Abstract
Oncogenic transformation of CD4+ T cells by human T-cell lymphotropic virus type 1 (HTLV-1) is understood as the initial step to adult T-cell leukemia/lymphoma, a process that is mainly initiated by perturbation of cellular signaling by the viral Tax oncoprotein, a potent transcriptional regulator. In search of novel biomarkers with relevance to oncogenesis, we identified the tumor marker and actin-bundling protein Fascin (FSCN1) to be specifically and strongly up-regulated in both HTLV-1–transformed and adult T-cell leukemia/lymphoma patient-derived CD4+ T cells. Fascin is important for migration and metastasis in various types of cancer. Here we report that a direct link can exist between a single viral oncoprotein and Fascin expression, as the viral oncoprotein Tax was sufficient to induce high levels of Fascin. Nuclear factor-κB signals were important for Tax-mediated transcriptional regulation of Fascin in T cells. This suggests that Fascin up-regulation by Tax contributes to the development of HTLV-1–associated pathogenesis.
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1), a widespread δ-retrovirus, induces an aggressive lymphoproliferative disorder, adult T-cell leukemia/lymphoma (ATLL), in approximately 5% of infected carriers; frequently, the neoplasm is associated with skin infiltrations.1 The virus transforms CD4+ T lymphocytes to permanent growth, a process that is mainly mediated by the viral Tax oncoprotein.2,3 Tax activates viral transcription through the HTLV-1 long terminal repeat and induces cellular genes through signaling pathways, including nuclear factor-κB (NF-κB), serum response factor/activator protein 1, and cyclic-AMP response element–binding protein (CREB).3,4 Activation of CREB by Tax is involved in immortalization of primary human T cells.5
Fascin (FSCN-1) stabilizes filamentous actin and is concentrated in cellular protrusions, such as filopodia, during cell migration.6 Fascin is expressed in dendritic, neuronal, mesenchymal, and endothelial cells, whereas it is absent from epithelial cells and lymphocytes.7,8 In many human carcinomas, including breast, lung, colon, esophagus, pancreatic, stomach, ovary, and skin cancers, Fascin is highly up-regulated. Moreover, the tumor marker Fascin is expressed in Hodgkin and Epstein-Barr virus–associated lymphomas and cutaneous CD30+ lymphoproliferative disorders.6-8 Fascin is concentrated in the leading edge of cancer tissue,6 stabilizes invadopodia,9 and mediates self-seeding of cancer cells.10 Silencing of Fascin decreases the migratory capacity of cancer cells.6,8 Beyond that, expression of Fascin can correlate with the extent of proliferation, migration, and invasion of cancer cells.6 Recently, Fascin has received attention as a potential prognostic marker and therapeutic target for metastasis.8,11 Here we report that Fascin is strongly induced by a single viral oncoprotein, HTLV-1/Tax, and this is mainly mediated by NF-κB-signals.
Methods
Cell culture
Cells were cultured as described.12,13 Peripheral blood mononuclear cells (PBMCs) were isolated from venipuncture samples taken from HTLV-1–infected ATLL patients attending the National Center for Human Retrovirology, London, with fully informed written consent in accordance with the Declaration of Helsinki. This study was approved by St Mary's NHS Trust Local Research Ethics committee.
Microarray data
Microarry data have been deposited in National Center for Biotechnology Information's Gene Expression Omnibus14 (GEO; www.ncbi.nlm.nih.gov/geo) and are accessible under GEO Series accession numbers GSE10508 and GSE17718.
Plasmids, antibodies, and chemicals
Expression plasmid for wt-Tax (pcTax),15 Tax mutants M7, M22, and M47,16 a dominant negative inhibitor of IκBα (pIκBα-DN),17 pcDNA3.1 (Invitrogen), pSiren-IRES-EGFP-shN (shNonsense),18 pSiren-IRES-EGFP-shFascin, and pVSV-G (Clontech) were used. Mouse monoclonal antibodies anti-Fascin (55K-2; Dako Deutschland), anti–β-actin (ACTB; AC-15; Sigma-Aldrich), anti-Hsp90α/β (F-8; Santa Cruz Biotechnology), Alexa Fluor 88–conjugated goat anti–mouse IgG secondary antibodies (Invitrogen), and Texas Red-X phalloidin (1:40; Invitrogen) detecting actin were used. An inhibitor of IκB kinase β (IKK-β), 2-amino-6-(2-(cyclopropylmethoxy)-6-hydroxyphenyl)-4-(4-piperidinyl)-3-pyridine carbonitrile (ACHP; Calbiochem/Merck), was solved in dimethyl sulfoxide.
RT-PCR and quantitative RT-PCR
Primers and probes for detection of Tax, β-actin (ACTB), and 4-1BB transcripts by reverse transcriptase-polymerase chain reaction (RT-PCR) and/or quantitative PCR have been described before.13 Fascin was quantified using a TaqMan Gene Expression Assay (Hs00979631_g1; Applied Biosystems).
Statistics
SPSS Version 16.0.2 (SPSS) was used for statistical analysis. P < .05 was considered to be significant.
Additional information about methods is available in the supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
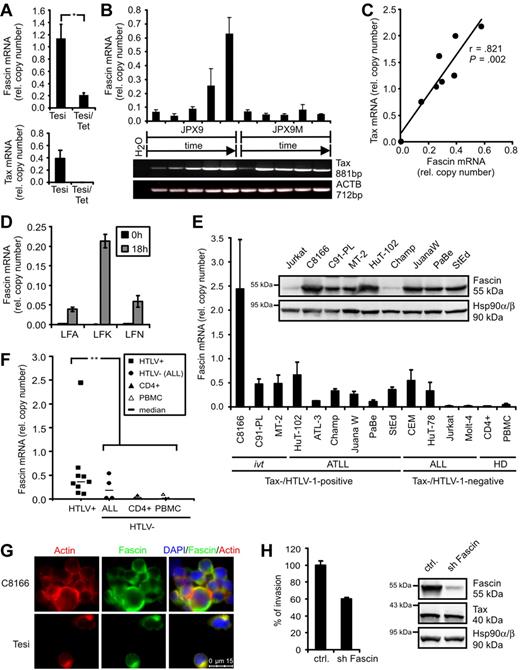
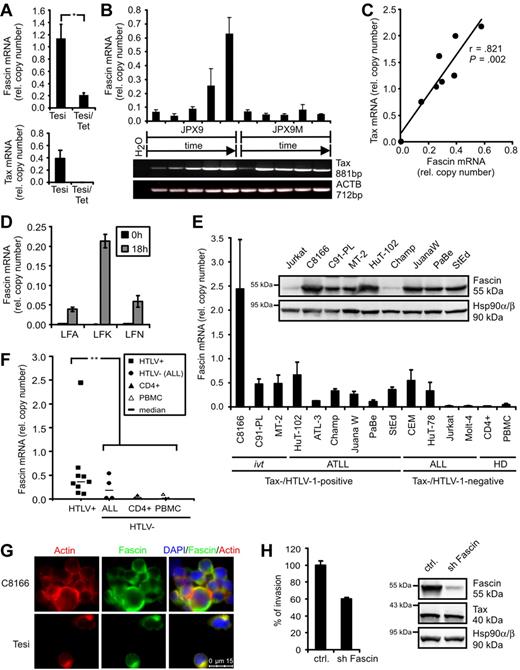
In the course of a dissection of novel Tax functions, transcriptome analyses were performed comparing HTLV-1–transformed cells (MT-2) and ATLL-derived cells (StEd) with normal primary CD4+ T-lymphocytes.12 In addition, Tesi cells, which are Tax-transformed lymphocytes that carry the viral Tax gene in a lymphotropic herpesvirus vector under control of a tetracycline-responsive repressor,19 were compared with Tesi/Tet cells where Tax was repressed.13 Transcripts were filtered according to their expression levels in Tax-positive cell lines (high) and uninfected CD4+ T-lymphocytes (absent/low). We found the actin-bundling protein Fascin to be specifically and strongly up-regulated in the presence of HTLV-1/Tax (supplemental Table 1). Data from microarrays were confirmed in quantitative PCR analyses. Fascin transcripts were significantly reduced (Figure 1A) in Tesi/tet cells when Tax was repressed. In addition, JPX9 cells that carry the Tax gene under control of a metallothionein-sensitive promoter were cultured in the presence of 20μM CdCl2 and analyzed after different time points (Figure 1B). In parallel to rising Tax mRNA levels, Fascin transcripts increased specifically, whereas in a mutant cell line (JPX9M) expressing nonfunctional Tax, Fascin transcripts were unaffected. Finally, transient transfection of Jurkat T cells with increasing amounts of pcTax (Figure 1C) revealed a dose-dependent increase of Fascin transcripts (r = .821; P = .002). From this we conclude that a single viral oncogene, Tax, is sufficient to induce the tumor marker Fascin. In freshly isolated CD4+ T lymphocytes from ATLL patients, both Tax (data not shown) and Fascin mRNAs (Figure 1D) were undetectable directly after isolation (t = 0 hours). After 18 hours in culture,20 Tax protein (data not shown) and Fascin mRNA (Figure 1D) increased. Beyond that, Fascin transcripts were strongly expressed in both HTLV-1-transformed and ATLL patient-derived cell lines (Figure 1E). The abundance of Fascin mRNA was similar to that of ACTB, indicating a strong expression of Fascin mRNA in context of HTLV-1 infection. Fascin was expressed in certain acute lymphoblastic leukemia (ALL)–derived cell lines, confirming previous observations in primary cells isolated from patients.21 Fascin transcripts were significantly elevated in HTLV-1–transformed cells (P = .002; Figure 1F) compared with uninfected CD4+ T cells and PBMCs. In addition, Fascin protein was detectable in high amounts in 8 of 9 HTLV-1-transformed cell lines, including ATLL patient-derived cells (Figure 1E). Immunofluorescence analysis revealed that Fascin was expressed in the cytoplasm of HTLV-1/Tax-transformed cells and colocalized with actin (Figure 1G), although it could not be detected in Jurkat T cells (supplemental Figure 1). This demonstrates high expression levels of Fascin as a consistent feature of HTLV-1–transformed T cells. Knockdown of Fascin in ATLL-derived HuT-102 cells using lentiviral vectors expressing shRNAs reduced the invasive capacity of HuT-102 cells into extracellular matrix to approximately 60% (Figure 1H).
HTLV-1 Tax induces Fascin in T cells. (A) Quantitative PCR of Fascin mRNA in the presence (Tesi) and absence (Tesi/Tet) of Tax; for complete Tax repression, cells were grown in medium containing 1 μg/mL tetracycline for 10 days (Tesi/Tet). Relative copy number was determined by normalizing Fascin and Tax transcripts to those of β-actin (ACTB). Mean values ± SE were compared using a paired t test (n = 4). *P < .05. (B) Representative diagram of Fascin (quantitative PCR) and Tax (RT-PCR) mRNA in Tax-inducible JPX9 cells (wt-Tax) and control cells JPX9M (inactive Tax mutant) after incubation with 20μM CdCl2 for 0, 6, 12, 24, and 48 hours. (C) Test of Fascin and Tax copy numbers (quantitative PCR) for correlation using Pearson test after transfection of Jurkat T cells with increasing amounts of pcTax (0, 5, 10, 15, 20, 25, 30, and 35 μg) replenished with pcDNA3.1 to 40 μg. (D) Quantitative PCR of Fascin mRNA in freshly isolated CD4+ T lymphocytes from ATLL patients LFA, LFK, and LFN directly after isolation (t = 0 hours) and 18 hours after cultivation. (E) Quantitative PCR and immunoblot of Fascin. Relative copy number was determined by normalizing Fascin transcripts to those of ACTB. The means of 3 independent experiments ± SE are shown. ivt indicates in vitro-transformed; ATLL, derived from adult T-cell leukemia/lymphoma; ALL, derived from acute lymphoblastic leukemia; and HD, healthy donors. In immunoblots, detection of Hsp90α/β served as loading control. (F) Comparison of Fascin copies between HTLV-1/Tax-positive cells shown in panel E and uninfected controls (ALL, CD4+, PBMCs) using a 2-tailed Mann-Whitney test (n = 3). **P < .01. Horizontal bars represent the median; ■, HTLV-1-transformed cells; ●, HTLV-1-negative ALL cells; ▴, CD4+ T lymphocytes from HD; and ▵, PBMCs from HD. (G) Immunofluorescence of C8166 (HTLV/Tax+) and Tesi (Tax+) cells using Phalloidin X-Texas Red for detection of actin and anti-Fascin and secondary anti–mouse Alexa Flour 488 antibodies. Images were acquired using a Leica LAS AF DMI 6000 fluorescence microscope equipped with a 63× ACX PL APO oil immersion lens and a Leica DFC 360 FX camera. Leica Application Suite 2.0.2 software was used. (H) Comparison of invasion of ATLL-derived, serum-starved HuT-102 cells, transduced with lentiviral vectors expressing either a nonsense shRNA (ctrl.) or shFascin, into extracellular matrix in transwell assays performed in quadruplicate. In immunoblots, protein expression of Fascin, Tax, and Hsp90α/β was controlled. One representative experiment is shown.
HTLV-1 Tax induces Fascin in T cells. (A) Quantitative PCR of Fascin mRNA in the presence (Tesi) and absence (Tesi/Tet) of Tax; for complete Tax repression, cells were grown in medium containing 1 μg/mL tetracycline for 10 days (Tesi/Tet). Relative copy number was determined by normalizing Fascin and Tax transcripts to those of β-actin (ACTB). Mean values ± SE were compared using a paired t test (n = 4). *P < .05. (B) Representative diagram of Fascin (quantitative PCR) and Tax (RT-PCR) mRNA in Tax-inducible JPX9 cells (wt-Tax) and control cells JPX9M (inactive Tax mutant) after incubation with 20μM CdCl2 for 0, 6, 12, 24, and 48 hours. (C) Test of Fascin and Tax copy numbers (quantitative PCR) for correlation using Pearson test after transfection of Jurkat T cells with increasing amounts of pcTax (0, 5, 10, 15, 20, 25, 30, and 35 μg) replenished with pcDNA3.1 to 40 μg. (D) Quantitative PCR of Fascin mRNA in freshly isolated CD4+ T lymphocytes from ATLL patients LFA, LFK, and LFN directly after isolation (t = 0 hours) and 18 hours after cultivation. (E) Quantitative PCR and immunoblot of Fascin. Relative copy number was determined by normalizing Fascin transcripts to those of ACTB. The means of 3 independent experiments ± SE are shown. ivt indicates in vitro-transformed; ATLL, derived from adult T-cell leukemia/lymphoma; ALL, derived from acute lymphoblastic leukemia; and HD, healthy donors. In immunoblots, detection of Hsp90α/β served as loading control. (F) Comparison of Fascin copies between HTLV-1/Tax-positive cells shown in panel E and uninfected controls (ALL, CD4+, PBMCs) using a 2-tailed Mann-Whitney test (n = 3). **P < .01. Horizontal bars represent the median; ■, HTLV-1-transformed cells; ●, HTLV-1-negative ALL cells; ▴, CD4+ T lymphocytes from HD; and ▵, PBMCs from HD. (G) Immunofluorescence of C8166 (HTLV/Tax+) and Tesi (Tax+) cells using Phalloidin X-Texas Red for detection of actin and anti-Fascin and secondary anti–mouse Alexa Flour 488 antibodies. Images were acquired using a Leica LAS AF DMI 6000 fluorescence microscope equipped with a 63× ACX PL APO oil immersion lens and a Leica DFC 360 FX camera. Leica Application Suite 2.0.2 software was used. (H) Comparison of invasion of ATLL-derived, serum-starved HuT-102 cells, transduced with lentiviral vectors expressing either a nonsense shRNA (ctrl.) or shFascin, into extracellular matrix in transwell assays performed in quadruplicate. In immunoblots, protein expression of Fascin, Tax, and Hsp90α/β was controlled. One representative experiment is shown.
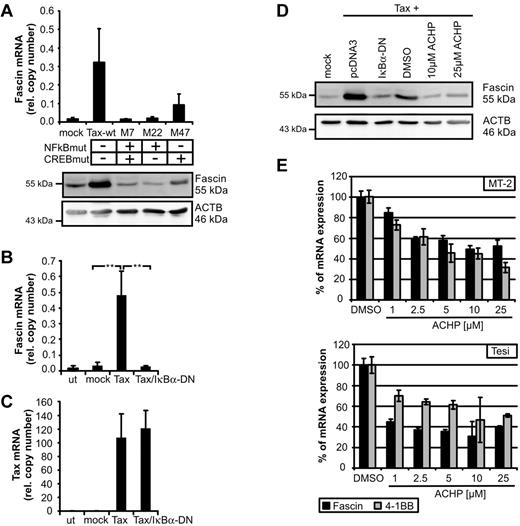
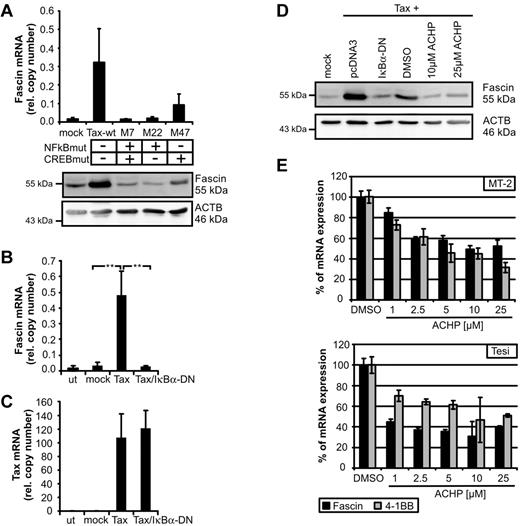
Thus far, little is known about transcriptional regulation of Fascin. Depending on the cellular context, Fascin can be regulated by CREB,22 tumor necrosis factor-α, insulin-like growth factor-1, interleukin-6,8 and ERK1/2.23 To identify signaling pathways leading to Tax-mediated induction of Fascin, we transfected expression plasmids of Tax-mutants in Jurkat T cells. Mutation of the NF-κB binding site (M7, M22) completely abolished Tax-mediated induction of Fascin, whereas mutation of the CREB-binding site led to intermediate expression levels of Fascin (Figure 2A; supplemental Figure 2). Cotransfection of Tax and the superinhibitor of IκBα-DN completely prevented Tax-mediated induction of Fascin mRNA (Figure 2B), whereas Tax copy numbers were not affected (Figure 2C). Not only Tax-mediated induction of Fascin transcripts, but also of protein (Figure 2D), could be prevented by IκBα-DN. Moreover, inhibition of IKK-β by ACHP (Figure 2D) abrogated Tax-mediated induction of Fascin. To analyze whether NF-κB signaling also plays a role in Tax-transformed and virus-transformed cells, Tax-transformed Tesi and HTLV-1–transformed MT-2 cells were cultured with increasing amounts of ACHP (Figure 2E). Block of IKK-β led to a strong decrease of Fascin transcripts. Specificity was confirmed by quantifying the NF-κB–responsive Tax target 4-1BB.13 These observations show, for the first time, that NF-κB signals are important for the regulation of Fascin.
NF-κB signaling is necessary for Tax-mediated induction of Fascin. (A) Fascin mRNA (quantitative PCR) and protein after transfection of Jurkat T cells with expression plasmids for NF-κB-deficient Tax-mutants M7 and M22 compared with wt-Tax (pcTax) and M47. The means of 3 independent experiments ± SE are shown in the upper panel. (B-C) Quantitative PCR of (B) Fascin and (C) Tax mRNA after transfection of expression plasmids for wt-Tax (20 μg) and IκBα-DN (2 μg) in Jurkat T cells. Mean values were compared using a paired t test (n = 3). **P < .01. (D) Immunoblot of Fascin after transfection of pcTax and cotransfection of pIκBα-DN or treatment with the IKK-β inhibitor ACHP solved in dimethyl sulfoxide. ACHP (10μM; 25μM) was added 24 hours after transfection for 24 hours. (E) Quantitative PCR of Fascin and 4-1BB mRNA after treatment of HTLV-1–transformed MT-2 cells and Tax-transformed Tesi cells with increasing amounts of ACHP (1, 2.5, 5, 10, and 25 μg) for 48 hours. Relative copy numbers were normalized on values obtained in dimethyl sulfoxide–treated cells.
NF-κB signaling is necessary for Tax-mediated induction of Fascin. (A) Fascin mRNA (quantitative PCR) and protein after transfection of Jurkat T cells with expression plasmids for NF-κB-deficient Tax-mutants M7 and M22 compared with wt-Tax (pcTax) and M47. The means of 3 independent experiments ± SE are shown in the upper panel. (B-C) Quantitative PCR of (B) Fascin and (C) Tax mRNA after transfection of expression plasmids for wt-Tax (20 μg) and IκBα-DN (2 μg) in Jurkat T cells. Mean values were compared using a paired t test (n = 3). **P < .01. (D) Immunoblot of Fascin after transfection of pcTax and cotransfection of pIκBα-DN or treatment with the IKK-β inhibitor ACHP solved in dimethyl sulfoxide. ACHP (10μM; 25μM) was added 24 hours after transfection for 24 hours. (E) Quantitative PCR of Fascin and 4-1BB mRNA after treatment of HTLV-1–transformed MT-2 cells and Tax-transformed Tesi cells with increasing amounts of ACHP (1, 2.5, 5, 10, and 25 μg) for 48 hours. Relative copy numbers were normalized on values obtained in dimethyl sulfoxide–treated cells.
Here we demonstrate that: (1) a viral oncogene, Tax, can induce the tumor marker Fascin, (2) Fascin is overexpressed in HTLV-1 transformed cells, and (3) NF-κB signaling plays an important role in Tax-mediated induction of Fascin. HTLV-1–induced changes in Fascin expression might contribute to the persistence of HTLV-1 or to the pathogenesis of the associated diseases. Expression of Fascin in the cytoskeleton of HTLV-1–transformed cells and its continuous expression indicate a possible role of Fascin in formation of the virologic synapse, which is a prerequisite for viral spread from cell to cell and involves microtubule polarization of the infected cell.24 Moreover, Fascin's capacity to induce migration and invasion of tumor cells could also contribute to the invasive capacity of HTLV-1–transformed cells in HTLV-1–associated disease: (1) In ATLL, skin infiltrations are common1 ; although chemokines, such as CCR4, may account for skin lesions,25 detailed mechanisms of infiltration are not exactly known. (2) In a neurologic disorder, such as HTLV-1–associated myelopathy/tropical spastic paraparesis, HTLV-1–infected cells infiltrate the spinal cord.26 To further characterize the role of Fascin in HTLV-1-associated disease, studies comparing the effects of Tax-1 (from HTLV-1) and its close relative Tax-2 (from HTLV-2) are meaningful.27 Our conclusion that NF-κB signals mediate induction of Fascin by Tax could explain the observation that Tax-M22-expressing mice did not develop skin lesions containing activated CD4+ T-cell infiltrates.28 Taken together, our findings suggest that Fascin plays an important role in HTLV-1–mediated pathogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This article is dedicated to the memory of Ralph Grassmann, who died on July 1, 2008.
The authors thank S. J. de Jong and K. Schmidt (Institute of Clinical and Molecular Virology, Erlangen, Germany) and K. Pichler (European Bioinformatics Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, United Kingdom) for helpful discussions; G. Taylor, C. R. M. Bangham, and L. Cook (Imperial College, St Mary's Hospital, London, United Kingdom) for providing ATLL patient samples; and F. Neipel (Institute of Clinical and Molecular Virology, Erlangen, Germany) for providing reagents.
This work was supported by the European Union (INCA, LSHC-CT-2005-018704), Deutsche Forschungsgemeinschaft (DFG GR 1224/3-1), and Akademie der Wissenschaften und der Literatur zu Mainz.
Authorship
Contribution: A.K.K. designed the study, performed experiments, analyzed data, and wrote the manuscript; M.K. performed experiments; A.G.R. contributed to experiments in ATLL patient samples; R.G. initiated the study; and B.F. analyzed data and participated in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea K. Kress, Institute of Clinical and Molecular Virology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Schlossgarten 4, 91054 Erlangen, Germany, e-mail: andrea.kress@viro.med.uni-erlangen.de.