Abstract
Dectin-1 is the major receptor for fungal β-glucans. The activation of Dectin-1 leads to the up-regulation of surface molecules on dendritic cells (DCs) and cytokine secretion. Furthermore, Dectin-1 is important for the recruitment of leukocytes and the production of inflammatory mediators. Peroxisome proliferator–activated receptor-γ (PPAR-γ) and its ligands, cyclopentenone prostaglandins or thiazolidinediones, have modulatory effects on B-cell, T-cell, and DC function. In the present study, we analyzed the effects of troglitazone (TGZ), a high-affinity synthetic PPAR-γ ligand, on the Dectin-1–mediated activation of monocyte-derived human DCs. Dectin-1–mediated activation of DCs was inhibited by TGZ, as shown by down-regulation of costimulatory molecules and reduced secretion of cytokines and chemokines involved in T-lymphocyte activation. Furthermore, TGZ inhibited the T-cell–stimulatory capacity of DCs. These effects were not due to a diminished expression of Dectin-1 or to a reduced phosphorylation of spleen tyrosine kinase; they were mediated by the inhibition of downstream signaling molecules such as mitogen-activated protein kinases and nuclear factor-κB. Furthermore, curdlan-mediated accumulation of caspase recruitment domain 9 (CARD9) in the cytosol was inhibited by TGZ. Our data demonstrate that the PPAR-γ ligand TGZ inhibits Dectin-1–mediated activation by interfering with CARD9, mitogen-activated protein kinase, and nuclear factor-κB signaling pathways. This confirms their important role as negative-feedback regulators of potentially harmful inflammatory responses.
Introduction
Dendritic cells (DCs) form a heterogenous population of antigen-presenting cells linking the innate and the adaptive immune systems.1 Immature DCs mostly reside in the body's tissues and travel through the blood and lymph stream. In their immature state, DCs take up particles and large amounts of extracellular fluids by receptor-mediated endocytosis, phagocytosis, and macropinocytosis. When they encounter foreign antigens, DCs undergo a variety of phenotypic and functional changes and differentiate into mature cells.2 DCs also up-regulate the expression of major histocompatibility complex class I and class II molecules; the costimulatory molecules CD80, CD83, CD86, and CD40; and adhesion molecules such as CD54, CD102, and CD209. They also secrete a variety of cytokines such as interleukin-6 (IL-6), IL-12, IL-1β, and tumor necrosis factor-α (TNF-α), and chemokines such as macrophage inflammatory protein-3α (MIP-3α) and regulated on activation normal T-cell expressed and secreted (RANTES) to generate an optimal environment for the following immune response and to attract lymphocytes.
A variety of antigenic structures recognized by DCs are pathogen-associated molecular patterns (PAMPs) that are shared by groups of pathogens. Well-known examples are lipopolysaccharides, which are characteristic of bacteria, or double-stranded RNA, which are typical for certain viruses. PAMPs are recognized by pattern-recognition receptors that are activated after binding of their ligand structures. The Dectin-1 receptor belongs to the group of pattern-recognition receptors and is mainly expressed on DCs and macrophages.3-5 It is a member of the C-type lectin receptor family and is able to recognize carbohydrates with its extracellular carbohydrate recognition domain. Due to alternative splicing, there are several isoforms of human Dectin-1 (hDectin-1) with different functions. Interestingly, only the isoforms hDectin-1a and hDectin-1b can bind zymosan, a β-glucan–rich particle from the cell wall of Saccharomyces cerevisiae.6 Isoform hDectin-1b was found to be the main form expressed in human monocyte–derived DCs (mDCs), whereas isoform hDectin-1a is mainly expressed in myeloid and plasmacytoid DCs.5 Other structural features of hDectin-1 are its transmembrane region and a cytoplasmic domain that contains an immunoreceptor tyrosine-activation motif (ITAM)-like motif. Through the ITAM-like motif, hDectin-1 initiates signaling cascades via activation of spleen tyrosine kinase (Syk) and mitogen-activated protein kinases (MAPKs).7,8 hDectin-1 generates proinflammatory cytokine responses together with Toll-like receptor 2 (TLR2) 2.9,10 However, the TLR2-dependent and the Syk-dependent pathways can operate independently from each other.11 Furthermore, hDectin-1 signaling induces the expression of the caspase recruitment domain (CARD)–containing adaptor protein Card9 and therefore activates nuclear factor-κB (NF-κB) in DCs.12-14 Card9 is a key adaptor for non-TLR pattern-recognition–receptor signal transduction and links hDectin-1/Syk activation with NF-κB activation. Card9-deficient DCs were reported to show defective activation of NF-κB and an impaired cytokine response upon stimulation with zymosan.12 NF-κB controls the expression of genes encoding inflammatory cytokines, chemokines, and cell-surface adhesion molecules, and hDectin-1 and its ligands transduce signals via the described pathways to raise an immune response.
Peroxisome proliferator–activated receptor-γ (PPAR-γ) is a nuclear hormone receptor and lipid-activated transcription factor. It is highly expressed in adipose tissue, but also in several immune cells such as DCs and macrophages.15-17 Natural ligands of PPAR-γ are polyunsaturated fatty acids, prostaglandin derivates such as 15-deoxy-Δ12−14-prostaglandin-J2 (15d-PGJ2) and linoleic acid metabolites.18,19 The synthetic ligands are, among others, thiazolidinediones such as troglitazone (TGZ).20
The activation of PPAR-γ by its natural or synthetic ligands affects DC differentiation and function.17,21,22 In mDCs, PPAR-γ activation inhibits differentiation processes, as shown by the impaired expression of CD1a.22 Furthermore, PPAR-γ agonists inhibit the maturation process: The expression of the costimulatory molecules CD80 and CD40 is decreased.17,22 The chemokine receptor CCR7, which is important for DC migration to draining lymph nodes, is down-regulated.22 The secretion of IL-12 and RANTES is inhibited.17 Finally, PPAR-γ activation impairs mDC stimulation via the TLR2, TLR3, TLR4, and TLR7 ligands.23
As the natural ligand of PPAR-γ, 15d-PGJ2 is produced during the late phase of inflammation, and because of the obvious anti-inflammatory effects of PPAR-γ activation, its role in the restriction of immune responses and induction of tolerance has been investigated.24-26 In the present study, we analyzed the effects of PPAR-γ activation on the hDectin-1–mediated stimulation of mDCs.
Methods
Generation of mDCs
mDCs were generated from adhering monocytes as described previously.27 Briefly, peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll/Paque (Biochrom AG) density-gradient centrifugation from buffy coats of healthy voluntary donors from the blood bank of the University of Bonn (Bonn, Germany). PBMCs were seeded into 75-cm2 cell-culture flasks (BD) in X-VIVO medium (Lonza) at a density of 108 cells/10 mL/flask. After 1.5 hours of incubation at 37°C and 5% CO2, nonadherent cells were removed by extensive washing. Adhering monocytes were cultured in RP10 medium (RPMI 1640 containing GlutaMAX supplemented with 10% heat-inactivated fetal calf serum and 100 units/mL of penicillin/streptomycin). Differentiation into DCs was induced by the addition of 100 ng/mL of granulocyte-macrophage colony-stimulating factor (sargramostim; Berlex) and 20 ng/mL of IL-4 (R&D Systems) every second day. Cells were harvested on day 7 after the start of culture.
Depending on the experiment, mDCs were treated with one or a combination of TGZ (5 μM; Biomol) every second day and curdlan (100 μg/mL; Wako) on day 6 of culture. Equal amounts of dimethyl-sulfoxide (DMSO) were added to nontreated cells as a control to exclude solvent effects.
Immunostaining and flow cytometric analysis
The phenotype of the generated mDCs was analyzed by flow cytometry. Cells were stained with fluorescein isothiocyanate- or phycoerythrin-conjugated mouse monoclonal antibodies against CD1a, CD40, CD54, CD80, CD83, human leukocyte antigen-DR (HLA-DR), and mouse immunoglobulin G (all purchased from Beckman Coulter) and Dectin-1, DC limbic system-associated membrane protein (DC-LAMP), and CC chemokine receptor 7 (CCR7; all purchased from R&D Systems). For intracellular staining, cells were fixed with a 2% formaldehyde solution and permeabilized with 0.1% Triton X-100, followed by staining with anti-bodies. All flow cytometric analysis was performed on a Cytomics FC 500 (Beckman Coulter) using CXP Analysis software Version 2.2 (Beckman Coulter).
Preparation of whole-cell lysates
Whole-cell lysates were prepared as described previously.23 mDCs were incubated in lysis buffer containing 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 2mM EDTA (ethylenediaminetetraacetic acid), 1mM phenylmethylsulfonyl fluoride (PMSF), 2 μg/mL of aprotinin, and 1mM sodium orthovanadate for 20-30 minutes. Lysates were centrifuged for 15 minutes at 20 000g and the protein-containing supernatant was harvested. The protein concentration was measured using a bicinchoninic acid assay (Pierce).
Preparation of nuclear extracts
Nuclear extracts were prepared as described previously.28 In brief, 106 mDCs were incubated in 400 μL of buffer A containing 10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.9, 10mM KCl, 0.1mM EDTA, 0.1mM EGTA (ethyleneglycoltetraacetic acid), 1mM dithiothreitol (DTT), and 0.5mM PMSF. Cellular membranes were destroyed by the addition of 10% Igepal CA-630 and vigorous vortexing. Nuclei were pelleted by centrifugation and resuspended in buffer C containing 20mM HEPES, pH 7.9, 0.4M NaCl, 1mM EDTA, 1mM EGTA, 1mM DTT, and 0.5mM PMSF. Nuclear proteins were recovered by centrifugation for 5 minutes at 20 000g.
PAGE and Western blotting
For analysis of cytosolic proteins, approximately 20 μg of whole-cell lysates were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane (Whatman). The blot was probed with antibodies recognizing hDectin-1 (rabbit polyclonal) and CARD9 (T-17, goat polyclonal; both from Santa Cruz Biotechnology); phospho-extracellular signal–regulated kinase 1/2 (phospho-ERK1/2; Thr202/Tyr204, rabbit polyclonal), ERK1/2 (137F5, rabbit monoclonal), phospho-p38 (Tyr180/Tyr182, rabbit polyclonal), and p38 (rabbit polyclonal; all purchased from Cell Signaling Technology); and phospho-Syk (pY348, mouse monoclonal) and Syk (mouse monoclonal; both from BD Biosciences). To ensure that equal amounts of protein had been loaded into each lane of the SDS gel, antibodies against glyceraldehyde-3-phosphate dehydrogenase (10B8, mouse monoclonal) and Actin (I-19, goat polyclonal; both from Santa Cruz Biotechnology) were used as controls.
To analyze nuclear proteins, 20 μL of nuclear extracts were separated on a 10% SDS-PAGE gel and blotted on a nitrocellulose membrane. Ponceau S staining was performed to ensure equal loading of the gel. The blot was probed with antibodies against RelB (C-19, rabbit polyclonal) and phospho-nuclear factor of activated T cells-c1 (phospho-NFATc1; Ser257, rabbit polyclonal; both from Santa Cruz Biotechnology).
For all Western blots, suitable secondary antibodies conjugated with horseradish peroxidase were used. Protein bands were visualized using the enhanced chemiluminescence staining system (GE Healthcare).
Determination of cytokine production
Secretion of cytokines and chemokines in mDC culture supernatants was measured using commercially available enzyme-linked immunosorbent assays (ELISAs) and following the manufacturer's instructions. The concentrations of IL-6, IL-1β, and TNF-α were analyzed with ELISA kits from Immunotech. The concentrations of MIP-3α and RANTES were analyzed with ELISAs from R&D Systems. The readout was performed using a Synergy2 microplate reader (BioTek).
MLR assay
PBMCs from allogeneic blood donors were seeded into flat-bottom, 96-well microtiter plates at 105 cells/well (Greiner Bio-One) and 105, 104, or 103 mDCs were added as stimulator cells and incubated for 5 days at 37°C and 5% CO2. The mixed-lymphocyte reaction (MLR) assay was performed in 4-fold replicates, as described previously.29 Cells were pulsed with 3H-thymidine (Hartmann Analytic) and incubated for another 16 hours at 37°C and 5% CO2. Cells were harvested using a FilterMate Harvester (PerkinElmer), and uptake of 3H-thymidine was measured with a MicroBeta TriLux (PerkinElmer).
Results
PPAR-γ activation inhibits Dectin-mediated alteration of cell-surface molecules
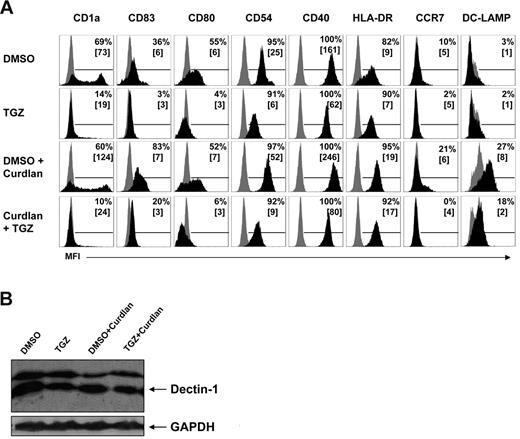
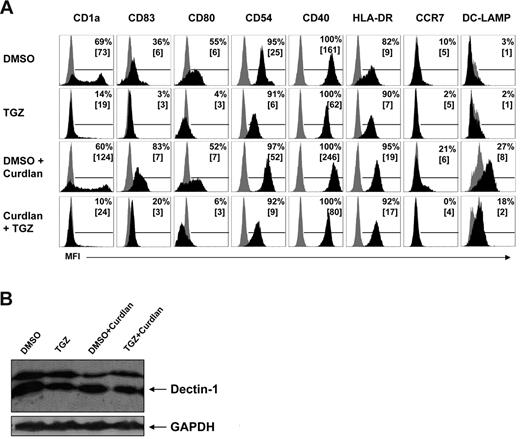
Activation of Dectin-1 with zymosan or the more specific ligand curdlan was shown to activate human DCs characterized by up-regulation of costimulatory molecules and CD83. To determine the effect of PPAR-γ activation on the expression of cell-surface molecules, we incubated DCs during their differentiation from peripheral blood monocytes with or without TGZ, a highly specific PPAR-γ ligand, and activated them with curdlan. On day 7 of culture, mDCs generated in the presence of granulocyte-macrophage colony-stimulating factor and IL-4 showed a strong expression of CD1a (Figure 1A) and a light expression of CD83 (Figure 1A), a phenotype characteristic of immature mDCs.25 Treatment with curdlan for 16 hours (stimulation on day 6 of culture) resulted in up-regulation of surface molecules associated with the mature DC phenotype as CD83, CD54, CD40, CCR7, HLA-DR, and DC-LAMP (Figure 1A). As expected, TGZ treatment during the generation of mDCs inhibited the expression of CD1a and costimulatory molecules. When curdlan was added to TGZ-treated mDCs, the Dectin-1–mediated up-regulation of CD83 and costimulatory molecules was dramatically inhibited (Figure 1A). To ensure that the inhibitory effect of TGZ was not due to a reduced expression of Dectin-1, we performed Western blot analysis and found no relevant differences in the protein expression of Dectin-1 in the cell populations (Figure 1B). We used Western blotting for analysis of Dectin-1 expression because activation of mDCs with β-glucans leads to the internalization of Dectin-1.30
PPAR-γ activation inhibits the hDectin-1–induced maturation in mDCs independently from hDectin-1 expression. mDCs were generated from adhering monocytes in the presence of the PPAR-γ ligand TGZ or DMSO as a control. Cells were stimulated with curdlan on day 6 of culture. (A) Expression of surface maturation markers was analyzed on day 7 by flow cytometry. Shown are the percentages of positive cells, along with mean fluorescence intensity values in square brackets. (B) The protein expression of hDectin-1 was analyzed on day 7 by Western blotting. The data shown are representative of at least 3 independent experiments.
PPAR-γ activation inhibits the hDectin-1–induced maturation in mDCs independently from hDectin-1 expression. mDCs were generated from adhering monocytes in the presence of the PPAR-γ ligand TGZ or DMSO as a control. Cells were stimulated with curdlan on day 6 of culture. (A) Expression of surface maturation markers was analyzed on day 7 by flow cytometry. Shown are the percentages of positive cells, along with mean fluorescence intensity values in square brackets. (B) The protein expression of hDectin-1 was analyzed on day 7 by Western blotting. The data shown are representative of at least 3 independent experiments.
PPAR-γ activation inhibits Dectin-mediated secretion of cytokines and chemokines
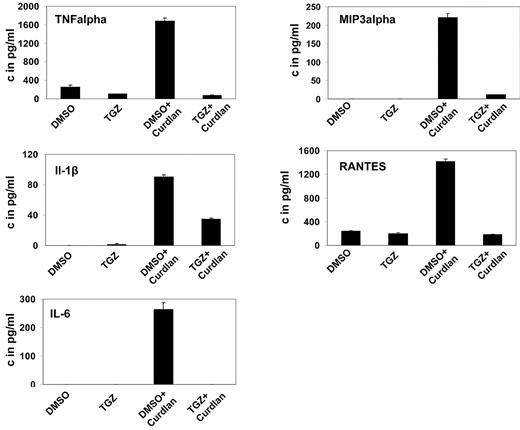
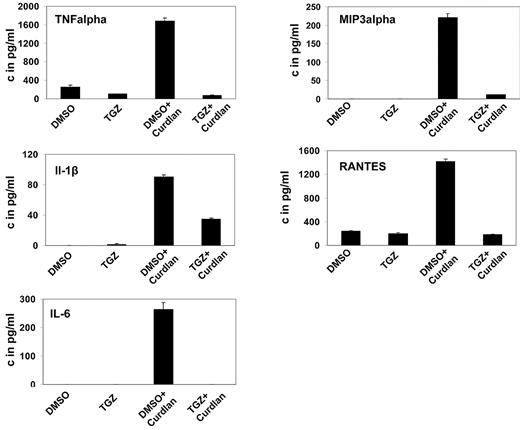
Upon activation, DCs secrete a variety of cytokines and chemokines to attract and stimulate T lymphocytes. Treatment of mDCs with curdlan induced secretion of IL-6, TNF-α, IL-1β, MIP-3α, and RANTES, unlike treatment with DMSO. Additional treatment with TGZ strongly decreased the secretion of all cytokines and chemokines to background levels or only slightly above (Figure 2).
PPAR-γ activation inhibits the hDectin-1–induced secretion of inflammatory cytokines and chemokines. mDCs were generated in the presence of the PPAR-γ ligand TGZ or DMSO as a control, and stimulated with curdlan on day 6 of culture. Cytokine and chemokine secretion in the culture supernatants was determined 24 hours later using ELISA. The data shown are representative of at least 3 independent experiments.
PPAR-γ activation inhibits the hDectin-1–induced secretion of inflammatory cytokines and chemokines. mDCs were generated in the presence of the PPAR-γ ligand TGZ or DMSO as a control, and stimulated with curdlan on day 6 of culture. Cytokine and chemokine secretion in the culture supernatants was determined 24 hours later using ELISA. The data shown are representative of at least 3 independent experiments.
PPAR-γ activation impairs the ability of Dectin-1–activated mDCs to stimulate lymphocyte proliferation
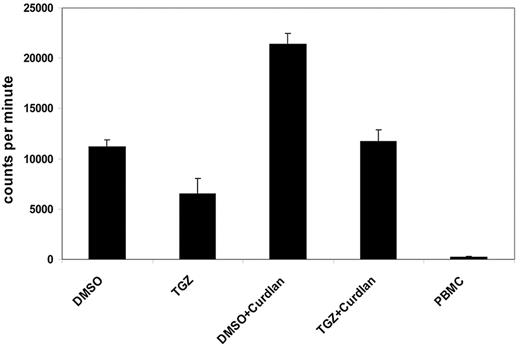
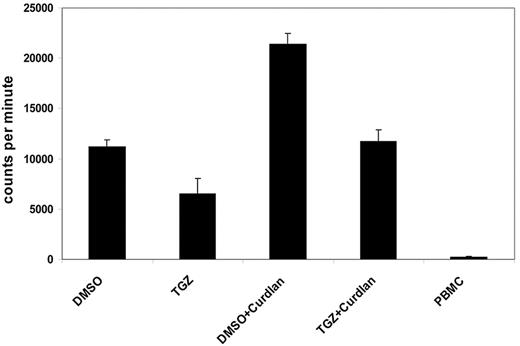
One characteristic feature of activated DCs is the induction of lymphocyte proliferation. We next analyzed the influence of PPAR-γ activation on the ability of Dectin-1–activated DCs to stimulate T cells in an MLR reaction. Curdlan-activated mDCs induced a strong proliferative response in allogeneic T cells compared with immature DCs. This effect was inhibited by additional treatment with TGZ, after which lymphocyte proliferation was comparable with immature mDCs (Figure 3).
PPAR-γ activation reduces the hDectin-1–induced proliferation of lymphocytes. DMSO- or TGZ-treated mDCs were stimulated with curdlan for 24 hours before using the cells as stimulators in an MLR assay. The incorporation of 3H-thymidine as a measurement of lymphocyte proliferation was analyzed. The data shown are representative of at least 3 independent experiments. The error bars represent the deviation of the quadruplicates.
PPAR-γ activation reduces the hDectin-1–induced proliferation of lymphocytes. DMSO- or TGZ-treated mDCs were stimulated with curdlan for 24 hours before using the cells as stimulators in an MLR assay. The incorporation of 3H-thymidine as a measurement of lymphocyte proliferation was analyzed. The data shown are representative of at least 3 independent experiments. The error bars represent the deviation of the quadruplicates.
Effects of PPAR-γ activation are mediated through MAPK signaling pathways but independently from Syk
Dectin-1 has an ITAM-like motif in its cytoplasmic tail, which mediates the downstream signaling by phosphorylation of Syk and activation of the MAPK pathways after stimulation with the Dectin-1 ligand curdlan.7
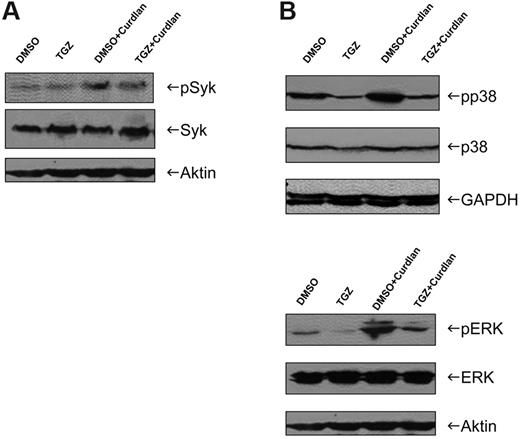
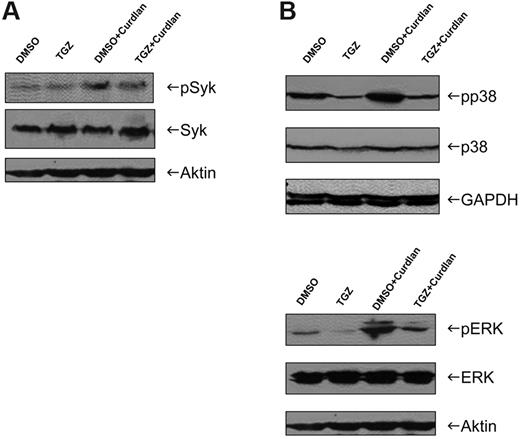
In the next set of experiments, we addressed the role of the tyrosine kinase Syk in TGZ-induced alteration of Dectin-1 signaling. Stimulation of mDCs with curdlan resulted in an increase in Syk phosphorylation that was not affected by TGZ (Figure 4A). However, we found that treatment of mDCs with curdlan induced phosphorylation, and thus activation of the MAPKs p38 and ERK1/2, which was inhibited by the addition of TGZ (Figure 4B).
Activation of MAPKs in response to hDectin-1 stimulation is impaired after PPAR-γ activation. DMSO- or TGZ-treated DCs were stimulated with curdlan for 4-24 hours and whole-cell lysates were prepared. The phosphorylation and thus activation of Syk (A) and p38 and ERK (B) were analyzed by Western blotting. The data shown are representative of at least 3 independent experiments.
Activation of MAPKs in response to hDectin-1 stimulation is impaired after PPAR-γ activation. DMSO- or TGZ-treated DCs were stimulated with curdlan for 4-24 hours and whole-cell lysates were prepared. The phosphorylation and thus activation of Syk (A) and p38 and ERK (B) were analyzed by Western blotting. The data shown are representative of at least 3 independent experiments.
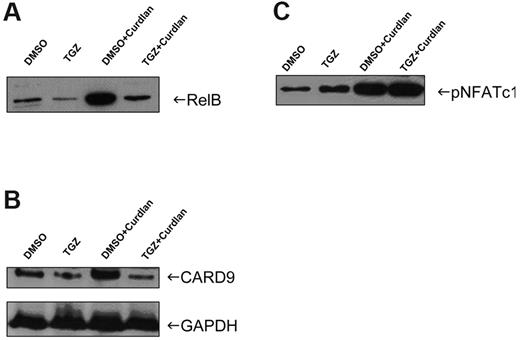
PPAR-γ activation impairs nuclear localization of NF-κB through inhibition of Card9 expression
We next used Western blotting to analyze the effects of TGZ on the nuclear expression of transcription factors involved in Dectin-1–mediated activation of DCs. The nuclear localization of the NF-κB family member RelB was strongly increased after treatment of mDCs with curdlan. Additional treatment of cells with TGZ inhibited the activation and nuclear localization of these NF-κB members (Figure 5A).
Inhibitory effects of PPAR-γ activation are mediated via the Card9/NF-κB signaling pathway. Nuclear extracts and whole-cell lysates were prepared from mDCs treated with TGZ or DMSO and stimulated with curdlan. The nuclear translocation of NF-κB member RelB was inhibited after PPAR-γ activation (A). The expression of Card9 was reduced after TGZ treatment and was correlated with the RelB translocation (B). The transcription factor NFAT was not affected by PPAR-γ activation (C). The data shown are representative of at least 3 independent experiments.
Inhibitory effects of PPAR-γ activation are mediated via the Card9/NF-κB signaling pathway. Nuclear extracts and whole-cell lysates were prepared from mDCs treated with TGZ or DMSO and stimulated with curdlan. The nuclear translocation of NF-κB member RelB was inhibited after PPAR-γ activation (A). The expression of Card9 was reduced after TGZ treatment and was correlated with the RelB translocation (B). The transcription factor NFAT was not affected by PPAR-γ activation (C). The data shown are representative of at least 3 independent experiments.
To further characterize the signaling processes leading to NF-κB translocation, we determined the protein expression of Card9 in nuclear extracts. Card9 is a known transducer of Dectin-1 signaling, which triggers NF-κB activation.12 As expected, stimulation of mDCs with curdlan induced the expression of Card9. When mDCs were also treated with TGZ, Card9 expression was dramatically inhibited in curdlan-stimulated cells (Figure 5B). This was not due to an increased proteasomal degradation of the protein, because the addition of bortezomib, a specific proteasome inhibitor, to the cell cultures had no effect on Card9 expression in TGZ-treated cells (data not shown). In addition, we did not find a relevant down-regulation of Card9 transcripts in these cells as analyzed by polymerase chain reaction (data not shown).
We further analyzed the activation of the transcription factor NFAT, which was demonstrated to be involved in Dectin-1–induced DC stimulation. Treatment with curdlan resulted in enhanced phosphorylation of NFAT in mDCs, which was not affected by TGZ (Figure 5C).
Discussion
Innate immune cells sense invading pathogens through pattern-recognition receptors, which recognize highly conserved molecular structures. In addition to TLRs and NOD proteins, dendritic cells express Dectin-1, a member of the C-type lectin family that represents the main receptor for fungal and bacterial β-glucans.4,31,32 Characterized ligands for hDectin-1 are zymosan, a β-glucan–rich particle from the cell wall of S cerevisiae, and curdlan, a linear β-1,3-glucan polymer from Alcaligenes faecalis.4,33 Because zymosan also stimulates TLR2 in addition to hDectin-1, it is difficult to differentiate between the effects that are induced via hDectin-1 from those induced via the TLR2/MyD88–signaling pathways.10,34 The polysaccharide curdlan is a more specific hDectin-1 agonist and mediates its effects independently from TLR2.14 We therefore used curdlan in our experiments to exclude TLR stimulation and to solely analyze hDectin-1 signaling.
The transcription factor PPAR-γ is activated by a variety of natural and synthetic ligands. Among its natural ligands is the cyclopentenone prostaglandin 15d-PGJ2, which is produced during the late phase of inflammation. The inhibitory effects of 15d-PGJ2 (or the more specific synthetic ligands such as TGZ) on the activation of DCs in regard to antigen presentation, migration, or cytokine secretion have been shown previously.23 Considering its inhibitory properties on antigen-presenting cells and the production of cyclopentenone prostaglandins by cyclooxygenase-2 during the late phase of inflammation, an assumed function of PPAR-γ in the immune system is the termination of initiated immune responses. Whereas the initiation of immune responses is essential for defending the organism against pathogens, its termination is pivotal for the body's integrity. Infinite inflammatory processes might lead to autoimmune diseases and cause severe damage. PPAR-γ activation might therefore play an important role as a negative-feedback mechanism in the regulation of immune responses.
We analyzed the modulation of Dectin-1–mediated DC activation using the PPAR-γ ligand. Whereas treatment of mDCs with the hDectin-1 ligand curdlan caused the up-regulation of CD83 and maturation-associated molecules, their expression was inhibited by the addition of TGZ. In agreement with these results, the secretion of proinflammatory cytokines and chemokines that were shown to be important for T-cell activation and attraction, such as IL-12, MIP-3α, and RANTES, was inhibited by TGZ.
Finally, the inhibitory effects of the PPAR-γ activation on the hDectin-induced maturation of mDCs were confirmed in MLR assays. The proliferation of T lymphocytes after stimulation with hDectin-1–activated mDCs was reduced when TGZ was administered, making an immune response toward a fungal pathogen impossible. These results demonstrate that TGZ interferes with the Dectin-1–induced activation of antigen-presenting cells, and can therefore prevent the generation of an effective immune response in curdlan-treated mDCs, simulating a fungal infection
In the next set of experiments, we analyzed the underlying mechanisms involved in the inhibitory effects of PPAR-γ. We showed that the effects are not caused by the induction of apoptosis by TGZ, and determined the activation and expression of several members of intracellular signaling cascades. It is well known that the activation of hDectin-1 by fungal PAMPs leads to the phosphorylation of Syk.8 In the next steps, the Card9/Bcl10/Malt-1 (CBM) complex is recruited and leads to the polyubiquitination of IκB kinase, followed by the phosphorylation and degradation of IκB, and finally the nuclear translocation of the transcription factor NF-κB.9,12 In addition, Syk mediates the activation of MAPK cascades and leads to the phosphorylation of p38 and ERK1/2, which activate several transcription factors as a consequence.
We found that the phosphorylation of Syk is induced after hDectin-1 stimulation with curdlan, and that Syk activation is not altered when PPAR-γ is activated by TGZ; therefore, the beginning of the signaling pathway is obviously not influenced by PPAR-γ activation. The phosphorylation of p38 and ERK1/2 MAPKs, however, is inhibited when TGZ-treated mDCs are stimulated with curdlan, indicating that the signaling cascade is inhibited between Syk and p38 or ERK1/2.
Looking at the nuclear translocation of the NF-κB member RelB, we found that the nuclear localization of the transcription factor was inhibited in curdlan-stimulated, TGZ-treated mDCs. The signaling cascade seemed to be restrained at some point between Syk and NF-κB. Because the activation of NF-κB depends on the CBM complex, we analyzed the expression of Card9 and found that it was induced in mDCs stimulated with curdlan, but inhibited when cells were costimulated with TGZ. These findings provide an explanation for the impaired nuclear translocation of NF-κB in TGZ- and curdlan-treated cells. In addition, our data show that after activation of hDectin-1, the signaling cascade Syk/CBM/NF-κB is not only activated by phosphorylation and ubiquitination processes, but is also strengthened by the up-regulated Card9 expression. The increased expression of the signal mediator Card9 might help to amplify the incoming signal.
It has been shown that the stimulation of hDectin-1 leads to the activation of the transcription factor NFAT in DCs.35 Similar to NFAT lymphocyte signaling, the signaling cascade is mediated via Syk, which then activates phospholipase C-γ, which in turn catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate and 1-diacylglycerol. Inositol 1,4,5-trisphosphate leads to intracellular Ca2+ release and activation of calcineurin. The phosphatase calcineurin dephosphorylates NFAT, allowing its nuclear translocation. Whereas NFAT has been found to be constitutively expressed in the nucleus of myeloid cells, it is induced after hDectin-1 stimulation. We found that mDC treatment with curdlan strongly increased the nuclear translocation of NFAT, which stayed unchanged when cells were cotreated with TGZ. These results show that the signaling pathway Syk/phospholipase C-γ/inositol 1,4,5-trisphosphate/Ca2+/calcineurin/NFAT is not inhibited by the activation of PPAR-γ.
It is still unclear how PPAR-γ activation mediates all of its effects on DC activation by fungal pathogens. PPAR-γ activation mainly decreases antigen presentation via HLA molecules and CD1a and inhibits cytokine secretion and the induction of lymphocyte proliferation. In this study, we show that activation of PPAR-γ leads to the inhibition of hDectin-1–induced activation of NF-κB and MAPK signaling cascades by reducing Card9 expression, whereas NFAT signaling remains unaffected.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank N. Gallala for her technical assistance.
Authorship
Contribution: G.K. performed research and wrote the paper; A.B. designed experiments, performed research, and wrote the paper; S.A.E.H. and S.D. performed research; A.H. analyzed data; and P.B. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Brossart, MD, Department of Hematology and Oncology, University of Bonn, Wilhelmstr 35-37, D-53111 Bonn, Germany; e-mail: peter.brossart@ukb.uni-bonn.de.
References
Author notes
G.K. and A.B. contributed equally to this study.