int, intermediate; yrs, years; CIC, conventional-intensity conditioning; RIC, reduced-intensity conditioning; allo-SCT, allogeneic stem cell transplantation. *Conventional drug therapy includes erythropoiesis-stimulating agents, androgens, danazol, corticosteroids, thalidomide, lenalidomide, hydroxyurea, and cladribine. Please see text about which agent is used when.
How I manage low- or intermediate 1–risk disease
According to the DIPSS-plus prognostic model,40 the respective median survival of patients with low- or intermediate 1–risk disease exceeds 15 and 6 years and even longer for patients younger than age 65 years. Therefore, the risk of allo-SCT–associated mortality and morbidity is not justified in such patients, and it is also not prudent to subject them to investigational drug therapy, considering the limited information about long-term safety of new therapeutic agents. Similarly, there is no evidence to support the value of conventional drug therapy in asymptomatic patients with low- or intermediate 1–risk disease. Therefore, I prefer a “watch and wait” treatment strategy in such instances, regardless of patient age.
Low-risk patients might occasionally experience symptoms associated with disease manifestations that do not necessarily affect DIPSS-plus risk stratification: splenomegaly, nonhepatosplenic extramedullary hematopoiesis, extramedullary hematopoiesis (EMH)–associated pulmonary hypertension, fatigue, bone (extremity) pain, pruritus, or thrombocytosis with a thrombosis history. Intermediate 1–risk patients might in addition display symptomatic anemia, marked leukocytosis, or constitutional symptoms such as drenching night sweats, fever, or weight loss (cachexia). However, such symptoms are much more prevalent in intermediate 2– and high-risk patients, and they are usually not severe enough to warrant therapeutic intervention in lower-risk patients. Nevertheless, if treatment is indicated in low- or intermediate 1–risk patients, it is reasonable to start with conventional drug therapy (see “Conventional drug therapy”) before rushing into treatment with experimental agents.
How I manage intermediate 2– or high-risk disease
Patients with MF with high- or intermediate 2–risk disease can be managed by conventional drug therapy, splenectomy, radiotherapy, allo-SCT, or experimental drug therapy. With each one of these treatment modalities except allo-SCT, the primary goal is palliation of anemia, symptomatic splenomegaly, constitutional symptoms, or disease complications from EMH.
Conventional drug therapy
For the purposes of this review, I define “conventional drugs” as those that are approved by the Food and Drug Administration, and their utility in a specific disease-related complication has been published in a peer-reviewed medical journal. My approach in managing symptomatic anemia depends on the presence or absence of associated splenomegaly. In the absence of splenomegaly, it is reasonable to try erythropoiesis-stimulating agents (ESAs; eg, darbepoetin, 150-300 μg weekly) on the basis of a potential response rate of ≤ 56% (lasting for an average of 1 year) in patients who are not transfusion dependent and show a hemoglobin level of < 10 g/dL.46 It should be noted, however, that ESAs are unlikely to benefit patients who are transfusion dependent46 or who display a serum erythropoietin level of > 125 U/L.47 In addition, the use of ESAs is discouraged in the presence of more than mild splenomegaly (ie, palpable spleen size > 5 cm below the left costal margin), because of the danger of drug-induced exacerbation of splenomegaly.
In anemic patients who are either not good candidates for ESA therapy (see above) or in whom such therapy was unsuccessful, one has a choice of several potentially effective conventional drugs, including corticosteroids (eg, prednisone 0.5 mg/kg/d),48 androgens (eg, fluoxymesterone 10 mg 3 times daily),49 danazol (600 mg/d),50 thalidomide (50 mg/d) with or without prednisone (0.25 mg/d)51,52 or lenalidomide (10 mg/d) with or without prednisone.53-55 Response rates and durations for each one of these treatment modalities are somewhat similar and estimated at 20% and 1 year, respectively. Thalidomide or lenalidomide should be avoided in women of childbearing age. Corticosteroid use should be avoided in the presence of diabetes or osteopenia; androgen or danazol use should be avoided in the presence of increased level of serum prostate-specific antigen or history of prostate cancer; thalidomide use should be avoided in the presence of neuropathy; and lenalidomide use should be avoided in the presence of moderate-to-severe neutropenia or thrombocytopenia.
I favor the use of lenalidomide in the presence of del(5q) and expect a response in both anemia and splenomegaly.56 In the absence of del(5q), the therapeutic benefit of lenalidomide, with or without prednisone,55 is too limited, and its myelosuppressive toxicity too high to warrant its use as first-line therapy. Patients receiving lenalidomide should be closely monitored for the occurrence of severe myelosuppression and thrombosis; I use concomitant aspirin, if the platelet count is > 50 × 109/L, to minimize lenalidomide- or thalidomide-associated thrombosis. Thalidomide alone can produce ∼ 20% response rates in MF-associated anemia, thrombocytopenia, or splenomegaly.52,57 Furthermore, its use in combination with prednisone reduces the severity of its short-term side effects and might enhance its therapeutic activity.51 Unfortunately, with longer-term usage, a substantial proportion of patients develop thalidomide-associated peripheral neuropathy. Therefore, in the absence of del(5q), I usually try androgens or danazol before resorting to thalidomide; side effects of androgen therapy include hepatotoxicity and virilizing effects.
Hydroxyurea (starting dose 500 mg by mouth twice daily) is my first-line drug of choice for the treatment of MF-associated splenomegaly.58,59 In the presence of marked splenomegaly (palpable at > 10 cm below the left costal margin), ∼ 35% of patients were reported to achieve ≥ 25% reduction in spleen size; 17% experienced 50% reduction in spleen size.58 Response rates were significantly lower (10%) in JAK2V617F-negative patients, compared with those with detectable JAK2V617F: 67% and 33% response rates in patients with mutant allele burdens of < or > 50%, respectively.58 Spleen responses to hydroxyurea last for an average of 1 year, and treatment side effects include myelosuppression, xerodermia, and mucocutaneous ulcers. At present, participation in clinical trials with JAK inhibitors (see “JAK inhibitors: value and limitations”) is advised for patients with hydroxyurea-refractory splenomegaly or hepatomegaly. Otherwise, one can try intravenous cladribine (5 mg/m2 by 2-hour infusion daily for 5 days and to be repeated monthly, depending on toxicity and response),60 thalidomide,52,57 or lenalidomide.53 Expected response rates for these latter agents range from 20% to 50%. Interferon-α is of limited value in the treatment of MF-associated splenomegaly.61
Splenectomy
Splenectomy remains a viable treatment option for drug-refractory symptomatic splenomegaly in MF.62 In general, I consider splenectomy in the presence of marked splenomegaly (> 10 cm palpable below the left costal margin) that is not responding to adequate doses of hydroxyurea and is associated with severe discomfort or pain, frequent red blood cell transfusions, severe thrombocytopenia, symptomatic portal hypertension, or profound cachexia. Most patients with MF who undergo splenectomy benefit from the procedure; more than one-half of transfusion-dependent patients become transfusion-independent, and most also experience resolution of mechanical symptoms and improvement in constitutional symptoms, cachexia, and platelet count if they were thrombocytopenic.63 The average duration of response is about a year.
The downside of splenectomy in MF includes a perioperative mortality rate of 5%-10% and morbidity rate of ∼ 25%.62,63 Abdominal vein thrombosis, operative site bleeding, and infections are particularly prevalent during the postoperative period, and close monitoring is critical for early diagnosis and treatment. In preparation for splenectomy, I usually place patients with platelet count of > 200 × 109/L on hydroxyurea to minimize the risk of postoperative extreme thrombocytosis and associated thrombosis.62 Postoperatively and once hemostasis is secured (∼ 5-8 days after surgery), I usually put patients on therapeutic systemic anticoagulation for ∼ 1 month to reduce the risk of postoperative splanchnic vein thrombosis. Postsplenectomy thrombocytosis and left-shifted granulocytosis, including an increase in circulating blast percentage, are frequent and do not necessarily imply disease progression. These redistribution changes are often effectively managed by cytoreductive therapy. In addition, ∼ 20% of patients might experience progressive hepatomegaly, and potentially useful drugs for such cases include hydroxyurea, cladribine, or JAK inhibitors (see above in the immediately preceding section). Median survival after splenectomy has been reported to be ∼ 2 years. In my opinion, leukemic transformation after splenectomy represents the natural progression of the disease rather than a treatment complication.64 Alternatives to splenectomy, in hydroxyurea-refractory splenomegaly, include participation in clinical trials, radiotherapy (see “Radiotherapy”), or transjugular intrahepatic portosystemic shunt in case of symptomatic portal hypertension (ie, ascites, recurrent variceal bleed). Because of the lack of adequately sized relevant studies in MF, I do not recommend laparoscopic total or subtotal splenectomy or splenic artery embolization.
Radiotherapy
In MF, radiotherapy is most useful in the setting of non–hepatosplenic EMH,65 pulmonary hypertension,66 or lower or upper extremity pain.67 Non–hepatosplenic EMH might involve the vertebral column (spinal cord compression), lymph nodes (lymphadenopathy), pleura (pleural effusion), peritoneum (ascites), skin (cutaneous nodules), or other tissues and is effectively treated with low-dose radiotherapy (100-500 cGy in 5-10 fractions).65
MF-associated pulmonary hypertension is suspected in the presence of clinical symptoms and signs, including dyspnea/hypoxia on exertion and peripheral edema, increased systolic pulmonary artery pressure on echocardiography, and an abnormal pulmonary uptake during a technetium 99m sulphur colloid scintigraphy. It is important to rule out alternative causes such as thromboembolic, infectious, or inflammatory lung processes (high-resolution computed tomographic scanning helps in this regard). In the absence of an alternative explanation for pulmonary hypertension, in a patient with MF, treatment with single-fraction (100 cGy) whole-lung irradiation is reasonable even if the technetium scan was negative.66
Single fraction of 100-400 cGy involved field radiotherapy has also been shown to benefit patients with MF-associated extremity pain.67 Finally, low-dose radiotherapy (100 cGy in 5-10 fractions) can be used in drug-refractory splenomegaly or hepatomegaly and is capable of inducing a transient (3-6 months) reduction in organ size.68 Such treatment, however, is often associated with severe and sometimes protracted pancytopenia and is best reserved for those patients who are poor surgical candidates for splenectomy and are unable to participate in a clinical trial.68
Allogeneic stem cell transplantation
Aside from case reports involving lenalidomide use in patients with del(5q)-associated MF,56 allo-SCT is currently the only treatment option in MF that is capable of inducing complete hematologic, cytogenetic, and molecular remissions.69 However, in considering allo-SCT as a treatment modality, one should be acutely aware of the risks involved. In the most recent study from the United Kingdom,70 51 patients with PMF (24%, 33%, and 43% with Dupriez low-, intermediate-, and high-risk disease)71 received mostly related conventional-intensity conditioning (CIC; ages 19-54 years) or reduced-intensity conditioning (RIC; ages 40-64 years) allo-SCT. Three-year OS was 44% for CIC transplantation and 31% for RIC transplantation, the corresponding relapse rates were 15% and 46%, non-relapse mortality rates were 41% and 32%, and extensive chronic GVHD rates were 30% and 35%.70
In an earlier study from the Center for International Bone Marrow Transplant Research involving 289 patients with PMF (ages, 18-73 years; 32%, 36%, and 31% with Dupriez low-, intermediate-, and high-risk disease),72 treatment-related mortality (TRM) was 27% at 1 year and 35% at 5 years. In the unrelated donor setting, TRM was 43% at 1 year and 50% at 5 years. Five-year OSs were 37% and 30% in related and unrelated donor settings, respectively. Outcome did not appear to be favorably affected by RIC when 3-year disease-free survival (DFS) was 39% and even lower (17%) in the unrelated donor setting.72 These results were similar to those of a multicenter study of 100 patients from Italy73 in which RIC transplantation did not affect the 3-year OS of 42% and TRM of 43%. A somewhat higher 5-year disease-free survival (51%) was reported from another RIC allo-SCT study74 in which relapse was predicted by high-risk disease and prior splenectomy.74 History of splenectomy did not affect outcome in the Center for International Bone Marrow Transplant Research study.72
On the basis of the above, I do not believe that the risk of allo-SCT is currently justified in patients with MF with DIPSS-plus40 low- or intermediate 1–risk disease patients (Figure 2). I am also not convinced that transplantation-related mortality and morbidity in MF have been favorably altered by the use of RIC transplantation when TRM, relapse, and chronic GVHD rates remain uncomfortably high. Therefore, if allo-SCT is indicated because of high- or intermediate 2–risk disease, I am inclined to favor the use of CIC transplantation in younger patients (age < 40-50 years), considering its association with a lower risk of relapse, compared with RIC transplantation. It is reasonable to offer RIC transplantation for older patients with high- or intermediate 2–risk disease, especially if a matched related donor is available. There is currently no hard data to support the need for splenectomy before transplantation. I prefer alternative means of reducing spleen size such as the use of cytoreductive therapy or JAK inhibitors (see “JAK inhibitors: value and limitations”), although I recognize the unknown effect of the latter agents on outcome after transplantation.
Experimental drug therapy
Investigational drugs in MF include pomalidomide, JAK inhibitor ATP mimetics, histone deacetylase inhibitors (eg, panobinostat, givinostat), and others (eg, hypomethylating agents, bevacizumab, plitidepsin). The most promising among these, so far, are pomalidomide and JAK inhibitors, and each is further elaborated.
Pomalidomide: Is it better than thalidomide or lenalidomide?
Pomalidomide is a thalidomide derivative classified with lenalidomide as an immunomodulatory drug. In vitro, immunomodulatory drugs antagonize angiogenesis and expression of tumor necrosis factor α and IL-6 while they facilitate production of IL-2 and interferon IFN-γ and enhance T-cell and natural killer–cell proliferation and activity; the precise mechanism of their action is not known but might include down-regulation cytokine signaling.75 All 3 drugs (thalidomide, lenalidomide, and pomalidomide) are active in both multiple myeloma and MF. In MF, thalidomide and lenalidomide, with or without prednisone, have comparable activity in alleviating anemia, splenomegaly, and thrombocytopenia; response rates for each were in the vicinity of 20%.51-54 Treatment was complicated by peripheral neuropathy or severe myelosuppression in patients receiving thalidomide52 or lenalidomide,55 respectively. Therefore, there was room for improvement in both therapeutic activity and side effect profile.
In a phase 2 randomized study, ∼ 25% of patients with anemia responded to pomalidomide alone (2 mg/d) or pomalidomide (0.5 or 2 mg/d) combined with prednisone.48 In a subsequent phase 2 study of single-agent pomalidomide (0.5 mg/d),76 anemia response was documented only in the presence of JAK2V617F (24% vs 0%) and predicted by the presence of pomalidomide-induced basophilia (38% vs 6%) or the absence of marked splenomegaly (38% vs 11%). Platelet response was seen in 58% of patients with baseline platelet count of 50-100 × 109/L, but the drug had limited activity in reducing spleen size.76 Unlike the case with thalidomide or lenalidomide, drug-associated neuropathy or myelosuppression was infrequent. However, higher doses of pomalidomide (> 2 mg/d) were myelosuppressive and not necessarily better in terms of efficacy.
In choosing between thalidomide, lenalidomide, and pomalidomide, I prefer to use lenalidomide in the presence of del(5q), because of the possibility of obtaining hematologic and cytogenetic remissions.56 In the absence of del(5q), my decision hinges on the presence or absence of JAK2V617F or marked splenomegaly. My choice is pomalidomide for the JAK2V617F-positive patient without marked splenomegaly.76 Otherwise, it is reasonable to give thalidomide plus prednisone a try.51 Of note, I would not use any of these 3 drugs in the absence of symptomatic anemia.
JAK inhibitors: value and limitations
JAK2 inhibitor ATP mimetics that are currently in clinical trials include INCB018424, TG101348, CEP-701, CYT387, AZD1480, SB1518, and LY2784544 (http://www.clinicaltrials.gov; Table 4). Results of these studies so far suggest substantial differences among these drugs in their toxicity and efficacy profiles, some of which might be linked to their pharmacokinetic properties and variable in vitro activity against other JAK and non-JAK kinase targets.
INCB018424 is a JAK1/JAK2 inhibitor. In a phase 1/2 study of 153 patients with MF,21 dose-limiting toxicity was thrombocytopenia, and the maximum tolerated dose was either 25 mg twice daily or 100 mg once daily. Adverse events included thrombocytopenia, anemia, and a “cytokine rebound reaction” on drug discontinuation. The latter is characterized by acute relapse of symptoms and splenomegaly, sometimes necessitating hospitalization. In a recent publication, 2 patients (1.3%) were reported to have experienced a systemic inflammatory response syndrome on drug discontinuation. To prevent or decrease the intensity of cytokine rebound reaction, I avoid abrupt drug discontinuation and instead use a 2-week tapering schedule. In addition, I sometimes use oral corticosteroid therapy (0.5 mg/kg/d), again in a tapering schedule, to help patients tolerate the event. Grade 3/4 thrombocytopenia or anemia in previously nontransfused patients occurred in 39% and 43% of patients receiving the drug at the recommended dose of 25 or 10 mg twice daily. At the lower dose level of 10 mg twice daily, the corresponding figures were 10% and 16%, but the response rate for splenomegaly (30%) was also lower at 10 mg twice daily compared with 25 mg twice daily (49%).21 Among all evaluable patients, 44% experienced ≥ 50% decrease in palpable spleen size. Improvement in constitutional symptoms (fatigue, pruritus, abdominal discomfort, early satiety, night sweats) and weight gain were seen in the majority of patients. Four of 28 transfusion-dependent patients (14%) became transfusion-independent. The drug's effect on JAK2V617F allele burden or bone marrow pathology was negligible, but a major reduction in proinflammatory cytokines (eg, IL-1RA, IL-6, tumor necrosis factor-α, macrophage-inflammatory protein-1β) was documented and coincided with improvement in constitutional symptoms.
TG101348 is a JAK2/fms-like tyrosine kinase 3 inhibitor. In a phase 1/2 study of 59 patients with MF,20 dose-limiting toxicity was a reversible and asymptomatic increase in serum amylase and lipase, and maximum tolerated dose was 680 mg/d. Grade 3/4 adverse events were all reversible and dose-dependent and included nausea (3%); vomiting (3%); diarrhea (10%); asymptomatic increases in serum lipase (27%), transaminases (27%) or creatinine (24%); thrombocytopenia (24%); and anemia (35%). After 6 and 12 months of treatment, 39% and 47% of patients, respectively, achieved a ≥ 50% decrease in palpable spleen size, and the majority also experienced improvement in early satiety, fatigue, night sweats, cough, or pruritus. Almost all patients with thrombocytosis and the majority with leukocytosis had drug-induced normalization of their counts. In general, responses were not affected by the presence or absence of JAK2V617F; however, a > 50% decrease in mutant allele burden was seen in 39% of patients with baseline JAK2V617F allele burden of > 20%. Effect on bone marrow pathology or plasma cytokine levels was unremarkable.
CEP-701 is a JAK2/fms-like tyrosine kinase 3 inhibitor. In a phase 2 study, 22 patients with JAK2V617F-positive MF received the drug orally at 80 mg twice daily.77 A > 50% spleen reduction was achieved in 4 of 18 evaluable patients (22%), and 2 of 8 transfusion-dependent patients (25%) became transfusion independent.77 Grade 3/4 side effects included anemia (14%), thrombocytopenia (23%), and diarrhea (9%). The drug did not affect bone marrow fibrosis or JAK2V617F allele burden. Nineteen different plasma cytokines, including proinflammatory cytokines, were measured at baseline and after treatment and showed no significant differences in their levels between responders and nonresponders and between baseline and posttreatment samples.
Results from other clinical trials of JAK inhibitors have not been published in full. However, glancing at the 2010 American Society of Hematology meeting abstracts shows encouraging preliminary observations from a phase 1/2 study that used another JAK1/JAK2 inhibitor, CYT387, in which an impressive 40% response rate in anemia accompanied equally remarkable response rates in splenomegaly and constitutional symptoms.78 The drug was well tolerated with infrequent grade 3/4 adverse events that included thrombocytopenia in 22% of patients and anemia in only 3%. Information about other JAK inhibitors (AZD1480, SB1518, and LY2784544) was either less impressive or not available.
On the basis of this discussion, 3 major points can be made about currently available JAK inhibitors and their value in MF. First, none of them were capable of inducing complete or partial remissions, but they definitely have palliative value. Second, they are significantly different from each other in terms of both therapeutic activity and side effect profile. The third point concerns their mechanism of action, which might involve down-regulation of proinflammatory cytokines for INCB018424 and clonal myeloproliferation for TG101348. It is possible that the balance of their composite effect on these parameters governs the spectrum of their activity. In my opinion, the therapeutically most-promising JAK inhibitors at this point are CYT387, TG101348, and INCB018424, but longer follow-up is needed to fully appreciate their safety profile.
The future: yes we can, but…
None of the currently known mutations in BCR-ABL1–negative MPN exhibit BCR-ABL1–like disease specificity or pathogenetic significance. However, the remarkably high JAK2 mutational frequency in these diseases justifies the flurry of current activity in preclinical and clinical evaluation of drugs that target JAK-STAT. I do not foresee a therapeutic success such as imatinib and CML anytime soon because molecular pathogenesis in MF is complex and involves multiple mutations and aberrant pathways. It is possible that these putatively secondary changes are derived from a common mutant stem cell with a specific disease-causing mutation. It is also possible that MF is not one but many diseases with different molecular signatures. Either way, it is becoming more and more evident that JAK inhibitors, by themselves, may not constitute adequate therapy in MF and have yet to show disease-modifying activity. Whether this will change by refining drug specificity to mutant as opposed to wild-type JAK remains to be seen. Nevertheless, JAK inhibitors have unquestionable palliative value,20,21 and our challenge in this regard is to figure out which JAK inhibitor is appropriate for which disease, which patient, and at what point in the disease course. There is also room for improvement in terms of both therapeutic activity and side effect profile; for example, in addition to the now well-established value of certain JAK inhibitors (eg INCB018424, TG101348)20,21 in reducing spleen size and alleviating constitutional symptoms, preliminary results suggest that CYT387, a newer JAK1/2 inhibitor, also induces anemia response in a substantial proportion of patients.78
Drug-induced complete remissions are possible in MF, as has been previously shown for lenalidomide, in the presence of isolated del(5q).56 It is therefore critical to continue laboratory investigations and new drug development with this goal in mind and not be content with what we have accomplished so far with JAK inhibitors.20,21 In this regard, phosphatidylinositol-3 kinase/Akt/mammalian target of rapamycin, Ras/mitogen-activated protein kinase and extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase, STAT3/5, and heat shock protein 90 are alternative MPN-relevant drug targets with some encouraging preclinical79-82 and clinical82 data. There is also an obvious interest in combining these drugs with each other83 or with conventional drugs such as hydroxyurea or ESAs. I am also intrigued by the significant antianemia activity of pomalidomide in JAK2V617F-positive MF.76 However, I have, thus far, not been impressed by the net value (benefit minus risk) of histone deacetylase inhibitors or hypomethylating agents.84-86 Finally, the recent demonstration of a correlation between JAK inhibitor therapy-induced down-regulation of proinflammatory cytokines and clinical benefit in MF,21 combined with new information on specific plasma cytokines in PMF and their DIPSS-plus–independent prognostic relevance,29 supports further evaluation of targeted anticytokine therapy.
Authorship
Contribution: A.T. was the single author of this paper.
Conflict-of-interest disclosure: The author declares no competing financial interests. He serves as principal investigator or co-investigator on many clinical trials, including those that are industry-sponsored. The latter include pomalidomide (Celgene), INCB018424 (Incyte), TG101348 (Targegene), CYT387 (YM Biosciences), and panobinostat (Novartis).
Correspondence: Ayalew Tefferi, Division of Hematology, Department of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: tefferi.ayalew@mayo.edu.