Abstract
MicroRNAs (miRs) are small noncoding RNAs that principally function in the spatiotemporal regulation of protein translation in animal cells. Although emerging evidence suggests that some miRs play important roles in osteoblastogenesis and skeletal homeostasis, much less is known in osteoclastogenesis. Here, we show that receptor activator of nuclear factor κB ligand (RANKL)–induced osteoclastogenesis is mediated by miR-21. MiR-21 was identified as an miR expression signature of RANKL-induced osteoclastogenesis that down-regulates programmed cell death 4 (PDCD4) protein levels. Diminished PDCD4 removes a repression from c-Fos, a critical transcription factor for osteoclastogenesis and osteoclast-specific downstream target genes. In addition, RANKL-induced c-Fos up-regulates miR-21 gene expression. Bone marrow–derived monocyte/macrophage precursors deficient of DiGeorge syndrome critical region gene 8, an RNA binding protein associated with miR biogenesis, and Dicer, an endoribonuclease in the RNaseIII family associated with miR biogenesis, possessed significantly decreased miR-21 levels and increased PDCD4 protein levels so that RANKL-induced osteoclastogenesis was impaired in those cells. However, forced expression of miR-21 rescued osteoclast development because of down-regulation of PDCD4 protein expression levels. Thus, our studies provide a new molecular mechanism, including a positive feedback loop of c-Fos/miR-21/PDCD4, regulating osteoclastogenesis.
Introduction
The osteoclast, the exclusive bone resorptive cell, is derived from hematopoietic stem cells through the common myeloid progenitor to the colony-forming unit for granulocytes and macrophages to the colony-forming unit for macrophages and into the osteoclast lineage.1 Osteoclast differentiation, function, and survival are regulated by several exogenous cytokines (macrophage colony-stimulating factor [M-CSF], receptor activator of nuclear factor κ B ligand [RANKL], tumor necrosis factor α, interleukin-1, and interleukin-6) and hormones (sex steroids, parathyroid hormone, vitamin D, insulinlike growth factor-1, calcitonin, and prostaglandins) through several transcription factors activities identified by studies in genetically engineered mice.2,3 The transcription factor c-Fos plays an important role in osteoclastogenesis because mice lacking c-Fos develop osteopetrosis.2-4 c-Fos is not required for normal osteoprogenitor development but is required for osteoclastogenesis.2-4 Nuclear factor of activated T cells cytoplasmic 1 (NFATc1) is also a critical transcription factor that was discovered as an early RANKL-inducible gene by gene expression profiling after RANKL stimulation.3 NFATc1 molecules act as cofactors with activator protein-1 (AP-1) composed of Fos/Jun proteins to bind to regulatory cis DNA elements,5 and osteoclast-specific markers such as tartrate-resistant acid phosphatase (TRAP) and cathepsin K have multiple sites recognized by NFATc1 as well as its partner AP-1.5
MicroRNAs (miRs), which regulate gene expression, are transcribed as primary miRs (pri-miRs) containing a 5′-cap structure and poly(A) tail that are processed to produce the mature miRs.6 Two nuclease enzymes, the nuclear RNaseIII Drosha and the cytosolic RNaseIII Dicer, are known to act sequentially to trim the miRs to mature form.6 Hundreds of miR genes have been identified in the human genome, and it is estimated that one-third of protein-coding genes are regulated by miRs. Hence, miRs constitute one of the most abundant classes of gene-regulatory molecules in animals, and they are implicated in almost every biologic process, including development timing, cell differentiation, cell proliferation, cell death, metabolic control, transposon silencing, and antiviral defense.6
Emerging evidence indicates that Dicer-generated miRs play important roles in osteoblastogenesis, chondrocyte proliferation and differentiation, and osteoclastogenesis.7-10 Recent studies have shown that ≥ 12 miRs, miR-26a, miR-125b, miR-133, miR-135, miR-29a, miR-141, miR-200a, miR-210, miR-29, miR-378, miR-2861, and miR-206, are implicated in osteoblast differentiation.11-20 In particular, miR-2861 was identified as a novel miR expressed in mouse osteoblasts promoting osteoblast differentiation by suppressing expression of histone deacetylase 5 at the posttranslational level and contributing to bone formation.19 Moreover, mutation of miR-2861 causes osteoporosis in humans, suggesting that miR-2861 plays a critical role in the pathogenesis of bone disease.19 Some miRs, miR-18a, miR-199a, miR-146a, miR-222, miR-140, miR-27b, and miR-140, have been related to chondrocyte differentiation and osteoarthritis.21-27 More recently, a putative role for miR-140 in the pathogenesis of osteoarthritis has been reported through regulation of a disintegrin and metalloproteinase with thrombospondin motifs 5.27 Furthermore, chondrocyte proliferation was impaired in mice lacking miR-140, suggesting that miR-140 has an important and nonredundant role in cartilage development and homeostasis.27
We have demonstrated for the first time that Dicer inactivation in the myeloid-osteoclast lineage causes osteopetrosis because of decreased osteoclast formation and bone resorption activity, suggesting the requirement of global miR expression in osteoclastic control of bone metabolism.9 Moreover, we found that miR-223 regulates osteoclast development through nuclear factor Ι-A and M-CSF receptor levels.9 The function of miRs on osteoclastogenesis is, however, less well understood. In this study, a high-throughput analysis showed that miR-21 is up-regulated during RANKL-induced osteoclastogenesis. Mir-21 has attracted the attention of many researchers and has become one of the most studied miRs.28 The mature miR-21 is highly conserved in mammals and is encoded by a single gene.28,29 The mouse miR-21 gene was mapped to chromosome 11, within the 3′-untranslated region of the protein-coding gene transmembrane protein 49 between the transmembrane protein 49 stop codon and poly A signal.28,29 In addition, miR-21 has its own promoter that has several conserved binding sites for transcription factors for osteoclastogenesis such as AP-1 and PU.1.29 However, nuclear factor I-B (NFIB) associates with miR-21 promoter and inhibits the transcription of primary miR-21.29 It is well known that miR-21 is up-regulated in almost all solid tumors such as glioblastoma and breast cancer.28 However, the functions of miR-21 in normal cells are still very limited.28
In this study, miR-21 silencing impaired osteoclastogenesis because of increased programmed cell death 4 (PDCD4) protein levels, a functional target of miR-21. Moreover, forced expression of PDCD4 impaired osteoclastogenesis because of decreased expression levels of miR-21. These new molecular mechanisms regulating osteoclastogenesis by miR-21 could lead to development of an ideal gene therapy for treatment of osteoporosis, a devastating bone disease.
Methods
Mice
C57BL/6J mice were purchased from The Jackson Laboratory. DGCR8-floxed (DGCR8F/F) mice were obtained from Dr Elaine Fuchs (Howard Hughes Medical Institute). Dicer-floxed (DicerF/F) mice were obtained from Dr Brian Harfe (University of Florida College of Medicine). CD-11b Cre mice were from J.V. c-Fos–deficient mice were from Dr Erwin Wagner (Spanish National Cancer Center). All animals were housed under pathogen-free conditions according to the guidelines of the Division of Comparative Medicine, Washington University School of Medicine. The animal ethics committee approved all experiments.
Generation of Dicer conditional-deficient mice
The myeloid-osteoclast lineage-specific deletion of Dicer was achieved by breeding DicerF/wt mice to mice expressing CD-11b Cre as described previously.9
RNA isolation and miR microarray profiling
Primary mouse bone marrow–derived monocyte/macrophage precursors (BMMs) were prepared from the femur and tibia of 4- to 6-week-old mice and cultured with 20 ng/mL M-CSF in the absence (control) or presence of 100 ng/mL RANKL for 24 hours. Total RNA with small RNA (< 200 nucleotides) was isolated with the use of the mirVana miRNA isolation kit (Ambion), following the manufacturer's suggestion, and immediately frozen in dry ice and submitted to LC Sciences. Dual-color hybridization microarray studies (P < .01; n = 3) for miR expression were performed with the LC Science array platform containing probes for 617 mature mouse miRs present in miRBase. All microarray data have been deposited to CIBEX under accession number CBX146.
Reverse transcription for miR real-time PCR
cDNA was generated with the use of the miScript reverse transcription kit (QIAGEN) according to the manufacturer's instructions.
miR real-time PCR
MiRNA real-time polymerase chain reaction (PCR) was performed with the use of miScript SYBR Green PCR Kit (QIAGEN) on an Mx4000 multiplex quantitative PCR System (Stratagene). The 20 μL of PCR mixture included 2 μL of reverse transcription (RT) product, 10 μL of 2X QuantiTect SYBR Green PCR master mix, 2 μL of 10X miScript universal primer, 2 μL of 10X miScript primer assay (for U6 or mir-21), and 4 μL of autoclaved distilled water. The reaction mixtures were incubated at 95°C for 10 minutes, followed by 40 amplification cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 70°C for 30 seconds. Each sample was run in triplicate. The relative expression for each miR was estimated from triplicate miR real-time PCRs (QRT-PCRs) after normalization to the small nucleolar RNA, U6 (RNU6B), using the δ-δ Ct method.
One step RT-PCR
Total RNA was isolated with the use of the mirVana miRNA isolation kit (Ambion) following the manufacturer's suggestion. One-step RT-PCR was performed in 50 μL of reaction volume with the use of SuperScript III One-Step RT-PCR System with Platinum Taq DNA polymerase kit (Invitrogen) according to the manufacturer's protocol. The primers for mouse c-Fos were sense (5′-AAACCGCATGGAGTGTGTTGTTCC-3′) and antisense (5′-TCAGACCACCTCGACAATGCATGA-3′), mousePU.1 were sense (5′-TCCCATGGTGCCACCCCACA-3′) and antisense (5′-CCAGGAGCCTGGCGGTCTCT-3′), mouse NFATc1 were sense (5′-TCTCGAAAGACAGCACTGGAGCAT-3′) and antisense (5′-ACGGGATCTCCAGGAATTTGGTGT-3′), mouse PDCD4 were sense (5′-AGCGGTTAGAAGTGGAGTTGCTGT-3′) and antisense (5′-ACAAGGTGATTGACAGGCTGTTGC-3′), mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were sense (5′-AACTTTGGCATTGTGGAAGGGCTC-3′) and antisense (5′-TGGAAGAGTGGGAGTTGCTGTTGA-3′).
Construction of lentiviral expression vector and infection
Synthetic antisense oligonucleotides of miR-21 were synthesized by Integrated DNA Technologies.30 The synthetic oligonucleotides were annealed and inserted into pLVX-shRNA1 lentiviral vectors (Clontech) digested with BamHI and EcoRI restriction enzymes (Invitrogen). Clones containing the oligonucleotide insertion were verified by DNA sequencing (The Protein and Nucleic Acid Chemistry Laboratories at Washington University School of Medicine). Subconfluent Lenti-X 293T cells (Clontech) were transfected with Lenti-X HT packaging mix (Clontech) and the lentiviral small interfering RNA expression vectors with the use of the Lenti-X lentiviral expression systems (Clontech). After 48 hours, the supernatant was collected and filtered through a 0.45-μm syringe filter. The virus titers averaged and typically ranged from 1 to 5 × 106 Inclusion Forming Units (IFU)/mL, which was measured by the Lenti-X p24 rapid titer kit, following the manufacturer's suggestion (Clontech). Target cells were infected with 5 mL of α-minimum essential medium (MEM; Sigma-Aldrich) containing 10% fetal bovine serum (FBS; Atlas) and 5 mL of the supernatant, including viruses with polybrene (8 μg/mL; Sigma-Aldrich) for 6-8 hours. After that, the media was exchanged with the fresh 10 mL of αMEM containing 10% FBS. Infected cells were cultured for an additional 3 days for puromycin (2 μg/mL) selection (Sigma-Aldrich), and the cells was harvested for assay.
Construction of retroviral expression vector and infection
c-Fos or PDCD4 open reading frame inserted pCMV6 entry vectors were purchased from OriGene. Open reading frame insert was shuttled into pMX-IRES-puromycin retroviral vectors (Cell Biolabs Inc) digested with BamHI and NotI restriction enzymes (Invitrogen). Precursor miR-21 (750 base pairs) inserted pCMV6-MIR vectors were purchased from OriGene. Precursor miR-21 insert was shuttled into pMX-IRES-puromycin retroviral vectors (Cell Biolabs Inc) digested with BamHI and NotI restriction enzymes (Invitrogen). Cre-inserted pCAGEN vectors were purchased from Addgene. Cre insert was shuttled into pMX-IRES-blasticidin retroviral vectors (Cell Biolabs Inc) digested with EcoRI and NotI restriction enzymes (Invitrogen). The sequence of each construct was confirmed by the Protein and Nucleic Acid Chemistry Laboratories at Washington University School of Medicine. Subconfluent PLAT-E packaging cells (Cell Biolabs Inc) were transfected with retroviral vectors with the use of FuGENE (Roche Applied Science). After 48 hours, the supernatant was collected and filtered through a 0.45-μm syringe filter, and the cells were infected with 5 mL of αMEM (Sigma-Aldrich) containing 10% FBS (Atlas) and 5 mL of the supernatant, including viruses with polybrene (8 μg/mL; Sigma-Aldrich) for 6-8 hours. After that, the media was exchanged with the fresh 10 mL of αMEM containing 10% FBS. Infected cells were cultured for an additional 3 days for puromycin (2 μg/mL) selection (Sigma-Aldrich) or blasticidin (2 μg/mL) selection (Invitrogen), and the cells were harvested for assay.
Immunoblotting
To visualize c-Fos (Cell Signaling), c-Fos phosphorylation threonine 232 (p-c-Fos; Invitrogen), NFATc1 (Thermo Scientific), cathepsin K (Cell Signaling), PDCD4 (Cell Signaling), NFIB (Abcan), and GAPDH (Santa Cruz), whole-cell lysates were prepared by the M-PER mammalian protein extraction reagent (Thermo Scientific). Proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electroblotted to polyvinylidene difluoride membrane (Millipore), blocked in 5% skim milk, and probed with primary antibodies (1:1000). After incubation with anti–mouse immunoglobulin G (IgG) horseradish peroxidase–linked antibody (1:2000; Cell Signaling) or anti–rabbit IgG horseradish peroxidase–linked antibody (1:2000; Cell Signaling) were detected with enhanced chemiluminescence (Thermo Scientific).
ChIP
Chromatin immunoprecipitation (ChIP) assay was performed with the imprint ChIP assay kit (Sigma-Aldrich) according to the manufacturer's suggestions with the use of antibodies against c-Fos (Santa Cruz), NFATc1 (Thermo Scientific), and normal IgG (Sigma-Aldrich). The purified DNA was analyzed by PCR with the use of primers that detect sequences containing the mouse NFATc1 promoter,31 the mouse TRAP promoter,31 and the mouse miR-21 promoter.32
In vitro osteoclastogenesis
BMMs were prepared as described previously.9 DGCR8F/F BMMs, Dicer wild-type (wt) BMMs, or Dicer-deficient BMMs were prepared from the femur and tibia of 4- to 6-week-old DGCR8F/F mice, Dicerwt/wt;CD11b-Cre (Dicer wt), or DicerF/F;CD11b-Cre (Dicer cKO) mice, respectively. c-Fos wt and deficient splenocytes were prepared from spleens of 3-week-old c-Fos wt and deficient mice, respectively. To generate osteoclasts, 20 ng/mL M-CSF and 100 ng/mL RANKL were added to αMEM containing 10% FBS for 3 days. The cells were stained for TRAP, according to the manufacturer's suggestions (Sigma-Aldrich). TRAP-positive cells containing > 3 nuclei were counted as osteoclasts under microscopic examination (Olympus BX60).
Results
miR expression profiles in RANKL-induced osteoclastogenesis
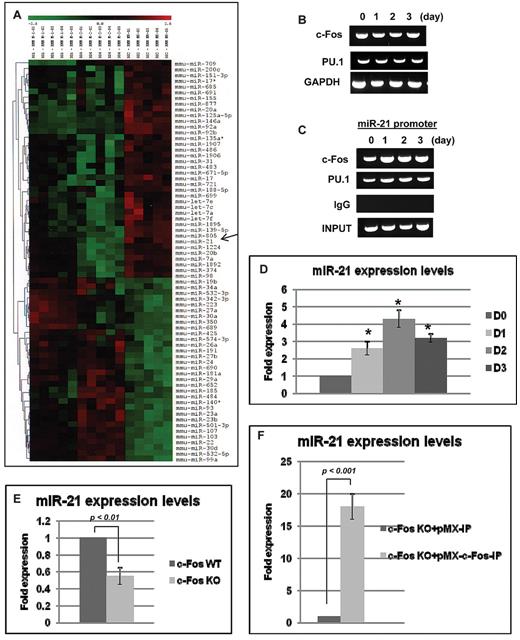
To identify a miR expression signature in RANKL-induced osteoclastogenesis, BMMs were cultured in the presence of 20 ng/mL M-CSF without or with 100 ng/mL RANKL for 24 hours and subjected to miR expression profile analysis. A total of 617 mouse miRs were analyzed, showing that 38 miRs were up-regulated by RANKL stimulation (red color far left third of array in Figure 1A), and 2 of them were robustly stimulated. One of the 2 was miR-21 (Figure 1A arrow). Thirty-three miRs were down-regulated by RANKL stimulation (green color far left third of array in Figure 1A). It has been reported that transcription factors for osteoclastogenesis such as c-Fos and PU.1 trigger miR-21 transcription via several binding sites of AP-1 and PU.1 present in the miR-21 promoter.29 RT-PCR analysis showed that c-Fos and PU.1 were stably expressed during RANKL-induced osteoclastogenesis (Figure 1B), and ChIP analysis showed that those transcription factors were found to associate to the miR-21 promoter (Figure 1C). In particular, the binding of c-Fos to miR-21 promoter was strongly enhanced after RANKL stimulation (Figure 1C). Consistent with those results, miR-21 was significantly up-regulated during RANKL-induced osteoclastogenesis (Figure 1D). Moreover, expression of miR-21 was significantly decreased in c-Fos–deficient splenocytes compared with wt cells (Figure 1E), and miR-21 expression levels were extremely up-regulated by overexpression of c-Fos in c-Fos–deficient cells (Figure 1F). Taken together, the data show that expression of miR-21 is controlled by transcription factors for osteoclastogenesis, particularly by c-Fos, and that RANKL-stimulated miR-21 expression might be obligatory for osteoclastogenesis.
Expression of miR-21 in osteoclastogenesis. (A) The heat map was generated with the use of hierarchical cluster analysis to show distinct miR expression patterns in primary cultured BMMs between M-CSF alone and M-CSF + RANKL treatment groups. The color bar was extracted to show the color contrast level of the heat map. Red and green indicate high expression levels and low expression levels, respectively. (B) RT-PCR analysis of genes related to osteoclastogenesis during RANKL-induced osteoclastogenesis for the indicated days. (C) ChIP assay was performed to study the association of c-Fos and PU.1 with miR-21 promoter. (D) QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate; *P < .01 compared with day 0. (E) Total RNA was prepared from c-Fos wt and knock-out (KO) splenocytes. QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (F) Total RNA was prepared from c-Fos KO splenocytes with retrovirus vectors (pMX-IP or pMX-c-Fos-IP). QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate.
Expression of miR-21 in osteoclastogenesis. (A) The heat map was generated with the use of hierarchical cluster analysis to show distinct miR expression patterns in primary cultured BMMs between M-CSF alone and M-CSF + RANKL treatment groups. The color bar was extracted to show the color contrast level of the heat map. Red and green indicate high expression levels and low expression levels, respectively. (B) RT-PCR analysis of genes related to osteoclastogenesis during RANKL-induced osteoclastogenesis for the indicated days. (C) ChIP assay was performed to study the association of c-Fos and PU.1 with miR-21 promoter. (D) QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate; *P < .01 compared with day 0. (E) Total RNA was prepared from c-Fos wt and knock-out (KO) splenocytes. QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (F) Total RNA was prepared from c-Fos KO splenocytes with retrovirus vectors (pMX-IP or pMX-c-Fos-IP). QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate.
Impaired osteoclastogenesis by miR-21 silencing
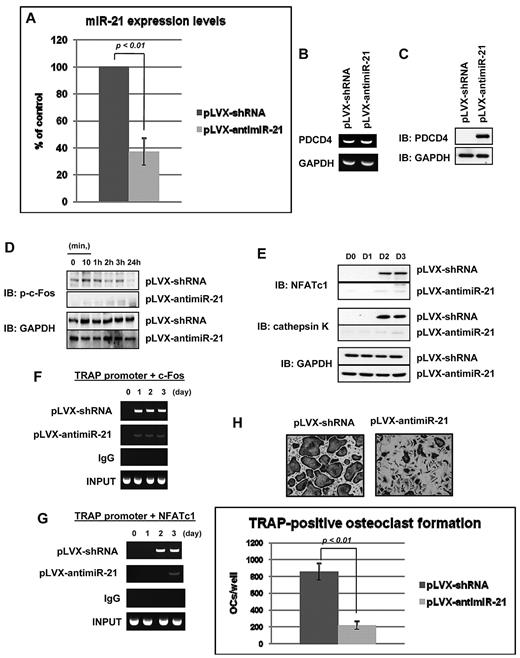
To study whether expression of miR-21 is of critical importance in osteoclastogenesis, we transduced BMMs with antisense miR-21–containing lentivirus. QRT-PCR analysis showed that antisense to miR-21 (pLVX-antimiR-21) significantly decreased expression levels of miR-21 by 62% compared with control BMMs (Figure 2A). In addition, we confirmed that RANKL did not stimulate miR-21 levels in cells expressing antisense miR-21 (data not shown). PDCD4, a target of miR-21, mRNA expression was detectable by RT-PCR analysis (Figure 2B), but PDCD4 protein was not detected by immunoblotting analysis in control cells (Figure 2C), compatible with miR-21 regulation of PDCD4 expression in BMMs at the posttranscriptional level. PDCD4 protein levels were extremely up-regulated by miR-21 silencing compared with control (Figure 2C). It is well known that RANKL-phosphorylated c-Fos enhances expression of NFATc1 and osteoclast-specific markers such as TRAP and cathepsin K during osteoclast development.2-4 Immunoblots showed that phosphorylation of c-Fos at the threonine 232 site was present without RANKL stimulation but was significantly enhanced by RANKL stimulation at 10 minutes (Figure 2D). c-Fos phosphorylation was decreased at 1 hour and stayed down for 2 hours (Figure 2D). As expected, in response of c-Fos stimulation, RANKL stimulated NFATc1 and cathepsin K expression by days 2 and 3 (Figure 2E). In contrast, RANKL-induction of c-Fos phosphorylation and NFATc1 and cathepsin K protein expression were remarkably down-regulated in the cells harboring reduced levels of miR-21 (Figure 2D-E). Moreover, ChIP analysis showed that, although c-Fos and NFATc1 bound to the TRAP promoter after RANKL stimulation by day 1 or day 2, respectively, those effects were also attenuated by miR-21 silencing (Figure 2F-G). In particular, the binding of NFATc1 to the TRAP promoter was delayed (Figure 2G). Consistent with those results, TRAP-positive osteoclast formation was strikingly impaired by miR-21 silencing compared with control (Figure 2H). In addition, osteoclast bone-resorbing activity was also reduced because of down-regulated osteoclast formation number and size (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These findings indicate that expression of miR-21 is relevant for the mechanism of osteoclast development.
Impaired osteoclastogenesis by miR-21 silencing. (A) Total RNA was prepared from BMMs harboring pLVX-shRNA or pLVX-antimiR-21. QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (B) RT-PCR analysis of PDCD4 in primary cultured BMMs without RANKL treatment. antimiR-21 indicates antisense miR-21; shRNA, short hairpin RNA. (C) Immunoblotting analysis of PDCD4 of primary BMMs without RANKL treatment. (D-E) Immunoblotting analysis of phosphorylated c-Fos (D), NFATc1 (E), or cathepsin K (E) of BMMs with antisense miR-21 or control for indicated times. antimiR-21 indicates antisense miR-21. (F-G) ChIP assay was performed to study the association of c-Fos (F) or NFATc1 (G) with TRAP promoter during RANKL-induced osteoclastogenesis for the indicated days. (H) TRAP-positive multinucleated cell count at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments.
Impaired osteoclastogenesis by miR-21 silencing. (A) Total RNA was prepared from BMMs harboring pLVX-shRNA or pLVX-antimiR-21. QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (B) RT-PCR analysis of PDCD4 in primary cultured BMMs without RANKL treatment. antimiR-21 indicates antisense miR-21; shRNA, short hairpin RNA. (C) Immunoblotting analysis of PDCD4 of primary BMMs without RANKL treatment. (D-E) Immunoblotting analysis of phosphorylated c-Fos (D), NFATc1 (E), or cathepsin K (E) of BMMs with antisense miR-21 or control for indicated times. antimiR-21 indicates antisense miR-21. (F-G) ChIP assay was performed to study the association of c-Fos (F) or NFATc1 (G) with TRAP promoter during RANKL-induced osteoclastogenesis for the indicated days. (H) TRAP-positive multinucleated cell count at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments.
Importance of down-regulation of PDCD4 by miR-21 during osteoclastogenesis
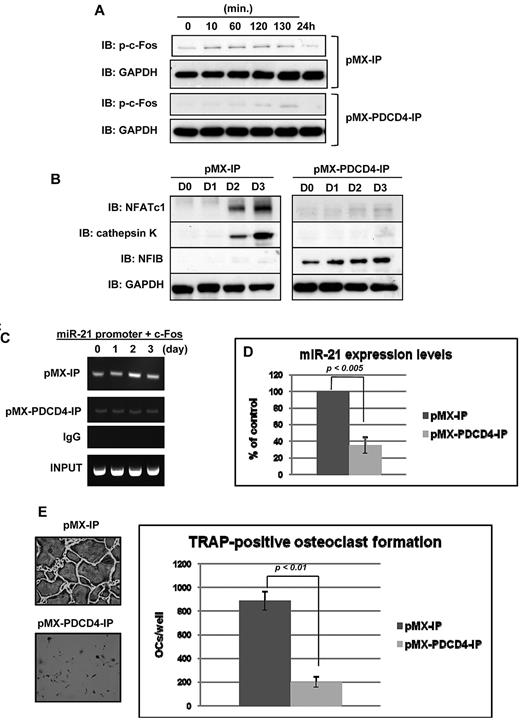
Because PDCD4 protein expression levels were robustly up-regulated by miR-21 silencing so that osteoclastogenesis was impaired (Figure 2C,H), we hypothesized that PDCD4 would negatively regulate osteoclastogenesis. To confirm our hypothesis, we transduced BMMs with a PDCD4-containing retrovirus. Similar to miR-21 silencing studies, RANKL stimulated phosphorylation of c-Fos and expression of NFATc1 and cathepsin K in control cells (Figure 3A-B). However, RANKL induction of these proteins was barely detectable in the cells harboring up-regulated levels of PDCD4 (Figure 3A-B). Interestingly, although NFIB protein expression was not detectable in control cells, its expression was strikingly up-regulated before RANKL stimulation and continued for 3 days by PDCD4 introduction (Figure 3B). Moreover, ChIP analysis showed that overexpression of PDCD4 significantly inhibited the binding of c-Fos into the miR-21 promoter during RANKL-induced osteoclastogenesis compared with control (Figure 3C), and, consequently, miR-21 expression levels were significantly decreased by PDCD4 introduction before RANKL stimulation (Figure 3D). Consistent with those findings, osteoclastogenesis was extremely impaired by forced expression of PDCD4 (Figure 3E), suggesting that PDCD4 negatively regulates osteoclastogenesis through down-regulation of miR-21 expression levels. In addition, osteoclast bone-resorbing activity was also reduced because of down-regulated osteoclast formation number and size (supplemental Figure 2). However, overexpression of PDCD4 did not induce apoptosis in our studies (data not shown). Taken together, the action of miR-21 to suppress PDCD4 protein expression levels in BMMs is a prerequisite for induction of osteoclastic transcription factors, specific markers, and osteoclastogenesis.
Impaired osteoclastogenesis by forced expression of PDCD4. (A-B) Immunoblotting analysis of phosphorylated c-Fos (A), NFATc1 (B), or cathepsin K (B) of BMMs with overexpressed PDCD4 or control for the indicated times. (C) ChIP assay was performed to study the association of c-Fos with miR-21 promoter during RANKL-induced osteoclastogenesis for the indicated days. (D) QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (E) TRAP-positive multinucleated cells count at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments.
Impaired osteoclastogenesis by forced expression of PDCD4. (A-B) Immunoblotting analysis of phosphorylated c-Fos (A), NFATc1 (B), or cathepsin K (B) of BMMs with overexpressed PDCD4 or control for the indicated times. (C) ChIP assay was performed to study the association of c-Fos with miR-21 promoter during RANKL-induced osteoclastogenesis for the indicated days. (D) QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (E) TRAP-positive multinucleated cells count at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments.
Osteoclast development rescued by miR-21 introduction
To confirm the importance of miR-21 expression during RANKL-induced osteoclastogenesis, rescue studies of osteoclast development by miR-21 introduction were performed in DGCR8-deficient BMMs. DGCR8 is the RNA binding protein that is exclusively related with primary miRs biogenesis together with the nuclear RNaseIII enzyme Drosha.6 DGCR8F/F BMMs were isolated from long bone tissues of DGCR8F/F mice. Cre-containing retroviruses were introduced into these BMMs, and infected cells were selected by blasticidin (Figure 4). The selected cells expressing Cre had no expression of DGCR8 at protein levels (Figure 4A). The selected cells were transduced by precursor miR-21 (760 base pairs) containing retrovirus, and infected cells were selected by puromycin. As expected, the cells lacking DGCR8 possessed significantly down-regulated levels of miR-21 so that PDCD4 protein expression levels had been robustly elevated in cells compared with control cells (Figure 4B-C). Thereby, RANKL-induced osteoclastogenesis was significantly impaired in DGCR8-deficient BMMs, even though RANKL induced TRAP-positive osteoclasts from DGCR8F/F BMMs (Figure 4D). Next, forced expression of miR-21 markedly reduced PDCD4 protein expression levels in DGCR8-deficient cells compared with control (Figure 4C). Interestingly, TRAP-positive osteoclast development was moderately rescued in DGCR8-deficient cells with miR-21 introduction compared with control (Figure 4D). This is because those cells possess c-Fos mRNA expression before RANKL stimulation, which in turn allows NFATc1 mRNA induction by RANKL stimulation (Figure 4E). In addition, elevated PDCD4 protein expression levels by decreased miR-21 expression were remarkably attenuated by miR-21 overexpression, indicating that expression of miR-21 for down-regulation of PDCD4 is physiologically pivotal in osteoclastogenesis.
miR-21 rescued osteoclastogenesis in DGCR8-deficient BMMs. (A) Immunoblotting analysis of DGCR8 of DGCR8F/F BMMs (pMX-IB) and DGCR8-deficient BMMs (pMX-Cre-IB) without RANKL treatment. (B) QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. WT indicates DGCR8 wt BMMs. (C) Immunoblotting analysis of PDCD4 in infected BMMs without RANKL treatment. (D) TRAP-positive multinucleated cells counted at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments. (E) RT-PCR analysis of the genes related to osteoclastogenesis. OC indicates osteoclast.
miR-21 rescued osteoclastogenesis in DGCR8-deficient BMMs. (A) Immunoblotting analysis of DGCR8 of DGCR8F/F BMMs (pMX-IB) and DGCR8-deficient BMMs (pMX-Cre-IB) without RANKL treatment. (B) QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. WT indicates DGCR8 wt BMMs. (C) Immunoblotting analysis of PDCD4 in infected BMMs without RANKL treatment. (D) TRAP-positive multinucleated cells counted at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments. (E) RT-PCR analysis of the genes related to osteoclastogenesis. OC indicates osteoclast.
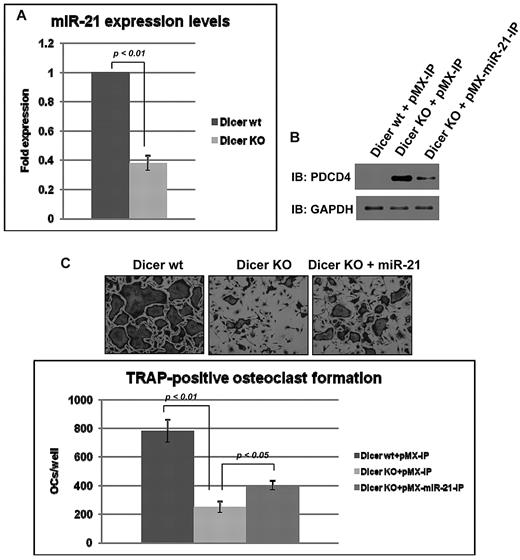
To further confirm the rescue of osteoclast development by miR-21 in the cells lacking DGCR8, we performed the same rescue study with the use of Dicer-deficient BMMs isolated from Dicer cKO mice. Dicer is the cytoplasmic RNaseIII enzyme that cleaves precursor miRs into mature miRs.6 We have demonstrated that Dicer cKO mice show mild osteopetrosis caused by decreased osteoclast number and bone resorption.9 We isolated BMMs from the long bone tissues of Dicer wt and cKO mice. Dicer protein expression levels were eliminated in Dicer-deficient BMMs as described previously9 (data not shown), and miR-21 levels were significantly down-regulated in Dicer-deficient BMMs as well as DGCR8-deficient BMMs (Figure 5A). Moreover, the rescue study of osteoclast development in the cells lacking Dicer was similar to that observed in the study that used DGCR8-deficient cells. RANKL-induced osteoclast development was impaired in Dicer-deficient cells because of up-regulation of PDCD4 protein expression levels (Figure 5B-C), and the impaired osteoclastogenesis was moderately rescued by miR-21 introduction because of decreased PDCD4 protein levels in the cells lacking Dicer (Figure 5B-C). In addition, RT-PCR analysis of c-Fos or NFATc1 mRNA in Dicer-deficient BMMs before or after RANKL stimulation, respectively (data not shown), was similar to the studies of DGCR8-deficient BMMs. Thus, osteopetrosis in Dicer cKO mice may be caused by impaired miR-21 levels and up-regulated PDCD4 protein levels in BMMs.
miR-21 rescued osteoclastogenesis in Dicer-deficient BMMs. (A) Total RNA was prepared from Dicer wt (Dicerwt/wt;CD11b-Cre) BMMs and Dicer KO (DicerF/F;CD11b-Cre) BMMs. QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (B) Immunoblotting analysis of PDCD4 in infected BMMs without RANKL treatment. (C) TRAP-positive multinucleated cells counted at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments. OC indicates osteoclast.
miR-21 rescued osteoclastogenesis in Dicer-deficient BMMs. (A) Total RNA was prepared from Dicer wt (Dicerwt/wt;CD11b-Cre) BMMs and Dicer KO (DicerF/F;CD11b-Cre) BMMs. QRT-PCR was performed with primer for miR-21. The PCR products were normalized to U6 level for each reaction. The data represent the means ± SDs of 3 experiments in triplicate. (B) Immunoblotting analysis of PDCD4 in infected BMMs without RANKL treatment. (C) TRAP-positive multinucleated cells counted at 3 days after RANKL treatment, cultured in 24-well plates (200×). Similar findings were obtained in 4 independent sets of experiments. OC indicates osteoclast.
Discussion
We found a miR signature of RANKL-induced osteoclastogenesis that showed 38 miRs elevated and 33 miRs down-regulated (Figure 1A). Two of the 38 miRs were extremely up-regulated, one of which was miR-21. The significant stimulation of miR-21 during RANKL-induced osteoclastogenesis was confirmed by QRT-PCR (Figure 1D). Thus, elevated miR-21 levels are observed in the myeloid-osteoclast lineage, but whether high expression levels of miR-21 are required for myeloid-osteoclast lineage-specific development in normal hematopoiesis is unknown.
We have demonstrated that miR-223 is expressed in the RAW264.7 osteoclast precursor cell line, which is M-CSF receptor positive (M-CSFR+) and receptor for RANKL positive (RANK+), and regulates osteoclastogenesis through regulation of NFIA and M-CSFR levels in primary cells.9,33 In addition, miR-223–deficient mice have an expanded granulocytic compartment because of enhanced proliferation and differentiation.34 Thus, miR-223 has been implicated in myeloid differentiation. Yet, nothing is known about the role of miR-21 in normal myelopoiesis.
Osteoclasts are thought to arise from the common myeloid progenitors (CMPs) through macrophage/dendritic cell progenitors or granulocyte/macrophage progenitors.1 PU.1 is expressed in hematopoietic stem cells, CMPs, and the common lymphoid progenitors at the same levels and is present at high levels in mature myeloid cells.35,36 PU.1 is one of the important transcription factors for osteoclastogenesis because PU.1-deficient mice have a decreased number of macrophages and no osteoclasts.2,3 CMPs are classified into ≥ 2 subpopulations which are PU.1-expressing CMPs and PU.1 nonexpressing CMPs.35,36 Intriguingly, PU.1-expressing CMPs have myeloid potential, whereas PU.1-negative CMPs have erythroid potential. Thus, PU.1-expressing CMPs are thought to give rise to osteoclasts. In addition, low levels of PU.1 are implicated in B-cell development and granulocyte production; whereas high levels of PU.1 are required for macrophage development.37-39 Taken together, high expression levels of PU.1 should be required for induction of osteoclasts in hematopoietic myeloid-osteoclast lineage. Moreover, c-Fos also contributes to monocyte lineage specification.40 Our high-throughput analysis showed that miR-21 was highly expressed before RANKL stimulation in primary BMMs (M-CSFR+/RANK+) (data not shown). This is because c-Fos and PU.1 were expressed before RANKL stimulation, and these transcription factors transcribed the miR-21 gene (Figure 1B-D). Moreover, miR-21 expression levels were significantly decreased in c-Fos–deficient BMMs, and overexpression of c-Fos markedly stimulated miR-21 levels in the cells lacking c-Fos (Figure 1E-F). Because c-Fos and PU.1 are highly expressed in the myeloid lineage,35-40 miR-21 also might be highly expressed in the myeloid lineage, suggesting that high expression levels of miR-21 might be required for not only osteoclastogenesis but also myeloid differentiation.
The next question was why high miR-21 levels are required in osteoclast development. One possibility is that the protein posttranscriptionally controlled by miR-21 inhibits osteoclastogenesis. Although hundreds of targets of miR-21 are indicated by bioinformatics, we focused on PDCD4 protein expression and function in osteoclastogenesis. PDCD4 is known to affect the activity of the transcription factor AP-1 directly.28,41 Several conserved enhancer elements, including the binding site for AP-1, have been identified in the miR-21 promoter region, and AP-1 triggers the transcription of pri-miR-21. It is well known that c-Fos is the principal contributor among the AP-1 components induced.29 In fact, overexpression of c-Fos markedly induced miR-21 levels (Figure 1F). In our studies, PDCD4 protein expression levels were not detectable in the BMMs without RANKL stimulation (Figure 2C). In addition, PDCD4 protein expression levels were barely detectable during RANKL-induced osteoclastogenesis (data not shown), and its overexpression remarkably impaired osteoclastogenesis in culture (Figure 3E). RANKL induces NFATc1 mRNA expression through activated c-Fos, in turn those transcription factors stimulate osteoclast-specific markers such as TRAP and cathepsin K directly.2,3 However, because expression of p-c-Fos was impaired by overexpressed PDCD4, NFATc1 and cathepsin K protein expression levels were also down-regulated (Figure 3A-B). Moreover, as expected, the recruitment of c-Fos to the miR-21 promoter was also attenuated so that miR-21 levels were also decreased (Figure 3C-D), and PDCD4 protein levels were up-regulated (data not shown). Furthermore, because DGCR8 or Dicer-deficient BMMs possess high protein expression levels of PDCD4 (Figures 4C and 5B), osteoclast development was impaired (Figures 4D and 5C). However, retroviral delivery of precursor miR-21 rescued impaired osteoclast development because of decreased PDCD4 protein levels, showing PDCD4 as a negative regulator of osteoclastogenesis. Hence, high expression levels of miR-21 are obligatory for at least down-regulation of PDCD4 in osteoclastogenesis.
Other proteins of principal miR-21 targets may also be implicated in suppression of osteoclastogenesis besides PDCD4. NFIB, a target of miR-21,29 is a member of the NFI gene family, which frequently functions as a transcriptional repressor of many promoters through competition with other transcriptional factors for recruitment.29 We found that, despite our failure to detect NFIB protein expression during osteoclastogenesis, it was gradually up-regulated by overexpression of PDCD4 (Figure 3B). Although we were not able to detect this novel mechanism of osteoclastogenesis repression, we speculate that NFIB increased by overexpressed PDCD4 may have eliminated the recruitment of c-Fos to the miR-21 promoter. The result was decreased miR-21 expression levels that contributed to strongly impaired osteoclastogenesis as shown in Figure 3E. Moreover, although forced expression of PDCD4 attenuated the binding of c-Fos to the miR-21 promoter and miR-21 expression levels (Figure 3C-D), elevated NFIB protein expression would also be implicated in those events as a synergistic interaction.
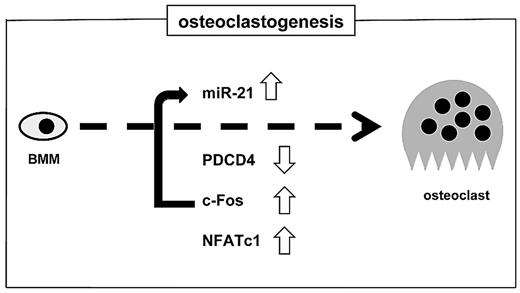
Taken together, our data show that PDCD4 may be one of the repressor proteins for osteoclastogenesis and that miR-21 expression is a critical prerequisite for down-regulation of PDCD4 so that transcription factors for osteoclastogenesis and osteoclast-specific markers are transcribed (Figure 6). These studies show a new role of miR-21 in osteoclastogenesis in culture. Although further studies in vivo are needed, miR-21 may represent a novel target for treatment of bone metabolic disorders with excessive osteoclast development and activity such as osteoporosis.
A positive feedback loop of c-Fos/miR-21/PDCD4 is critical during stimulation of osteoclastogenesis by RANKL. c-Fos triggers the miR-21 gene, resulting in down-regulation of PDCD4 protein levels, which in turn further stimulates c-Fos transactivation, which leads to NFATc1 expression and BMMs differentiating into osteoclasts.
A positive feedback loop of c-Fos/miR-21/PDCD4 is critical during stimulation of osteoclastogenesis by RANKL. c-Fos triggers the miR-21 gene, resulting in down-regulation of PDCD4 protein levels, which in turn further stimulates c-Fos transactivation, which leads to NFATc1 expression and BMMs differentiating into osteoclasts.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Elaine Fuchs (Howard Hughes Medical Institute, Rockefeller University, New York, NY) for the gift of DGCR8 flox mice, Dr Brian D. Harfe (University of Florida College of Medicine, Gainesville, FL) for the Dicer flox mice, J.V. for the CD-11b Cre mice, and Dr Erwin F. Wagner (Spanish National Cancer Center, Madrid, Spain) for the c-Fos deficient mice.
This work was supported by the National Institutes of Health (grant DK070790; K.A.H.)
National Institutes of Health
Authorship
Contribution: T.S. designed and performed research, analyzed data, and contributed the primary draft of the manuscript; J.V. generated CD11-b Cre transgenic mice; and K.A.H. revised and produced the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshifumi Sugatani, Department of Pediatrics, Washington University School of Medicine, 5th Fl, MPRB Bldg, Campus Box 8208, 660 S Euclid Ave, St Louis, MO 63110; e-mail: sugatani_t@kids.wustl.edu.