Abstract
Children with primary immunodeficiency diseases, particularly those less than 1 year of age, experience significant toxicity after hematopoietic stem cell transplantation, with busulfan- or melphalan-based conditioning. Treosulfan causes less veno-occlusive disease than busulfan and does not require pharmacokinetic monitoring. We report its use in 70 children. Children received 42 g/m2 or 36 g/m2 with cyclophosphamide 200 mg/kg (n = 30) or fludarabine 150 mg/m2 (n = 40), with alemtuzumab in most. Median age at transplantation was 8.5 months (range, 1.2-175 months); 46 (66%) patients were 12 months of age or younger. Donors were as follows: matched sibling donor, 8; matched family donor, 13; haploidentical, 4; and unrelated, 45. Median follow-up was 19 months (range, 1-47 months). Overall survival was 81%, equivalent in those age less or greater than 1 year. Skin toxicity was common. Veno-occlusive disease occurred twice with cyclophosphamide. Eighteen patients (26%) had graft-versus-host disease, and only 7 (10%) greater than grade 2. Two patients rejected; 24 of 42 more than 1 year after transplantation had 100% donor chimerism. The remainder had stable mixed chimerism. T-cell chimerism was significantly better with fludarabine. Long-term follow-up is required, but in combination with fludarabine, treosulfan is a good choice of conditioning for hematopoietic stem cell transplantation in primary immunodeficiency disease.
Introduction
Hematopoietic stem cell transplantation (HSCT) remains the only curative option for many children with primary immunodeficiency disorders (PIDs) or severe immune dysregulatory disorders. The aim of HSCT is to produce stable donor engraftment after partial or full ablation of the recipient's marrow and immune system using a combination of chemotherapy, antibody therapy, and a graft-versus-marrow effect.1 Apart from a graft-versus-marrow effect, there is no advantage in producing significant graft-versus-host disease (GVHD) in these patients as no graft-versus-tumor effect is required, GVHD can adversely affect thymic function, and stable mixed chimerism can lead to cure.2 In recent years, survival rates for allogeneic HSCT have improved because of a number of factors, including better human leukocyte antigen (HLA) matching and greater availability of closely matched unrelated donors, including the use of cord blood donations, improved monitoring for viral and fungal infections with preemptive treatment, and better supportive care.3 The introduction of reduced intensity conditioning regimens, such as fludarabine and melphalan, has reduced treatment-related toxicity in some PID patients,4 but toxicity remains a problem for children less than 1 year of age5 and there have been specific cardiac toxicities associated with melphalan.6 Minimal intensity conditioning, for instance, with fludarabine and cyclophosphamide can reduce toxicity even further but may be associated with poor donor myeloid chimerism or an increased incidence of GVHD.7 Consequently, there is a need to explore new conditioning regimens for PID, which enable adequate myeloablation with limited toxicity, particularly in patients less than 1 year of age.
Treosulfan (L-treitol-1,4-bis-methanesulfonate) is the prodrug of L-epoxybutane, a water-soluble bifunctional alkylating agent with myeloablative and immunosuppressive properties,8 and has been shown to provide effective HSCT conditioning with reduced risk of toxicities, particularly veno-occlusive disease (VOD), compared with busulfan.9-13 In addition, unlike busulfan, it may not be necessary to measure drug levels, as stable linear pharmacokinetics up to the clinically effective dose of 42 g/m2 have been shown.10 Beelen et al demonstrated a low rate of organ toxicities and favorable one-year nonrelapse mortality rate combining treosulfan with cyclophosphamide in a group of 18 adult patients with hematologic malignancies considered ineligible for other myeloablative preparative regimens.10 Casper et al combined treosulfan with fludarabine in a group of 30 adult patients with hematologic malignancies considered as unacceptable risks for conventional conditioning and achieved a good outcome with respect to toxicity, achievement of complete donor chimerism, low GVHD rate, and low treatment-related mortality and relapse rate.12 There is less published evidence on the use of treosulfan in children,13 in particular those with PID. Early studies using treosulfan for HSCT in children with PID (n = 18) look promising with 17 of 18 surviving to a median follow-up of 429 days (range, 156-722 days),9 including successful engraftment in some forms of PID prone to graft rejection, such as chronic granulomatous disease.9,14 Here we describe a large cohort of children who have received treosulfan-based conditioning regimens for PID and examine outcomes with particular reference to age younger or older than 1 year.
Methods
Patients
A retrospective study of 70 consecutive patients with PID or severe immune dysregulatory disorder who underwent HSCT at United Kingdom supra-regional referral centers for PID, Newcastle on Tyne General Hospital (n = 40) and Great Ormond Street Hospital (n = 30), between February 2006 and December 2009 was performed. Information was collected regarding patient demographics, diagnosis, donor match and stem cell source, conditioning regimen, transplantation-related complications, GVHD, chimerism, immune reconstitution, outcome, and length of follow-up. Patients were not randomized, and the choice of conditioning was based on clinical decision. Informed consent was taken from all parents according to the local center and European Blood and Marrow Transplantation guidelines and the Declaration of Helsinki.
HLA typing was performed by molecular typing for HLA class I and II loci. The unrelated donors were all 7 to 10 of 10 HLA matched. Bone marrow (BM, n = 40), peripheral blood stem cells (PBSCs, n = 9), and cord blood (CB, n = 17) were used as a stem cell source. Peripheral blood was used for the 4 haploidentical transplants, using the Clinimacs (Miltenyi Biotec) systems for CD3/CD19 depletion in 3 and CD34+ stem cell selection in one.
Treosulfan was given at a dose of 42 g/m2 (n = 43) or 36 g/m2 (n = 27) in 3 divided doses on 3 consecutive days. Sixteen of 30 patients (53%) who received cyclophosphamide were given the higher dose of treosulfan as were 24 of 40 (68%) who received fludarabine. The lower dose was given in young babies generally less than 1 year of age. Alemtuzumab 0.3 to 1.0 mg/kg total dose was given to all the patients except those who received a matched sibling donor (MSD) graft (n = 8), 1 who had a second transplant from an matched family donor (MFD) after a previous unconditioned transplant, recipients of haploidentical CD3/CD19-depleted PBSCs (n = 3), or haploidentical CD34+ selected PBSCs who received OKT3 (n = 1) and 7 recipients of CB, 3 of whom received antithymocyte globulin. GVHD prophylaxis in the majority of patients (53) consisted of cyclosporine with mycophenolate mofetil, which was weaned from day 28 in the absence of any GVHD. Ten received cyclosporine alone, 3 cyclosporine and methotrexate, 2 cord transplant recipients cyclosporine and methylprednisolone, 1 haploidentical recipient mycophenolate mofetil and OKT3, and 1 died before transplantation.
Patients had weekly polymerase chain reaction testing of blood for adenovirus, Epstein-Barr virus (EBV), and cytomegalovirus (CMV) in both centers and human herpesvirus 6 (HHV6) in Newcastle. Acute GVHD was assessed using the Seattle criteria. Chronic GVHD was defined as GVHD occurring 100 days or more after HSCT and was graded as extensive or limited.
Chimerism
Donor chimerism was measured by labeling blood with anti-CD3, anti-CD19, or anti-CD15 microbeads, and cell lines were separated using an autoMACS automated bench-top magnetic cell sorter (Miltenyi Biotec). Separated cells were assayed using variable number of tandem repeat or XY fluorescence in situ hybridization analysis for sex-mismatched donor-recipient transplants.
Statistics
Groups were compared using Fisher exact test with a 2-tailed P value, except where numbers were small, when the χ2 test with Yates correction was used (GraphPad Prism, Version 5, GraphPad Software). P values equal to or less than .05 were considered statistically significant.
Results
Diagnoses were as follows: severe combined immunodeficiency (SCID, n = 26), Wiskott-Aldrich syndrome (WAS, n = 7), Omenn syndrome (n = 7), hemophagocytic lymphohistiocytosis (HLH, n = 4), combined immunodeficiency (CID, n = 4), leukocyte adhesion deficiency (LAD, n = 4), chronic granulomatous disease (CGD, n = 3), severe immune dysregulation (n = 3), cartilage hair hypoplasia (CHH, n = 2), immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX syndrome, n = 2), major histocompatibility class II deficiency (MHC II, n = 2), and 1 of each: T-cell activation defect, X lymphoproliferative-like syndrome (XLP-like), CD40 ligand deficiency, autoimmune lymphoproliferative syndrome (ALPS), severe congenital neutropenia (SCN), and immunodeficiency, centromeric instability, facial dysmorphism (ICF) syndrome.
The median age at transplantation was 8.5 months (range, 1.2-175 months). Forty-six of 70 (66%) were 12 months or younger at the time of transplantation. Patients received HSCT from an unrelated donor (n = 45), MSD (n = 8), MFD (n = 13), or haploidentical donor (n = 4) using treosulfan in combination with fludarabine 150 mg/m2 (n = 40) or cyclophosphamide 200 mg/kg (n = 30; Table 1). Transplantation-related complications are summarized in Table 2.
Survival
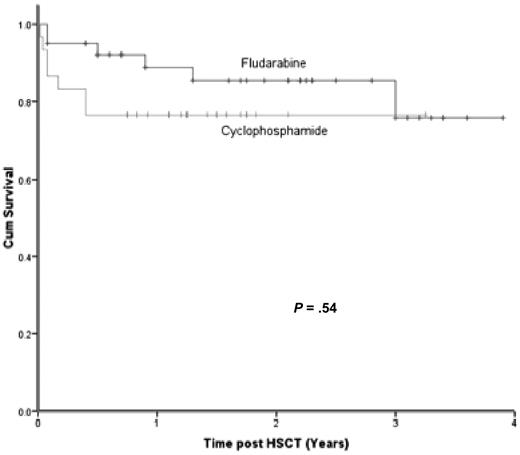
Thirteen children died, giving an overall survival (OS) of 81%. There was no significant difference in survival between those who received fludarabine compared with those who received cyclophosphamide (Figure 1). In the fludarabine group, there were 6 deaths (OS = 85%). None of these appeared to be directly related to toxicity of the conditioning regimen: patient 1 (Table 1) died 16 months after transplantation of chronic GVHD and infection; patient 2 died of refractory HLH on day −1; patient 3 with ALPS rejected a haploidentical transplant, reactivated CMV, and died before retransplant; patient 4 after transplantation for Griscelli syndrome developed myelodysplasia/acute myeloid leukemia with monosomy 7 in recipient cells, declined a second HSCT, and died 3 years after transplantation; patient 5 with T-B-SCID and intestinal atresia died of Pseudomona sepsis at 2 months; and patient 6 with Omenn syndrome died 11 months after transplantation with GVHD and cerebral infarcts. In the cyclophosphamide group, there were 7 deaths (OS = 77%). The first 4 of these were possibly related to the conditioning drugs: patient 42 (Table 1) died on day 7 with a cerebral hemorrhage; patient 43 died of multiorgan failure in association with HHV6 disease; patient 44 died of VOD of the liver; patient 45 had severe pneumonitis and pulmonary hypertension; patient 41 died 5 months after transplantation with HHV6; patient 46 had chronic lung damage before transplantation because of multiple infections and died of pulmonary hemorrhage; and patient 47 had severe gut GVHD after withdrawal of cyclosporine because of mixed chimerism and died.
Kaplan-Meier survival curve. There was no significant difference in survival between those that received fludarabine compared with cyclophosphamide.
Kaplan-Meier survival curve. There was no significant difference in survival between those that received fludarabine compared with cyclophosphamide.
In those 12 months of age or younger at the time of transplantation, there were 8 of 46 deaths (OS = 83%), which was not statistically different compared with 5 of 24 deaths (OS = 79%) in those older than 12 months. Twelve of 44 patients (27%) transplanted at age less than 1 year and 5 of 26 (19%) age more than 1 year required admission to an intensive care unit.
Toxicity
Skin toxicity was common, including perianal ulceration, pigment changes, and occasional peeling. Mucositis was mild. Four children had seizures after cessation of treosulfan: 2 were already on cyclosporine at the time of seizures, and all were less than 4 months of age. Two patients had severe VOD: both received the higher dose of treosulfan in combination with cyclophosphamide, and both had enterovirus in their feces. Both patients were treated with defibrotide: 1 recovered after ventilation and dialysis, but the other died and VOD was confirmed at postmortem.
GVHD
Eighteen (26%) patients had GVHD, but only 7 (10%) had greater than grade 2 GVHD. There were 3 deaths from GVHD. Four patients had limited chronic skin GVHD.
Viral reactivation
Eighteen patients had evidence of 1 or more viruses (26%). CMV was detected in 7, EBV in 4, adenovirus in 8, and HHV6 in 3 children after transplantation. In 5 cases, the viral infection contributed to the death of the child.
Chimerism
Median follow-up is 19 months (range, 1-47 months). Two patients had graft rejection: Patient 20 with MHC II deficiency rejected marrow from an MSD despite a top-up procedure but was successfully retransplanted using busulfan and cyclophosphamide; patient 3 had ALPS and rejected after a haploidentical transplant with CMV reactivation and died before retransplantation. Patient 8 with SCN had mixed chimerism after MSD marrow, with ongoing neutropenia and was successfully retransplanted using busulfan and cyclophosphamide. Two further patients had top-up procedures because of mixed chimerism; patient 14 with CGD had a neutrophil oxidative burst of 27% continuing to fall and an unconditioned PBSC top-up and developed grade 4 GVHD, but recovered well with 100% donor chimerism; and patient 16 with RAG SCID had stable mixed chimerism after top-up, with good immune reconstitution.
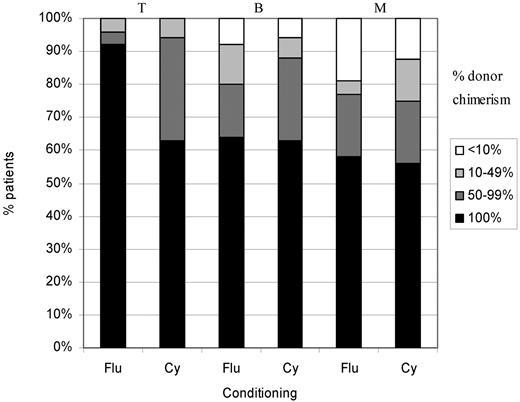
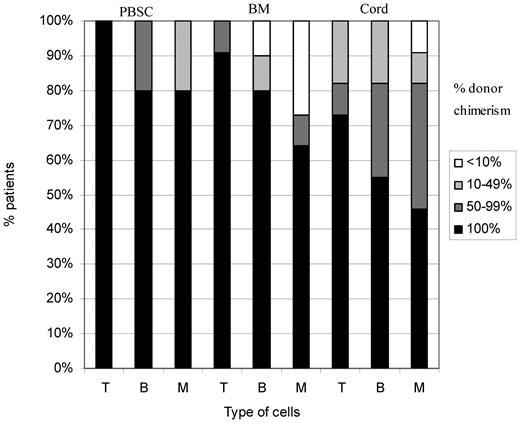
Forty-two were more than 1 year after transplantation, including patient 1 who died 16 months after transplantation. Twenty-four (57%) had 100% donor chimerism in all cell lines: 15 of 26 (58%) in the fludarabine group and 9 of 16 (56%) in the cyclophosphamide group. The rest had stable mixed chimerism. There was no very low level chimerism (< 10%) in the T-cell lineage and very little in the B and myeloid cell lineages (Figure 2).
Split cell chimerism in patients more than 1 year after HSCT. Of 42 patients, 24 (57%) had 100% donor chimerism in all cell lines: 15 of 26 (58%) in the fludarabine group and 9 of 16 (56%) in the cyclophosphamide group. The rest had stable mixed chimerism. There was no very low level chimerism (< 10%) in the T-cell lineage and very little in the B and myeloid cell lineages. There was significantly better T-cell chimerism in the group receiving fludarabine (P = .038). T indicates T-cell lymphocytes; B, B-cell lymphocytes; M, myeloid cells; Flu, fludarabine; and Cy, cyclophosphamide.
Split cell chimerism in patients more than 1 year after HSCT. Of 42 patients, 24 (57%) had 100% donor chimerism in all cell lines: 15 of 26 (58%) in the fludarabine group and 9 of 16 (56%) in the cyclophosphamide group. The rest had stable mixed chimerism. There was no very low level chimerism (< 10%) in the T-cell lineage and very little in the B and myeloid cell lineages. There was significantly better T-cell chimerism in the group receiving fludarabine (P = .038). T indicates T-cell lymphocytes; B, B-cell lymphocytes; M, myeloid cells; Flu, fludarabine; and Cy, cyclophosphamide.
There was significantly better T-cell chimerism in the group receiving fludarabine (24 of 26 had 100% donor) compared with cyclophosphamide (10 of 16; P = .038).
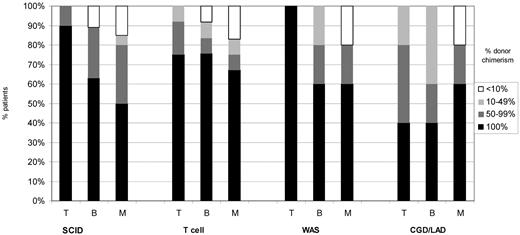
Lineage specific chimerism by disease type for 42 patients with at least 1 year of follow-up is shown in Figure 3. Although it is difficult to draw firm conclusions from small numbers, as might be expected, there was a tendency to greater donor T-cell chimerism in T-cell deficient diseases, SCID, other T-cell deficiency, and WAS compared with CGD and LAD. Donor myeloid chimerism was similar between the 4 groups, and B-cell chimerism roughly mirrored myeloid chimerism.
Split cell chimerism in 42 patients more than 1 year after HSCT by disease. Numbers are small, but there is a tendency to greater donor T-cell chimerism in T cell-deficient diseases, SCID, other T-cell deficiency, and WAS compared with CGD/LAD. Donor myeloid chimerism is similar between the 4 groups and B-cell chimerism approximately mirrors myeloid chimerism.
Split cell chimerism in 42 patients more than 1 year after HSCT by disease. Numbers are small, but there is a tendency to greater donor T-cell chimerism in T cell-deficient diseases, SCID, other T-cell deficiency, and WAS compared with CGD/LAD. Donor myeloid chimerism is similar between the 4 groups and B-cell chimerism approximately mirrors myeloid chimerism.
Donor and stem cell source
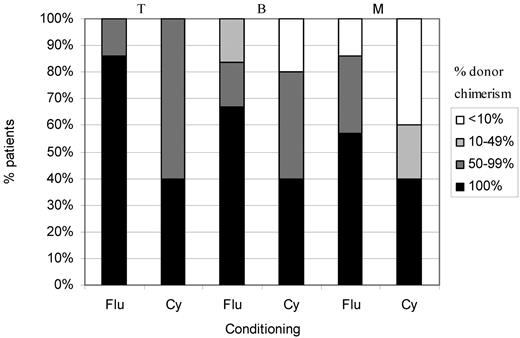
Twenty-one patients had MSD (n = 8) or MFD (n = 13) transplants (BM, n = 19; PBSCs, n = 1; CB, n = 1). There were 6 deaths (OS = 71%). Patients 20 and 8 required a second procedure and were successfully retransplanted using busulfan and cyclophosphamide as described in “Chimerism.” Two are less than 12 months after transplantation. Six of 12 (50%) have 100% donor chimerism in all cell lineages. There was no significant difference in chimerism between this group and those that had unrelated donor transplants. There are only 7 in the fludarabine group (including 1 death) and 5 in the cyclophosphamide group who are more than 12 months after transplantation (Figure 4). There was no significant difference in chimerism between these 2 groups.
Split cell chimerism in MSD/MFD recipients more than 1 year after HSCT. Numbers are small (7 in fludarabine group and 5 in cyclophosphamide group), but there is a suggestion that chimerism is better after fludarabine in all cell lineages.
Split cell chimerism in MSD/MFD recipients more than 1 year after HSCT. Numbers are small (7 in fludarabine group and 5 in cyclophosphamide group), but there is a suggestion that chimerism is better after fludarabine in all cell lineages.
Fifteen of 17 patients who had CB survived (OS = 88%). There was no significant difference in survival between this group, and 42 of 53 who survived after BM or peripheral blood. There are 6 in the fludarabine group and 5 in the cyclophosphamide group who are more than 12 months after transplantation. Four are less than 12 months after transplantation.
Forty-five patients had unrelated donor transplants. Survival was as follows: PBSCs, 8 of 8; BM, 17 of 21; and CB, 15 of 16. Of these, 27 are more than 12 months after transplantation (PBSCs, n = 5; BM, n = 11; and CB, n = 11). Chimerism is shown in Figure 5. Numbers are small, but there is a suggestion that chimerism is better with PBSCs without a significant increase in GVHD (1 > grade 2 after top-up in the PBSCs and 3 > grade 2 in the BM group).
Split cell chimerism in unrelated donor recipients more than 1 year after HSCT according to stem cell source. Numbers are small (PBSCs, n = 5; BM, n = 11; CB, n = 11), but there is a suggestion that chimerism is better with PBSCs.
Split cell chimerism in unrelated donor recipients more than 1 year after HSCT according to stem cell source. Numbers are small (PBSCs, n = 5; BM, n = 11; CB, n = 11), but there is a suggestion that chimerism is better with PBSCs.
Immune reconstitution
Of 41 who are more than 1 year after transplantation, 9 currently remain on IVIg. They have all had GVHD, except 1 common γ-chain-deficient SCID with recipient B cells. CD4 counts are all normal at last follow-up, except patient 18 who has poor immune reconstitution despite having 100% donor chimerism.
Discussion
HSCT with treosulfan-based conditioning regimens achieved excellent OS of more than 80% in this group of children with PID or severe immune dysregulatory disorder with a high level of complete or stable mixed chimerism in the diseased lineage sufficient to cure disease; this includes specific types of PID (eg, CGD), in whom secure engraftment has been compromised with other reduced intensity conditioning regimens as reported previously.2,14 In particular, there was a high survival rate in children transplanted less than 1 year of age in whom toxicity continues to be a problem with conventional and other reduced intensity conditioning regimens.
Toxicity was generally low. Perineal ulceration was common, presumably because of the urinary excretion of active treosulfan metabolites, but resolved in all cases with frequent napkin changes, use of barrier creams, and pain relief. Four babies had seizures; and although it cannot be proved that treosulfan was the cause, the use of clonazepam prophylaxis for those younger than 1 year might be considered. The combination of treosulfan with fludarabine was particularly well tolerated with no occurrence of VOD, which has been confirmed in other studies.9,15 There were no toxic deaths related to the combination of treosulfan and fludarabine. Two patients who developed VOD received treosulfan in combination with cyclophosphamide, and a strong correlation between blood levels of cyclophosphamide metabolites and VOD has previously been shown as a result of depletion of glutathione from the liver.16 Combinations of cyclophosphamide with reduced-dose busulfan may also lead to severe hepatic toxicity and VOD.17,18 There was no cardiac toxicity, which can occur with melphalan, and no pulmonary fibrosis, which can be a complication of the use of busulfan.19-21 Twelve of 44 patients (27%) transplanted at age younger than 1 year and 5 of 26 (19%) at age more than 1 year required admission to an intensive care unit. As a comparison, 146 patients undergoing fludarabine with melphalan conditioning largely for PID have recently been analyzed (Rao et al, manuscript in preparation): although the survival (76%) of those transplanted aged less than 1 year was similar to that of the rest of the group, the incidence of serious events needing intensive care support was higher in this group: 17 of 30 patients (57%) needed intensive care management. Bacterial and viral infections were the most common reasons for transfer to intensive care (n = 11) followed by conditioning-related toxicity (n = 4) and T-cell lung sequestration (n = 2). The incidence of PICU admission in the rest of the group was 26 of 118 (22% P < .0001). There were significantly fewer less than 1 year olds admitted to intensive care in this study (12 of 44) compared with those who received melphalan (17 of 30; P = .0155).
Rates of GVHD were generally low using this protocol. Standard conditioning agents lead to tissue damage, which causes cytokine release, which is involved in the pathogenesis of GVHD and VOD. It has been suggested that the immunosuppressive properties of treosulfan coupled with less tissue destruction decrease the likelihood of a cytokine storm.8,22,23 This may explain why, unlike other reports using PBSCs with modified conditioning,24 the use of PBSCs in this study was not associated with an increased incidence of GVHD.
There were significantly more patients with 100% donor T-cell chimerism after the combination treosulfan with fludarabine rather than cyclophosphamide. T-cell chimerism is important for the majority of patients with PID apart from those with phagocytic disorders in whom secure donor myeloid engraftment was also achieved. Outcome in terms of survival with donor chimerism remained good regardless of stem cell source used. The number of patients receiving PBSCs was fairly small, and firm conclusions are difficult, but there was a suggestion that these patients were more likely to achieve full donor chimerism. Conversely, patients receiving cord blood stem cells in conjunction with serotherapy had a tendency to more mixed donor chimerism. Although donor myeloid chimerism was sufficiently high to cure most patients with CGD and WAS with all donor types/stem cell sources, where 100% donor chimerism might be preferred in all cell lineages (eg, WAS),25 then the use of PBSCs or full myeloablation with busulfan might be required.
By chance, there were more HLH and WAS patients in the treosulfan plus fludarabine group than the treosulfan plus cyclophosphamide group. Rather than reducing toxicity, the increase in HLH patients in the former group might have been expected to increase transplantation-related toxicity because of the propensity of HLH patients to autoinflammatory responses. It is doubtful that the inclusion of these 2 conditions would explain an increase in donor T-cell chimerism in the treosulfan plus fludarabine group.
Viral infection before transplantation and viral reactivation after transplantation is common in patients with primary immunodeficiency; the rate of viral reactivation in this study (26%) was similar to that of 33% found in a group of 33 children with PID who received fludarabine, melphalan, and alemtuzumab in a previous study.4
There are only 2 published reports concerning treosulfan pharmacokinetics in children,26,27 and further studies are warranted to determine whether pharmacokinetic studies could further increase efficacy and reduce toxicity. However, even without pharmacokinetic data, the combination of treosulfan and fludarabine appears to be a suitable choice of conditioning for HSCT in PID, regardless of age and stem cell source. Prospective studies are required comparing this combination with more conventional busulfan and cyclophosphamide, or perhaps with busulfan and fludarabine28-30 where early reports also indicate reduced transplantation-related toxicities. In this latter respect, long-term follow-up is required with the treosulfan cohort to monitor long-term toxicity, and in particular infertility, as the gonadal toxicity of busulfan is already well documented.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.S., K.R., A.R.G., and P.V. designed the study and wrote the paper; M.A.S. and A.R.G. analyzed data; and A.C., H.B.G., W.Q., G.D., N.G., Z.N., S.H., M.A., T.F., and P.A. contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary A. Slatter, Ward 3, Children's Bone Marrow Transplant Unit, Level 4, Great North Children's Hospital, Queen Victoria Rd, Newcastle upon Tyne, NE1 4LP, United Kingdom; e-mail: mary.slatter@nuth.nhs.uk.