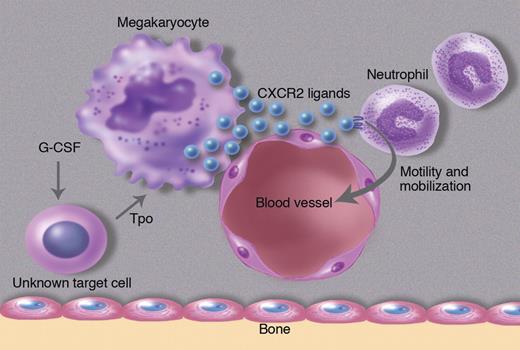
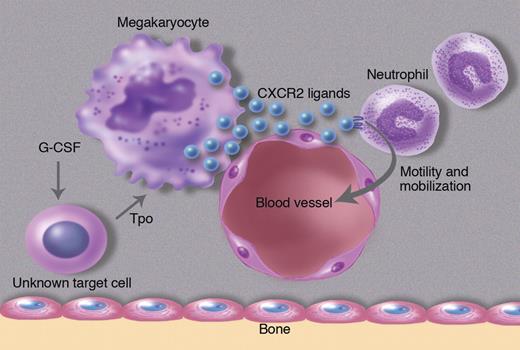
In this issue of Blood, Köhler and colleagues show that thrombopoietin, a hematopoietic cytokine best known for its regulation of platelet production, may also control neutrophil release from the bone marrow by regulating expression of CXCR2 ligands in endothelial cells and megakaryocytes.1
Neutrophils are an essential component of the innate immune response and a major contributor to inflammation. Accordingly, the number of neutrophils in the blood is tightly controlled through a balance of neutrophil production, release from the bone marrow, and clearance from the circulation. At baseline, only a small fraction of total body neutrophils circulate in the peripheral blood, while the majority remains in reserve in the bone marrow. However, in response to certain infections, neutrophils are rapidly mobilized from the bone marrow to blood.
Granulocyte colony-stimulating factor (G-CSF) is the principal cytokine regulating neutrophil homeostasis and is widely used in the clinical setting to treat neutropenia. The primary mechanism by which G-CSF increases the number of circulating neutrophils is through enhanced release from the bone marrow, rather than increased neutrophil production. Previous studies have established that G-CSF treatment works, in part, by disrupting an important neutrophil retention signal, the CXCL12-CXCR4 axis2,3 by both decreasing expression of CXCL12 by stromal cells in the bone marrow4-6 and by decreasing expression of its receptor, CXCR4, on neutrophils themselves.3 It is currently unclear how loss of CXCR4 signaling results in the directed movement of neutrophils from the bone marrow into the peripheral blood.
Here, Köhler et al show, using intravital 2-photon microscopy, that within hours of treating mice with G-CSF, neutrophils greatly increase their motility in the bone marrow and subsequent egress into the circulation. Concurrent with neutrophil mobilization, they note increased expression of 2 chemokines, KC (CXCL1, Groα) and MIP-2 (CXCL2, Groβ), both of which bind to the chemokine receptor CXCR2. CXCR2 ligands are potent chemoattractants for neutrophils and play a key role in the emigration of neutrophils from the blood to sites of tissue inflammation. Moreover, a recent study suggested that CXCR2 signaling may also regulate neutrophil mobilization from the bone marrow.2 Consistent with this observation, Köhler and colleagues show that G-CSF induced neutrophil mobilization is blunted in CXCR2 deficient mice or by treatment of mice with blocking antibodies to CXCR2.
The authors' investigation into the cellular source of CXCR2 ligands in the bone marrow led to a surprising cell type, megakaryocytes. They show by immunostaining of bone marrow sections that KC and MIP-2 are constitutively expressed in megakaryocytes and endothelial cells. Interestingly, KC and MIP-2 staining in these cell populations was reduced after G-CSF, while KC and MIP-2 mRNA expression in the bone marrow increased. The authors interpret these data to suggest that G-CSF is inducing secretion of KC and MIP-2 protein from megakaryocytes and endothelial cells in the bone marrow. Interestingly, G-CSF does not appear to directly stimulate megakaryocytes to release CXCR2 ligands. Rather the authors provide evidence that G-CSF works through thrombopoietin (Tpo). They show that G-CSF induces Tpo expression in the bone marrow, which in turn results in the secretion of CXCR2 ligands from megakaryocytes and possibly endothelial cells (see figure). In support of this model, injection of Tpo into mice results in rapid neutrophil mobilization. Conversely, G-CSF induced neutrophil mobilization is attenuated in mice lacking the Tpo receptor (c-Mpl).
G-CSF acts on an unknown cell population to produce thrombopoietin, which in turn induces megakaryocytes and endothelial cells to secrete the CXCR2 ligands KC and MIP-2. These CXCR2 ligands stimulate neutrophil migration in the bone marrow, ultimately leading to their release into the blood. Professional illustration by Marie Dauenheimer.
G-CSF acts on an unknown cell population to produce thrombopoietin, which in turn induces megakaryocytes and endothelial cells to secrete the CXCR2 ligands KC and MIP-2. These CXCR2 ligands stimulate neutrophil migration in the bone marrow, ultimately leading to their release into the blood. Professional illustration by Marie Dauenheimer.
This study suggests a novel signaling cascade connecting megakaryocyte signaling with neutrophil release from the bone marrow, and it raises several important questions. What are the cell population(s) in the bone marrow that secrete Tpo in response to G-CSF? How does increased CXCR2 ligand expression lead to neutrophil mobilization? Given that endothelial cells and perivascular megakaryocytes are major sources of CXCR2 ligands, perhaps a gradient exists between the central marrow and the vasculature. Finally, what is the clinical relevance of this pathway in syndromes with altered megakaryocytes or Tpo signaling? For example, in essential thrombocytosis, does the marked increase in megakaryocyte number (or more rarely activating mutations of MPL) contribute to the leukocytosis often present in these patients?
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■