Abstract
The present study characterized platelet secretion and surface expression of proangiogenic stromal cell-derived factor-1α (SDF-1α) and vascular endothelial growth factor (VEGF) and antiangiogenic PF4 and endostatin on activation. The angiogenic factors presented in randomly distributed granules in resting platelets, which were peripherized on activation. Confocal and immunogold electron microscopy demonstrated that SDF-1α/CXCL12 and PF4/CXCL4 mostly present in different granules. Platelet activation induced marked SDF-1α and endostatin but mild PF4 or no VEGF surface expression. PAR1-activating peptide (PAR1-AP), adenosine diphosphate (via P2Y1/P2Y12), and glycoprotein VI-targeting collagen-related peptide induced massive SDF-1α and VEGF but modest PF4 or no endostatin release. In contrast, PAR4-AP triggered marked PF4 and sole endostatin release but limited SDF-1α or VEGF secretion. Distinct platelet release of SDF-1α and endostatin involved different engagements of intracellular signaling pathways. In conclusion, different platelet stimuli evoke distinct secretion and surface expression of proangiogenic and antiangiogenic factors. PAR1, adenosine diphosphate, and glycoprotein VI stimulation favors proangiogenic, whereas PAR4 promotes antiangiogenic, factor release.
Introduction
Platelets contribute importantly to multiple physiologic/pathophysiologic processes beyond thrombosis and hemostasis, such as inflammation, angiogenesis, and atherogenesis.1-4 Platelets store and release a number of regulators that modulate angiogenesis.5-7 It has recently been shown that platelets store proangiogenic and antiangiogenic factors in separate α-granules8 and release them selectively on the thrombin receptor PAR1 and PAR4 stimulation.8,9 Platelets can also selectively sequester angiogenic regulators.10,11 We have further characterized differential release and surface expression of platelet proangiogenic stromal cell-derived factor-1α (SDF-1α/CXCL12) and vascular endothelial growth factor (VEGF) and antiangiogenic platelet factor 4 (PF4/CXCL4) and endostatin in response to different platelet stimuli.
Methods
Study subjects
Twenty-four healthy and medication-free volunteers (12 females, 25-64 years of age) gave informed consent to participate in the study, which was approved by Ethics Committee of the Karolinska Institutet.
Immunohistochemistry and confocal microscopy
Immunofluorescent staining of citrated platelet-rich plasma (PRP) was performed as previously described.8
Immunogold electron microscopy
Flow cytometry
Citrated whole blood or PRP was labeled at 22°C without or with platelet agonists and intracellular signaling inhibitors. Flow cytometric platelet analyses were performed as previously described.13
Platelet secretion of angiogenic proteins and immunoassay
Washed platelets (3 × 108/mL) were stimulated, and the supernatant was collected and aliquoted after centrifugation (14 000g, 5 minutes, 4°C). The levels of VEGF, SDF-1α, PF4, and endostatin were determined using corresponding DuoSet ELISA kits (R&D Systems).
Statistics
Data are presented as mean plus or minus SEM. Comparisons were performed with Student paired t test.
Results and discussion
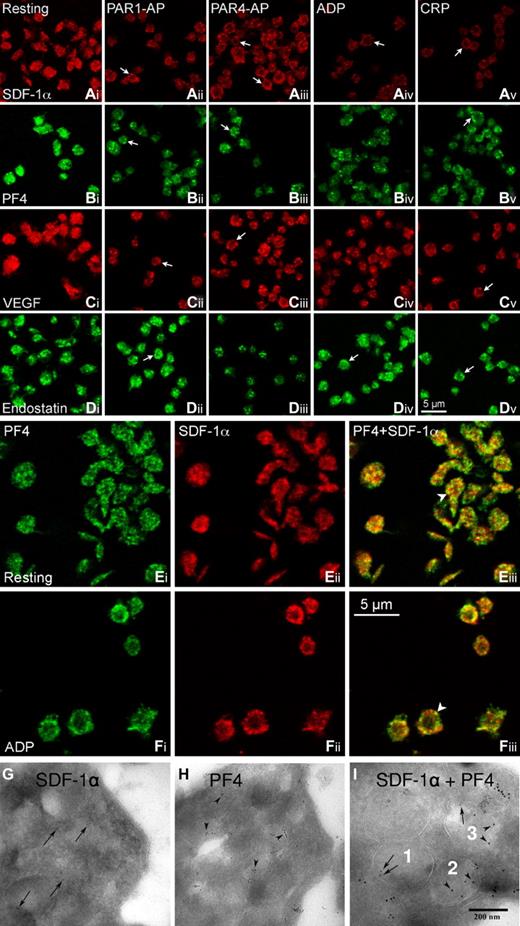
Immunofluorescent staining showed that each of the angiogenic factors was stored in granules randomly distributed in unstimulated platelets (Figure 1Ai,Bi,Ci,Di). On stimulation, the granules were mobilized toward cell periphery, often seen in a ring-like staining pattern (arrows) and with reduced immunofluorescence intensity compared with resting platelets (Figure 1Aii-v,Bii-v,Cii-v,Dii-v). Double immunofluorescence labeling demonstrated that most SDF-1α (red) and PF4 staining (green) was in separate granules, with some colocalization seen in yellow (arrowheads) in both resting (Figure 1Eiii) and adenosine diphosphate (ADP)-stimulated platelets (Figure 1Fiii). Immunogold electron microscopy revealed that SDF-1α (Figure 1G) or PF4-probing gold particles (Figure 1H) do not present in all α-granules and that only some α-granules contain both SDF-1α (arrows) and PF4 (arrowheads) gold particles (Figure 1I), supporting our findings with confocal microscopy and the notions that the counteracting factors are mostly segregated in separate α-granules8 and that different α-granules are packed with different proteins.14
Platelets store proangiogenic and antiangiogenic regulators in separate α-granules. For immunofluorescent staining, PRP (2 × 108 cells/mL) was subjected to the treatment with vehicle (resting), PAR1-activating peptide (PAR1-AP 10μM; SFLLRNPNDKYEPF-OH from Calbiochem), PAR4-AP (100μM; AYPGKF-NH2, from Sigma-Aldrich), ADP (10μM, Sigma-Aldrich), and CRP (1 μg/mL, from Dr R. Farndale, Cambridge, United Kingdom) for 5 minutes at room temperature. Thereafter, PRP was fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS), applied to 0.01% poly-L-lysine-coated coverslips, and then permeabilized with 0.5% Triton X-100. After blocking with 1% bovine serum albumin-PBS, the samples were incubated overnight at 4°C with respective primary antibodies: mouse anti–human SDF-1α/chemokine (C-X-C motif) ligand 12; MAB350 from R&D Systems at 1:20 (Ai-v,E,F); rabbit anti–human PF4/CXCL4 Ab sc-50301 from Santa Cruz Biotechnology at 1:25 (Bi-v,E,F); rabbit anti–human VEGF Ab-1 from Thermo Scientific at 1:500 (Ci-v); and rabbit anti–human endostatin Ab53702 from AbCam at 1:50 (Di-v). After washing, they were incubated with corresponding fluorescent secondary antibodies (DyLight 549 goat antimouse IgG at 1:50, Jackson ImmunoResearch Laboratories; AlexaFluor-488 goat anti–rabbit IgG at 1:100, AlexaFluor-546 goat anti–rabbit IgG at 1:500, and AlexaFluor-488 goat anti–rabbit IgG at 1:100/AlexaFluor-647 donkey anti–mouse IgG at 1:100 for double labeling, all from Invitrogen) for 2 hours at room temperature. After thorough washings with PBS containing 0.3% Triton X-100 and 0.1% Tween-20, the coverslips were mounted with Prolong Gold antifade mounting medium (Invitrogen). Single immunofluorescence (A-D) and SDF-1α/PF4-double immunofluorescence (E-F) platelet images were acquired using a Leica confocal microscope TCS SP2 equipped with an 100× NA1.4 objective. The digital images were assembled into composite images using Adobe PhotoShop Version 10.0.1. The arrows indicate angiogenic factor-staining that was marginized to form a ring-like distribution on platelet activation. The arrowheads indicate the colocalization (yellow) of SDF-1α and PF4. The images are representatives from 4 or 5 experiments. Single immunogold labeling of SDF-1α (MAB350 at 1:20; followed by 5-nm gold protein A probing, arrows; G) and PF4 (sc-50 301 antibody at 1:25, followed by 10-nm gold protein A probing, arrowheads; H) was performed on cryo-ultrathin sections of unstimulated platelets. SDF-1α-PF4 double immunogold labeling (I) was first probed with the SDF-1α antibody, which was indicated by 5-nm gold protein A (arrows) and then probed with the PF4 antibody, which was indicated with 10-nm gold protein A (arrowheads). The samples were visualized with a Leo 906 transmission electron microscope (Leo GmbH) operating with an accelerating voltage of 80 kV and at 60 000× original magnification. Digital images were taken with a Morada camera (SiS Münster). (I) SDF-1α–containing (numbered 1), PF4–containing (2), and SDF-1α-PF4–containing (3) α-granules within 1 platelet. The results shown are representative images from 3 separate experiments.
Platelets store proangiogenic and antiangiogenic regulators in separate α-granules. For immunofluorescent staining, PRP (2 × 108 cells/mL) was subjected to the treatment with vehicle (resting), PAR1-activating peptide (PAR1-AP 10μM; SFLLRNPNDKYEPF-OH from Calbiochem), PAR4-AP (100μM; AYPGKF-NH2, from Sigma-Aldrich), ADP (10μM, Sigma-Aldrich), and CRP (1 μg/mL, from Dr R. Farndale, Cambridge, United Kingdom) for 5 minutes at room temperature. Thereafter, PRP was fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS), applied to 0.01% poly-L-lysine-coated coverslips, and then permeabilized with 0.5% Triton X-100. After blocking with 1% bovine serum albumin-PBS, the samples were incubated overnight at 4°C with respective primary antibodies: mouse anti–human SDF-1α/chemokine (C-X-C motif) ligand 12; MAB350 from R&D Systems at 1:20 (Ai-v,E,F); rabbit anti–human PF4/CXCL4 Ab sc-50301 from Santa Cruz Biotechnology at 1:25 (Bi-v,E,F); rabbit anti–human VEGF Ab-1 from Thermo Scientific at 1:500 (Ci-v); and rabbit anti–human endostatin Ab53702 from AbCam at 1:50 (Di-v). After washing, they were incubated with corresponding fluorescent secondary antibodies (DyLight 549 goat antimouse IgG at 1:50, Jackson ImmunoResearch Laboratories; AlexaFluor-488 goat anti–rabbit IgG at 1:100, AlexaFluor-546 goat anti–rabbit IgG at 1:500, and AlexaFluor-488 goat anti–rabbit IgG at 1:100/AlexaFluor-647 donkey anti–mouse IgG at 1:100 for double labeling, all from Invitrogen) for 2 hours at room temperature. After thorough washings with PBS containing 0.3% Triton X-100 and 0.1% Tween-20, the coverslips were mounted with Prolong Gold antifade mounting medium (Invitrogen). Single immunofluorescence (A-D) and SDF-1α/PF4-double immunofluorescence (E-F) platelet images were acquired using a Leica confocal microscope TCS SP2 equipped with an 100× NA1.4 objective. The digital images were assembled into composite images using Adobe PhotoShop Version 10.0.1. The arrows indicate angiogenic factor-staining that was marginized to form a ring-like distribution on platelet activation. The arrowheads indicate the colocalization (yellow) of SDF-1α and PF4. The images are representatives from 4 or 5 experiments. Single immunogold labeling of SDF-1α (MAB350 at 1:20; followed by 5-nm gold protein A probing, arrows; G) and PF4 (sc-50 301 antibody at 1:25, followed by 10-nm gold protein A probing, arrowheads; H) was performed on cryo-ultrathin sections of unstimulated platelets. SDF-1α-PF4 double immunogold labeling (I) was first probed with the SDF-1α antibody, which was indicated by 5-nm gold protein A (arrows) and then probed with the PF4 antibody, which was indicated with 10-nm gold protein A (arrowheads). The samples were visualized with a Leo 906 transmission electron microscope (Leo GmbH) operating with an accelerating voltage of 80 kV and at 60 000× original magnification. Digital images were taken with a Morada camera (SiS Münster). (I) SDF-1α–containing (numbered 1), PF4–containing (2), and SDF-1α-PF4–containing (3) α-granules within 1 platelet. The results shown are representative images from 3 separate experiments.
Platelet activation evokes distinct secretion and surface expression of angiogenic factors. Stimulation with PAR1-AP, ADP, and collagen related peptide (CRP, targeting glycoprotein VI [GPVI]) induces massive release of SDF-1α and VEGF, as evidenced by marked reductions of immunofluorescent intensities (Figure 1Aii, iv,v;Cii,iv,v) and significant elevations of SDF-1α (Figure 2A) and VEGF levels (Figure 2B) in the supernatant of washed platelets. Semiquantitative flow cytometric analysis of permeabilized platelets showed that SDF-1α staining index (positive % × mean fluorescent intensity) was reduced by 50% in PAR1-stimulated (210.8 ± 37.8 vs 459.4 ± 76 in resting platelets) and by 35% in PAR4-stimulated platelets (240.7 ± 39.0; n = 3). These agonists induced, however, much less release of the antiangiogenic PF4 (Figures 1Bii,iv,v,2C) or actually no release of endostatin (Figure 2D), although endostatin-containing α-granules were still mobilized toward the periphery of platelets (Figure 1Dii,iv,v). The data indicate that PAR1-AP, ADP, and CRP selectively enhance proangiogenic factor release. Furthermore, the combination of ADP and a P2Y12 antagonist showed that ADP-induced VEGF and PF4 release was mainly via P2Y12 activation, whereas ADP-induced SDF-1α secretion was primarily via P2Y1 receptor engagement. In contrast, PAR4-AP triggered marked release of PF4 (Figures 1Biii, 2C) and endostatin (Figures 1Diii,2D) but limited release of SDF-1α (Figures 1Aiii,2A) and VEGF (Figures 1Ciii,2B). Thus, PAR4-AP stimulation promotes a selective release of platelet antiangiogenic factors. Furthermore, platelet secretion of angiogenic factors seems to be a rapid process. The maximal PAR1-stimulated VEGF release was reached in less than 5 minutes, with VEGF levels increased from undetectable of resting platelets to 49.8 ± 1.8, 50.6 ± 0.6, and 49.9 ± 0.6 pg/mL at 5, 15, and 30 minutes of stimulation, respectively. Similarly, PAR4-stimulated endostatin release peaked at 5 minutes (89.1 ± 10.6 vs 0.9 ± 0.1 ng/mL of resting platelets, and 81.3 ± 11.9 ng/mL after 30 minutes stimulation; n = 3).
Distinct secretion and surface expression of platelet proangiogenic and antiangiogenic factors. (A-D) Platelet-released SDF-1α (A), VEGF (B), PF4 (C), and endostatin (D) were determined in the supernatants of washed platelets (3 × 108 cells/mL) that had been treated with vehicle, PAR1-AP 10μM, PAR4-AP 100μM, ADP 10μM, ADP 10μM plus the P2Y12 antagonist AZD1283 10μM (AstraZeneca R&D Systems) and CRP 1 μg/mL, using the corresponding DuoSet ELISA kits from R&D Systems. Data are mean ± SEMs. *P < .05, compared with other treatments, except that P2Y1-elevated VEGF level (C) did not differ from PAR4 (P = .46). nd indicates not detectable; n = 3. (E-H) Platelet surface expression or binding of angiogenic factors and P-selectin were monitored by whole blood flow cytometry. Whole blood samples were incubated without or with increasing concentrations of PAR1-AP (E), PAR4-AP (F), ADP (G), or CRP (H) and in the presence of different antibodies (phycoerythrin-conjugated anti-P-selectin monoclonal antibody, BD Biosciences; SDF-1α monoclonal antibody; phycoerythrin-VEGF monoclonal antibody; phycoerythrin-PF4 monoclonal antibody, all from R&D Systems; and antiendostatin monoclonal antibody, Hycult Biotechnology) for 20 minutes at room temperature. The samples incubated with unconjugated antibodies were further labeled with a goat anti–mouse IgG1 fluorescein isothiocyanate-conjugated antibody (AbD Serotec). After washing with 1% bovine serum albumin–PBS and fixed with 0.5% formaldehyde saline, the samples were analyzed using a Beckman Coulter XL-MCL or FC500 flow cytometer. Data plotted are means from 6-9 subjects. (I-K) Signaling mechanisms underlying distinct angiogenic factor release. SDF-1α (I) and endostatin (J) release from washed platelets was induced by 10μM PAR1-AP or 100μM PAR4-AP in the presence of vehicle (0.1% dimethyl sulfoxide in PBS) or signaling inhibitors (−): the tyrosine kinase Src inhibitor < Src (−) > PP2 (final concentration 5μM; Calbiochem), the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (25μM; Cell Signaling Technology), the serine/threonine protein kinase Akt inhibitor SH-6 (30μM; Calbiochem), the PKC inhibitor ro-31-8220 (10μM; Sigma-Aldrich), the mitogen-activated protein kinase kinase inhibitor U0126 (10μM; Cell Signaling Technology), and the p38 mitogen-activated protein kinase inhibitor SB203580 (10μM; Calbiochem). SDF-1α and endostatin levels in the supernatant were determined using corresponding DuoSet ELISA kits. Data are presented as percentage of the control: Control % = ([SDF-1α]PAR+(−) − [SDF-1α]resting)/([SDF-1α]PAR+vehicle − [SDF-1α]resting) × 100%. In parallel with the platelet release assay, platelet surface expression of P-selectin was monitored by flow cytometry. These data are also presented as the percentage of control (K). *P < .05 compared with control (vehicle); n = 5.
Distinct secretion and surface expression of platelet proangiogenic and antiangiogenic factors. (A-D) Platelet-released SDF-1α (A), VEGF (B), PF4 (C), and endostatin (D) were determined in the supernatants of washed platelets (3 × 108 cells/mL) that had been treated with vehicle, PAR1-AP 10μM, PAR4-AP 100μM, ADP 10μM, ADP 10μM plus the P2Y12 antagonist AZD1283 10μM (AstraZeneca R&D Systems) and CRP 1 μg/mL, using the corresponding DuoSet ELISA kits from R&D Systems. Data are mean ± SEMs. *P < .05, compared with other treatments, except that P2Y1-elevated VEGF level (C) did not differ from PAR4 (P = .46). nd indicates not detectable; n = 3. (E-H) Platelet surface expression or binding of angiogenic factors and P-selectin were monitored by whole blood flow cytometry. Whole blood samples were incubated without or with increasing concentrations of PAR1-AP (E), PAR4-AP (F), ADP (G), or CRP (H) and in the presence of different antibodies (phycoerythrin-conjugated anti-P-selectin monoclonal antibody, BD Biosciences; SDF-1α monoclonal antibody; phycoerythrin-VEGF monoclonal antibody; phycoerythrin-PF4 monoclonal antibody, all from R&D Systems; and antiendostatin monoclonal antibody, Hycult Biotechnology) for 20 minutes at room temperature. The samples incubated with unconjugated antibodies were further labeled with a goat anti–mouse IgG1 fluorescein isothiocyanate-conjugated antibody (AbD Serotec). After washing with 1% bovine serum albumin–PBS and fixed with 0.5% formaldehyde saline, the samples were analyzed using a Beckman Coulter XL-MCL or FC500 flow cytometer. Data plotted are means from 6-9 subjects. (I-K) Signaling mechanisms underlying distinct angiogenic factor release. SDF-1α (I) and endostatin (J) release from washed platelets was induced by 10μM PAR1-AP or 100μM PAR4-AP in the presence of vehicle (0.1% dimethyl sulfoxide in PBS) or signaling inhibitors (−): the tyrosine kinase Src inhibitor < Src (−) > PP2 (final concentration 5μM; Calbiochem), the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (25μM; Cell Signaling Technology), the serine/threonine protein kinase Akt inhibitor SH-6 (30μM; Calbiochem), the PKC inhibitor ro-31-8220 (10μM; Sigma-Aldrich), the mitogen-activated protein kinase kinase inhibitor U0126 (10μM; Cell Signaling Technology), and the p38 mitogen-activated protein kinase inhibitor SB203580 (10μM; Calbiochem). SDF-1α and endostatin levels in the supernatant were determined using corresponding DuoSet ELISA kits. Data are presented as percentage of the control: Control % = ([SDF-1α]PAR+(−) − [SDF-1α]resting)/([SDF-1α]PAR+vehicle − [SDF-1α]resting) × 100%. In parallel with the platelet release assay, platelet surface expression of P-selectin was monitored by flow cytometry. These data are also presented as the percentage of control (K). *P < .05 compared with control (vehicle); n = 5.
In parallel with increased platelet P-selectin expression, all tested agonists markedly and dose-dependently elevated platelet surface expression of SDF-1α and endostatin but induced mild PF4 or no VEGF expression (Figure 2E-H). Interestingly, platelet surface expression of these angiogenic factors, especially SDF-1α and endostatin, did not completely synchronize with their milieu release. For instance, all agonists markedly enhanced platelet SDF-1α and endostatin expression (Figure 2E-H), but only PAR4-AP (D) induced endostatin secretion. This may account for the poor correlation between platelet surface expressed and plasma levels of SDF-1α.15 Enhanced platelet SDF-1α surface expression is angiogenically important and can enhance endothelial progenitor cell recruitment at the arterial injured sites.16
Platelet secretion involves multiple signaling pathways.17,18 We show here that, despite equally activated (as measured by platelet P-selectin expression, 81.3% ± 1.1% by PAR1 and 81.2% ± 0.4% by PAR4) and triggering similar intracellular signalings,19 PAR1- and PAR4-induced SDF-1α and endostatin secretion showed different dependency on multiple signaling pathways. Thus, Src blockade markedly inhibited PAR1-induced, but only modestly attenuated PAR4-induced, SDF-1α secretion. In contrast, blockade of phosphatidylinositol 3-kinase, Akt, protein kinase C (PKC), mitogen-activated protein kinase kinase, and p38 showed more profound inhibition on PAR4-induced SDF-1α secretion, whereas mitogen-activated protein kinase kinase or p38 blockade did not influence the PAR1 effect (Figure 2I). The better inhibitions on PAR4 effect are not simply because PAR4 stimulation induces less SDF-1α secretion. PAR4-induced massive endostatin release was also markedly inhibited, and Akt and PKC blockade almost abolished the release (Figure 2J, in which PAR1 induced no endostatin release, 3.6 ± 0.4 ng/mL vs 3.5 ± 0.4 ng/mL of unstimulated platelets, and effects of the inhibitors are thus not plotted). Notably, none of these signaling regulators markedly attenuated platelet surface expression of P-selectin (Figure 2K), SDF-1α (eg, 25.3% ± 6.0% and 26.7% ± 6.4% by PAR1 stimulation without or with PKC blockade; n = 5), or endostatin (28.4% ± 6.6% and 26.8% ± 6.1% by PAR4 stimulation without or with PKC blockade; n = 5). This is probably because P-selectin is a general marker of platelet secretion, and a trace of platelet-released SDF-1α and endostatin can efficiently bind on their specific receptors (eg, CXCR4 and integrin α4β1) on platelet surface.16,20,21 It should also be noted that those inhibitors did inhibit P-selectin expression at lower agonist concentrations (eg, 5μM PAR1-AP or 50μM PAR4-AP), which are insufficient to induce optimal release of platelet angiogenic factors (data not shown).
Taken together, PAR1, P2Y12, P2Y1, and GPVI stimulation favors platelet release of proangiogenic factors. PAR4 stimulation selectively induces platelet secretion of antiangiogenic factors. PAR1-induced SDF-1α secretion depends on mitogen-activated protein kinase signaling little, whereas Akt and PKC signaling is critical for PAR4-induced endostatin release. Distinct platelet secretion of angiogenic factors may be an important angiogenesis-regulating mechanism. Platelets adhere at the sites of vascular injury. They react to the early stimuli (eg, subendothelial collagen, low concentrations of initially generated thrombin that activates platelets via the high-affinity PAR1, and self-released ADP) and release proangiogenic factors. As thrombus builds up, platelets react to the late stimulus, high concentrations of thrombin that accumulate within platelet clots, via the low-affinity PAR4 to promote antiangiogenic regulator release. Hence, platelets may locally and dynamically modulate angiogenic balance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maud Daleskog and Ragnhild Stålesen for expert technical assistance.
This work was supported by grants from the Swedish Research Council, the Swedish Heart-Lung Foundation, the Stockholm County Council, the Swedish Society of Medicine, and the Karolinska Institutet.
Authorship
Contribution: M.C., Z.H., and W.Z. designed and performed the research, interpreted the data, and drafted the manuscript; L.J., L.Z., and H.H. performed the research, interpreted the data, and revised the manuscript; K.H. performed the research and interpreted the data; G.P.N. designed the research, interpreted the data, and revised the manuscript; and N.L. designed and supervised the research, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nailin Li, Karolinska Institutet, Department of Medicine-Solna, Clinical Pharmacology Unit, Karolinska University Hospital (Solna), SE-171 76 Stockholm, Sweden; e-mail: Nailin.Li@ki.se.

![Figure 2. Distinct secretion and surface expression of platelet proangiogenic and antiangiogenic factors. (A-D) Platelet-released SDF-1α (A), VEGF (B), PF4 (C), and endostatin (D) were determined in the supernatants of washed platelets (3 × 108 cells/mL) that had been treated with vehicle, PAR1-AP 10μM, PAR4-AP 100μM, ADP 10μM, ADP 10μM plus the P2Y12 antagonist AZD1283 10μM (AstraZeneca R&D Systems) and CRP 1 μg/mL, using the corresponding DuoSet ELISA kits from R&D Systems. Data are mean ± SEMs. *P < .05, compared with other treatments, except that P2Y1-elevated VEGF level (C) did not differ from PAR4 (P = .46). nd indicates not detectable; n = 3. (E-H) Platelet surface expression or binding of angiogenic factors and P-selectin were monitored by whole blood flow cytometry. Whole blood samples were incubated without or with increasing concentrations of PAR1-AP (E), PAR4-AP (F), ADP (G), or CRP (H) and in the presence of different antibodies (phycoerythrin-conjugated anti-P-selectin monoclonal antibody, BD Biosciences; SDF-1α monoclonal antibody; phycoerythrin-VEGF monoclonal antibody; phycoerythrin-PF4 monoclonal antibody, all from R&D Systems; and antiendostatin monoclonal antibody, Hycult Biotechnology) for 20 minutes at room temperature. The samples incubated with unconjugated antibodies were further labeled with a goat anti–mouse IgG1 fluorescein isothiocyanate-conjugated antibody (AbD Serotec). After washing with 1% bovine serum albumin–PBS and fixed with 0.5% formaldehyde saline, the samples were analyzed using a Beckman Coulter XL-MCL or FC500 flow cytometer. Data plotted are means from 6-9 subjects. (I-K) Signaling mechanisms underlying distinct angiogenic factor release. SDF-1α (I) and endostatin (J) release from washed platelets was induced by 10μM PAR1-AP or 100μM PAR4-AP in the presence of vehicle (0.1% dimethyl sulfoxide in PBS) or signaling inhibitors (−): the tyrosine kinase Src inhibitor < Src (−) > PP2 (final concentration 5μM; Calbiochem), the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (25μM; Cell Signaling Technology), the serine/threonine protein kinase Akt inhibitor SH-6 (30μM; Calbiochem), the PKC inhibitor ro-31-8220 (10μM; Sigma-Aldrich), the mitogen-activated protein kinase kinase inhibitor U0126 (10μM; Cell Signaling Technology), and the p38 mitogen-activated protein kinase inhibitor SB203580 (10μM; Calbiochem). SDF-1α and endostatin levels in the supernatant were determined using corresponding DuoSet ELISA kits. Data are presented as percentage of the control: Control % = ([SDF-1α]PAR+(−) − [SDF-1α]resting)/([SDF-1α]PAR+vehicle − [SDF-1α]resting) × 100%. In parallel with the platelet release assay, platelet surface expression of P-selectin was monitored by flow cytometry. These data are also presented as the percentage of control (K). *P < .05 compared with control (vehicle); n = 5.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/14/10.1182_blood-2010-12-327007/4/m_zh89991169410002.jpeg?Expires=1769370784&Signature=c3lGfTA4lWiSHTQLEurJ9o8I6-NoeX8rx~sXRjlrUn1FNPSWmqa~6R6uN8i6RY1-QK0G6Qbt76DzUohphwnYtlki5P3VtknH7W9aGejLEOtMDAGMVGY2lSAcR3f7Kw91IFWvVoPXY4f8mihr453ggq0Xd-PVJI31zR8LdiRekWwuVwTcn~eFWjdN7orGwEG9bXXjq6B~obiPtXyJ0aDaIZGAPBAL8EQfSeDRkMy76Hz8OUk0p6bHwFNueV7syzrM09fSV7x1HlfZll197GgK4kZrOFNk7mYCrrZhWoz7gND7PnCprTf1gdDmyIXy3qOvjo6RMQmuXvLgmqNp-Mhzzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
