Abstract
The understanding of the hierarchical organization of the human hematopoietic system is of major biologic and clinical significance. The validity of the conventional model in which hematopoiesis is solely maintained by a pool of multipotent long-term hematopoietic stem cells (LT-HSCs) has been recently challenged by several mouse studies. These new data point to the existence of a heterogeneous stem cell population that consists of distinct subsets of LT-HSCs, which include stem cells biased toward lineage-specific differentiation programs. This review attempts to discuss the balanced versus biased patterns of lineage output of human LT-HSCs gathered in 3 different gene therapy trials on the basis of vector integration site analysis by deep sequencing. The distribution of integration sites observed tends to support the validity of the revised model.
Introduction
For more than 50 years now, it has been known that the BM comprises a population of multipotent long-term hematopoietic stem cells (LT-HSCs) that are defined by their extensive self-renewal capacity and ability to differentiate into short-term HSCs and lineage-restricted progenitors and finally to give rise to all blood lineage. After massive proliferation and sequential differentiation, the progenitors produce all the terminally differentiated, mature blood cells. This perspective highlights recent data, gathered in animal model studies and clinical trials in humans, which may challenge this model and point to the possible coexistence of multipotent LT-HSCs with HSCs capable of sustained hematopoiesis although with lineage biases in the progeny they generate.
It is first important to examine the historical context in which the model of hematopoiesis solely derived from multipotent LT-HSCs arose. After the seminal observation of hematopoietic colony forming units in the spleen by Till and McCulloch in 1961,1 a range of approaches have shown convincingly that normal BM in humans and other animals contains rare cell populations capable of multilineage differentiation and with various degrees of self-renewal potential both in vitro (eg, granulocyte, erythroid, megakaryocyte, and macrophage colony forming unit, long-term culture-initiating cell [LTC-IC]) and after long-term reconstitution of myeloablated hosts.2-5 Gene marking experiments (that used retroviral vector integration) and tracking of spontaneous genetic mutations (eg, bcr-abl in chronic myeloid leukemia) have reinforced the hypothesis that all blood lineages are derived from the same HSC population.2,3,6-9 The combination of immunophenotypic purification of cell subsets with transplantation assays has further refined the classification and hierarchy of multipotent HSCs with various degrees of self-renewal capability. Taken as a whole, these data led to establishment of the well-accepted linear branching model of hematopoiesis.10,11 Without challenging the existence of the multipotent LT-HSC population, recent data generated in animal studies and human clinical trials now beg the following question: is normal and pathologic hematopoiesis maintained solely by multipotent LT-HSCs?
Until recently, the only available experimental approaches for probing the hierarchical composition of the hematopoietic system were mainly based on the use of limited numbers of cells (ie, limited dilution or purification or both) in transplantation experiments. Faced with (1) the lack of experimental clarity as to what constitutes extensive self-renewal capacity and (2) the absence of a well-defined method for isolating LT-HSCs, a general consensus was reached in which LT-HSCs were defined on the basis of their functional ability to perform long-term hematopoietic reconstitution in myeloablated hosts, rather than on their phenotype.2-4,12 A sustained output of ≥ 1% of all the circulating white blood cells (WBCs) for ≥ 4 months was set as the de facto “gold standard” definition of a multipotent LT-HSC population in mice.13
Several recently published studies have begun to challenge this model by providing convincing evidence that there is a certain degree of heterogeneity in lineage restriction among LT-HSCs14 and thus in their offspring.
Mice
After analyzing > 90 single-cell or clonal transplantations, Dykstra et al14 identified 4 subtypes of adult BM HSCs (referred to as α, β, γ, and δ). The α and β patterns are associated with robust self-renewal activity and stable perpetuation of the original repopulating pattern in their progeny. The α cells display an unbalanced daughter cell output toward myeloid cells and maintain their lineage bias through 3 serial transplantation generations, even under nonlimiting conditions. This emphasizes the high fidelity of the retained pattern in daughter HSCs. The γ cells are also multipotent and make a significant (> 1%) contribution to the myeloid lineage for ≥ 16 weeks. However, the subsequent decline of γ-cell self-renewal activity within 6-7 months suggests that this population represents an intermediate type of repopulating cell, thus invalidating the above-mentioned definition of a cell with HSC activity (ie, 1% of circulating WBCs for ≥ 4 months). Although δ cells are able to contribute to the circulating WBC pool for ≥ 16 weeks, this lymphoid-biased population lacks extensive self-renewal activity and show even less sustained myeloid potential than γ cells.
On the basis of repopulation kinetics in mice that received a transplant with limiting numbers of whole BM cells, another group of researchers suggested the existence of myeloid-biased, lymphoid-biased, and balanced HSCs that generate myeloid and lymphoid cells in the same ratio as seen in the blood of nonmanipulated mice.15-17 Furthermore, on the basis of differential responses to cytokines18 or transcription factor expression pattern,19 a wider variety of hematopoietic differentiation pathways than previously thought has been reported, and the heterogeneity of HSCs (in terms of differentiation potential) has become acknowledged.
As a consequence, the classic model of hematopoietic hierarchy (in which all mature blood circulating cells are the progeny of a single multipotent LT-HSC population) is being modified. It has become legitimate to propose that the normal murine multipotent LT-HSC pool is merely a heterogeneous cell population with predetermined but distinct differentiation potentials, despite a common, stable, self-renewal capacity (for a review, see Iwasaki and Akashi20 ). Certain classes of LT-HSCs thus have sustained lineage biases, and their frequency may change with the age of the individual.21 Finally, it has been suggested that the donor-derived myeloid/lymphoid ratio in reconstituted mice indicates the self-renewal potential of the transplanted HSCs.14,16
The heterogeneity of HSCs has been largely confirmed by the prospective isolation of cells for clonal analysis. The discovery that CD150 expression can be used to enrich for LT-HSCs with distinct reconstitution patterns and in vivo functions enabled the field to move faster toward their in-depth characterization.22 CD34− kit+ Sca1+ lineageneg (KSL) cells were subdivided into CD150high, CD150med, and CD150neg fractions, and the functions of these cells were compared at the clonal level with the use of single-cell transplantation and cultures. The Eaves group further confirmed and expanded these data by showing that murine HSCs can be distinguished on the basis of CD150 expression as follows: CD150highCD34neg KSL cells have the highest self-renewal potential, whereas in their reconstitution pattern the myeloid progeny largely predominates over lymphoid cells. Conversely, CD150negCD34neg KSL cells exhibit a low self-renewal potential, perform lymphoid-biased reconstitution, and lose their erythroid and megakaryocytic potentials.21,23,24 Note that the cells with the highest self-renewal capacity provide very-late-onset repopulation (> 4 months after transplantation).14,24,25 The lineage bias and latency thus challenge the current experimental definition of HSCs: multilineage reconstitution in primary recipients 12-16 weeks after transplantation. Goodell et al26 further showed that combining Hoechst dye efflux and CD150 labeling allows identification of HSC subsets with a gradient of self-renewal capacity. They also observed that the myeloid-biased subtype is characterized by the CD150+ lower side population KLS phenotype.26 However, despite many data suggesting that the highest self-renewal mice HSC capacity resides in the CD150 population, a recent study showed that signaling lymphocyte activation molecule markers do not select long-term reconstitution HSC population in humans and rhesus macaques.27
Altogether, these results emphasize the need to redefine the criteria for HSC identification, and, as a consequence, novel assays that permit rapid and efficient detection of all sorts of HSCs are awaited.
The clinical use of hematopoietic cell transplantation (HCT) over the past 40 years to treat a variety of cancers and inherited diseases would benefit from a better characterization of the HSC content in the transplant, and it would also help to understand the BM microenvironment more fully. Furthermore, it is unknown whether the ex vivo treatment of HSCs can modify their fate and lineage commitment. Future studies should clarify the physiologic and pathologic roles of these HSC subsets in the hematopoietic system of mice and establish whether it is possible to modify their expansion behavior ex vivo.
Humans
The novel model of HSC clonal diversity has important clinical implications in the fields of HCT and ex vivo stem cell gene therapy. The new model will influence both the choice of transduced cell populations for optimal gene transfer and the interpretation of patterns of hematopoietic reconstitution (whether lineage-biased or not). In addition, clinical trials of hematopoietic gene transfer provide a unique opportunity for probing the new model in human patients by scoring the lifetime and distribution of cells bearing an integrated vector. This can be performed by deep sequencing analysis of vector integration sites (ISs), followed by specific, quantitative PCR (qPCR)–based quantification of junctions between integrated vector and flanking chromosomal regions.
SCIDs comprise a heterogeneous group of genetic disorders characterized by a profound impairment of T-lymphocyte differentiation and a concurrent direct or indirect deficiency in B-cell immunity. This profound impairment of adaptive immunity makes it possible to perform HSCT in the absence of any conditioning regimen. This life-saving therapy can now be performed either with allogeneic, healthy HSCs from a donor (whether related or not to the affected child)28 or with autologous, gene-modified HSCs29,30 for ≥ 2 forms of SCID. Patients with SCID-X1 carry a mutation in the gene that encodes the γ common chain (γc) of hematopoietic cytokines. SCID mutation results in a very early block in T-cell differentiation (probably the earliest T-cell block of all the different SCIDs). Transplantation of allogeneic or autologous genetically modified cells (in unconditioned hosts) results in split chimerism in which all the T cells derive from the donor, whereas the myeloid compartment is derived from the host. The in vivo ability of the thymus to support T-cell development can be monitored with the use of the TCR excision circle (TREC) assay to quantifying the episomal DNA circles generated during rearrangement of gene segments encoding the TCR. Continuous production of this naive T-cell population in patients with SCID-X1 that received a transplant > 10 years ago (even in the absence of detectable donor-derived myeloid engraftment) is the criterion used to define stable thymic function.29,31,32
We have documented the sustained presence of circulating T cells with normal TREC levels in patients 10 years after allotransplantation or autotransplantation with gene-corrected hematopoietic cells.29,31 Transduced B lymphocytes and myeloid cells were no longer detected 6-10 years after gene transfer in the SCID-X1 gene therapy trial.29 In light of the HSC heterogeneity recently described in mice, is this evidence of sustained lymphoid-biased hematopoiesis in humans? A caveat could be that a small number of transduced nonbiased multipotent LT-HSCs may yield several vector-bearing myeloid cells below the detection threshold, whereas their equally small T-lymphoid output is amplified because of the lack of competing T-cell production originating from noncorrected cells. The persistence of TREC+ naive T cells and their reappearance in patients who had undergone chemotherapy 3-6 years after gene therapy strongly suggests that hematopoietic cells with self-renewal capacity persist and are performing sustained thymopoiesis. Moreover, this particular subpopulation is clearly unable to give rise to myeloid cells in vivo, given the fact that after chemotherapy-induced aplasia, no myeloid-positive γ-c cells could be detected. Finally, despite the low numbers of patients treated by gene therapy in comparison to the greater experience with allogeneic HSCT, > 10 years later, biologic and clinical results are highly comparable, being actually undistinguishable. Whether the ex vivo culture of HSCs in the presence of various cytokines is able to modify their fate and lineage commitment remains to be established.
Results from the transplantation of lentivirally transduced HSCs in the disease adrenoleukodystrophy (ALD) provide a seemingly contrasting picture.33 In both ALD gene therapy patients, ∼ 10% of both myeloid and lymphoid cells express the transgene stably > 3 years after the transplantation of autologous gene-modified CD34+ cells. Patients' myeloid and lymphoid cells share common ISs, according to deep sequencing analysis. Does this reflect the apparently balanced output of lentivirally marked myeloid and lymphoid cells and thus argue in favor of the previous model of nonbiased hematopoiesis? Given the polyclonality of hematopoietic reconstitution with the transduced cells observed here, one cannot rule out the coexistence of multipotent LT-HSCs and LT-HSCs with lineage-biased progeny. It is thus suggested that during the follow-up of patients treated for ALD, special attention should be paid to ISs that are not shared by all lineages.
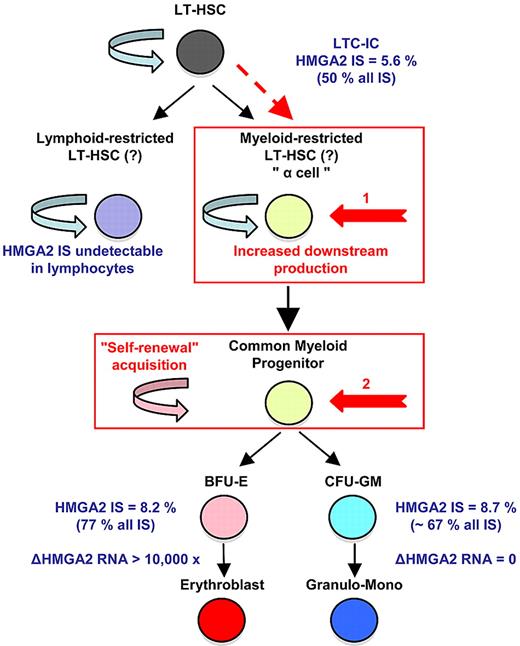
A recent lentiviral gene therapy trial in β-thalassemia (Figure 1)34 has shed additional light on this issue. In the study, a patient with severe, transfusion-dependent βE/β0-thalassemia was monitored for 3 years after a transplantation of autologous CD34+ cells transduced with a lentiviral vector expressing a normal, tagged β-globin. The clinical efficacy was remarkable, with high, sustained expression of the therapeutic globin gene and conversion to complete transfusion independence. Deep-sequencing analysis identified 24 ISs in both lymphoid and myeloid lineages. Cross-contamination was excluded with the use of qPCR to screen for specific provirus/flanking DNA junctions in highly purified cell subsets. Two of the most abundant and stable multilineage ISs (lymphoid and myeloid) lay within the Rfx3 and Zzef1 genes. Their contribution (according to the qPCR results) remained < 1% of whole blood cells.
Homeostatic myeloid-biased cell expansion. In the human β-thalassemia trial, most of therapeutic effect results from a dominant myeloid-biased cell clone. In this clone, the HMGA2 integration site (IS) is present in similar proportions among erythroblasts, granulocyte, monocyte, and LTC-IC cells, but not in lymphocytes (LT); whereas its expression is strictly erythroid specific. Therefore, the HMGA2 IS-initiating cell is probably a myeloid-biased LT-HSC19/a cell with increased downstream cell production (1, in red) or a common myeloid progenitor with acquired self-renewal capability (2, in red). From Cavazzana-Calvo et al.34
Homeostatic myeloid-biased cell expansion. In the human β-thalassemia trial, most of therapeutic effect results from a dominant myeloid-biased cell clone. In this clone, the HMGA2 integration site (IS) is present in similar proportions among erythroblasts, granulocyte, monocyte, and LTC-IC cells, but not in lymphocytes (LT); whereas its expression is strictly erythroid specific. Therefore, the HMGA2 IS-initiating cell is probably a myeloid-biased LT-HSC19/a cell with increased downstream cell production (1, in red) or a common myeloid progenitor with acquired self-renewal capability (2, in red). From Cavazzana-Calvo et al.34
Importantly, there was dominance over time of an IS at the high-mobility group AT-hook 2 (HMGA2) locus in both granulocytes and erythroblasts. However, the HMGA2 IS was not detected at all in lymphocytes. In myeloid cells, a single vector had integrated within the third intron of the HMGA2 gene. The qPCR analysis showed that ∼ 45% (plateau) of the vector-bearing WBCs were positive for the HMGA2 IS. However, nontransduced cells continued to predominate, so that < 10% circulating cells and clonogenic progenitors were HMGA2 IS positive. Three years after transplantation, the contribution of the HMGA2 IS clone has remained stable below this threshold, without any detectable hematopoietic abnormalities or breach of homeostasis. Importantly, the proportion of LTC-ICs positive for the HMGA2 IS was 5.6%, accounting for ∼ 50% of the vector-bearing LTC-ICs. Overexpression of HMGA2 was found to be restricted to erythroid cells; this is consistent with the erythroid-specific transcriptional control system of the integrated vector (ie, the β-locus control region).
The presence of the HMGA2 IS in similar proportions of erythroblasts, granulocytes, and LTC-IC subsets but not in lymphocytes prompted the suggestion that hematopoiesis (deriving from the transduced clone-initiating cell) is myeloid-biased in this patient. The considerable departure from polyclonal distribution exhibited by the HMGA2 IS clone (> 50% of all ISs in myeloid cells, including LTC-ICs) can be explained by (1) a stochastic event with a low initial number of transduced HSCs and strict erythroid-specific HMGA2 expression driven by vector transcriptional activation, (2) differential expansion/production of myeloid-biased HSCs triggered by dysregulated expression of HMGA2, or (3) acquisition of myeloid-biased stemness by dysregulated expression of HMGA2 (Figure 1).
Conclusions
A comprehensive model of human hematopoiesis lags behind what has been established for the mouse, because of the lack of cell surface markers to distinguish pure subpopulations of human hematopoietic cells and the absence of assays to detect multilineage outputs from single cells with high-cloning efficiency. Progress has been made recently by Dick et al35,36 who have used immune-compromised mouse strains such as the NOD/L7-SCID/IL2Rγnull triple-knockout mice to sustain a greater degree of marrow engraftment by human cells than all other strains. They have thus been able to show that human hematopoiesis does not follow, in contrast to the mouse, a rigid model of myeloid-lymphoid segregation.35,36 In light of these data together with the advent of deep sequencing of vector IS, in-depth studies should be designed to resolve the topical question we have attempted to review here of balanced versus biased lineage output of human LT-HSCs. This central biologic and clinical issue should be further clarified by the analysis of human gene therapy trial outcomes and nonhuman primate studies. As a corollary and until this issue is resolved, it is difficult to answer the simple question often raised in the context of these trials: have we transduced a “true” HSC?
Acknowledgments
We thank patients' families for their continuous support of our research, as well as the medical and nursing staff of the Unité d'Immunologie et d'Hématologie Pédiatriques, Hôpital des Enfants Malades, for patient care. We thank Inserm research units 768, 745, and 962; CEA; bluebird bio (France and US); and Isabelle Andre-Schmutz, Laure Coulombel, and C. Eaves for their constant involvement in our work and fruitful discussions and collaborations.
This work was funded by grants from Inserm, National Institutes of Health (grant HL090921 to P.L.), the Association Française contre les Myopathies (AFM), the EC Consert (contract 005242), the EC CELL-PID (grant agreement 261387), the Inherinet (contract QLK3-CT-2001-00427), the Agence Nationale de la Recherche (ANR; grant 05-MRAR-004), the Progamme Hospitalier de Recherche Clinique (PHRC AOM 08064-P071204) from the French Ministry of Health, Assistance Publique-Hôpitaux de Paris, the Fondation de l'Avenir, and the Fondation pour la Recherche Médicale (FRM).
National Institutes of Health
Authorship
Contribution: M.C.-C. and P.L. wrote the paper; S.H.-B.-A. made and coordinated revisions and prepared the final draft; and A.F., F.D.B., and E.P. participated in the discussion and revisions together with M.C.-C., P.L., and S.H.-B.-A.
Conflict-of-interest disclosure: E.P. and P.L. have declared a financial interest in the company bluebird bio (Cambridge, MA). The remaining authors declare no competing financial interests.
Correspondence: Marina Cavazzana-Calvo, Department of Biotherapy, Hopital Necker Enfants Malades, 149 rue de Sevres, F-75015 Paris, France; e-mail: m.cavazzana@nck.aphp.fr; or Philippe Leboulch, Institute of Emerging Diseases and, Innovative Therapies, CEA Fontenay Bat 60, 18 route du Panorama BP-6, 92265 Fontenay-aux-Roses, France; e-mail: pleboulch@rics.bwh.harvard.edu.