To the editor:
We read with interest the results of the comparison between reduced-intensity conditioning (RIC) and conventional myeloablative conditioning (MAC) allogeneic stem cell transplantation (allo-SCT) for patients with acute lymphoblastic leukemia (ALL) in complete remission (CR), reported by Mohty et al.1 They concluded that RIC allo-SCT was a potential therapeutic option for ALL.
Although we agree in general with their conclusion, our major concern is that the cytogenetic background between MAC and RIC might differ. Adjustment for cytogenetic risk groups was not performed in a multivariate analysis, because there was no difference in the cytogenetic distribution between MAC and RIC when analyzed among 3 risk groups: t(9;22), t(4;11), or other (P = .10). However, when analyzed among 4 groups, including NA/failed as one group, a significant difference was noted between MAC and RIC (P = .02). In fact, the number of Philadelphia chromosome–positive [Ph+] ALL was smaller in MAC than in RIC [104/449 (23%) vs 41/127 (32%), P = .049]. Since the relapse incidence (RI) was higher among Ph+ ALL patients than in the whole study population (40% ± 5% vs 31% ± 2% in MAC, and 49% ± 9% vs 47% ± 5% in RIC), lower RI in MAC might be associated with a smaller number of Ph+ ALL. Allo-SCT has been recognized as the only curative therapy for Ph+ ALL,2 and there are already several reports of RIC allo-SCT for Ph+ ALL.3,4 It may be better to treat Ph+ ALL and Philadelphia chromosome-negative (Ph−) ALL as different diseases because their treatment would differ in an era of tyrosine kinase inhibitors. Therefore, it would be more practical to present data only from patients with Ph− ALL.5
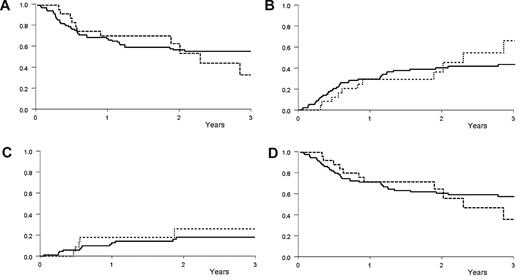
The results of our 121 HLA-matched allo-SCT for adult Ph− ALL in first (81 MAC, 21 RIC) or second (14 MAC, 5 RIC) CR for patients aged ≥ 45 years (between 1998 and 2007 using the Japan Society for Hematopoietic Cell Transplantation and the Japan Marrow Donor Program database) were comparable between MAC and RIC (Figure 1A-D). In a multivariate analysis, RIC was not a significant risk factor for relapse (Hazard ratio [HR] 1.66, 95% confidence interval [CI] 0.63-4.37, P = .30). The variables considered in our multivariate analyses were conditioning (MAC vs RIC), age (> 50 years vs ≤50 years), sex, white blood cell counts (< 30 000/μL vs ≥30 000/μL), lineage (T vs B), disease status (first vs second CR), donor source (sibling vs unrelated), and graft-versus-host disease prophylaxis (cyclosporine-based vs tacrolimus-based). Similarly, RIC posed no significant risk factor for leukemia-free survival (HR 1.00, 95% CI 0.51-1.96, P = .99), nonrelapse mortality (HR 1.05, 95% CI 0.53-2.05, P = .89), and overall survival (HR 1.06, 95% CI 0.64-2.07, P = .87).
Survival probabilities. (A) Leukemia-free survival according to conditioning regimen: 58% ± 5% in myeloablative conditioning (MAC) vs 63% ± 11% in reduced-intensity conditioning (RIC) at 2 years (P = .90). (B) Nonrelapse mortality according to conditioning regimen: 40% ± 5% in MAC vs 36% ± 11% in RIC at 2 years (P = .79). (C) Relapse incidence according to conditioning regimen: 18% ± 5% in MAC vs 26% ± 11% in RIC at 2 years (P = .27). (D) Overall survival according to conditioning regimen: 59% ± 5% in MAC vs 63% ± 11% in RIC at 2 years (P = .82). Solid curve indicates MAC; dashed curve, RIC; x-axis, years after transplantation; and y-axis, probability.
Survival probabilities. (A) Leukemia-free survival according to conditioning regimen: 58% ± 5% in myeloablative conditioning (MAC) vs 63% ± 11% in reduced-intensity conditioning (RIC) at 2 years (P = .90). (B) Nonrelapse mortality according to conditioning regimen: 40% ± 5% in MAC vs 36% ± 11% in RIC at 2 years (P = .79). (C) Relapse incidence according to conditioning regimen: 18% ± 5% in MAC vs 26% ± 11% in RIC at 2 years (P = .27). (D) Overall survival according to conditioning regimen: 59% ± 5% in MAC vs 63% ± 11% in RIC at 2 years (P = .82). Solid curve indicates MAC; dashed curve, RIC; x-axis, years after transplantation; and y-axis, probability.
There are several ways to deal with missing data.6 Given that the cytogenetics of 54% (244/449) for MAC and 43% (55/127) for RIC were missing in the study by Mohty et al, differences in how to handle missing data may produce different results. Because there was a difference in the cytogenetic distribution between MAC and RIC when analyzing missing data as one category, data adjusted for cytogenetic risk groups, or those of Ph− ALL, are of considerable interest.
Authorship
Contribution: S.N., Y.I., and K.M designed the research; S.N., Y.I., and R.S. performed the statistical analysis and interpreted the data; M.I., H.T., and K.H. provided the data of patients; K.K., and R.S. collected the data of patients; and S.N., Y.I., and R.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satoshi Nishiwaki; Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan; e-mail: n-3104@tf7.so-net.ne.jp.