Abstract
Pediatric immune thrombocytopenia (ITP) is usually self-limited. However, approximately 20% of children develop chronic ITP, which can be associated with significant morbidity because of long-term immunosuppression and splenectomy in refractory cases. To explore the molecular mechanism of chronic ITP compared with acute ITP, we studied 63 pediatric patients with ITP. Gene expression analysis of whole blood revealed distinct signatures for acute and chronic ITP. Oxidative stress–related pathways were among the most significant chronic ITP-associated pathways. Overexpression of VNN1, an oxidative stress sensor in epithelial cells, was most strongly associated with progression to chronic ITP. Studies of normal persons demonstrated VNN1 expression in a variety of blood cells. Exposure of blood mononuclear cells to oxidative stress inducers elicited dramatic up-regulation of VNN1 and down-regulation of PPARγ, indicating a role for VNN1 as a peripheral blood oxidative stress sensor. Assessment of redox state by tandem mass spectrometry demonstrated statistically significant lower glutathione ratios in patients with ITP versus healthy controls; lower glutathione ratios were also seen in untreated patients with ITP compared with recently treated patients. Our work demonstrates distinct patterns of gene expression in acute and chronic ITP and implicates oxidative stress pathways in the pathogenesis of chronic pediatric ITP.
Introduction
Immune thrombocytopenia (ITP) is an immune-mediated hematologic disorder in which increased platelet destruction and decreased platelet production lead to thrombocytopenia and, thus, mucocutaneous bleeding. The pathophysiology of ITP has been extensively investigated. It is generally accepted that a complex multifactorial immune dysregulation, loss of immune tolerance, and generation of platelet autoantibodies account for the primary mechanism. Nevertheless, the underlying pathogenic events leading to the breakdown of immune tolerance in ITP remain elusive.1-3 Molecular mimicry and epitope spreading theories provide plausible explanations for the appearance of autoantibodies; however, most patients have antibodies against multiple platelet surface glycoproteins at the time the disease becomes clinically evident, and the factors that initiate autoantibody production as well as the reason for derivation of cryptic epitopes in vivo are still unknown.4 Protein modification as a result of free radical–-mediated oxidative damage has been shown to elicit antibodies in several autoimmune diseases, including systemic lupus erythematosus, type 1 diabetes mellitus, rheumatoid arthritis, and systemic sclerosis.5 More recently, evidence from a murine model also confirmed the role of reactive oxygen species (ROS) in triggering the autoimmune reaction in autoimmune hemolytic anemia.6 Griffiths7 pointed out in his review that, although low levels of oxidants are important as signaling molecules, oxidant overproduction in the absence of adequate antioxidant defense may cause irreversible changes to biomolecules and contribute to disease progression; generation of antigenic determinants by ROS and reactive nitrogen species may contribute to epitope spreading in autoimmunity.
In children, ITP is typically preceded by a viral illness; and although the majority of patients resolve spontaneously within 6 months of diagnosis, approximately 20% of patients develop chronic disease. The underlying mechanism distinguishing self-limited acute ITP from chronic ITP is unknown. Knowledge of these differences would not only contribute to a better understanding of the pathogenesis of ITP but also identify potential targets for therapeutic interventions in the group at risk for chronic ITP.
In the present study, we used whole transcriptome cDNA microarray analysis of peripheral blood as a tool to analyze the gene expression profiles of patients with acute and chronic ITP. Oxidative stress–related pathways were revealed to be among the most significantly perturbed canonical pathways in chronic ITP, and this was a distinguishing feature of chronic versus acute disease. Of particular interest was the increased expression of the gene vanin-1 (VNN1) in patients with chronic ITP during the acute phase and patients with treatment-resistant chronic ITP. VNN1 is known to play a role in oxidative stress response and license the production of inflammatory mediators in murine intestinal epithelial cells by antagonizing peroxisome proliferator-activated receptor-γ (PPARγ).8 PPARγ is known to be an anti-inflammatory checkpoint in many inflammatory settings and various cell types.9 We demonstrate the expression distribution of VNN1 in the major subsets of human blood cells and, furthermore, a similar role of VNN1 as an oxidative stress sensor in human peripheral blood mononuclear cells (PBMCs). Thus, VNN1 in particular and oxidative stress pathways in general appear to be associated with the development of chronic ITP in children. The increased ratio of reduced to oxidized glutathione (GSH/GSSG), which is indicative of oxidative stress, was also observed in patients with chronic ITP compared with healthy controls.
Methods
Patient enrollment and blood specimen collection
Pediatric patients (age < 18 years) diagnosed as having primary ITP (platelet count < 150 000/μL), as well as pediatric controls (normal platelet count and no concurrent illnesses or medications at the time of blood draw) were enrolled into the study with an Institutional Review Board–approved consent in accordance with the Declaration of Helsinki. Although the new diagnostic ITP guidelines10 were recently published, our study was well advanced before these guidelines appeared; and for purposes of maintaining consistency, we used conventional ITP criteria throughout this study. For microarray analysis and real-time polymerase chain reaction (PCR) validation, 2.5-mL whole blood samples (8 samples from patients with self-limited acute ITP and active disease, 14 samples from patients with chronic ITP and active disease after 6 months of diagnosis, 7 samples from patients with chronic ITP and active disease within 6 months of diagnosis, 6 samples from patients with resolved acute ITP, and 5 samples from healthy pediatric controls) were collected into PAXgene Blood RNA tubes (PreAnalytiX). Patients with acute ITP were followed for at least 6 months to determine clinical outcomes. For glutathione measurement, whole blood specimens from 5 patients with self-limited acute ITP and active disease, 4 patients with chronic ITP within 6 months of diagnosis, 2 patients with ITP within 6 months of diagnosis with outcome status pending, 16 patients with chronic ITP and active disease after 6 months of diagnosis, and 15 pediatric healthy controls were collected in ethylenediaminetetraacetic acid anticoagulant tubes and kept on ice. Table 1 shows the clinical characteristics of patients in the study.
Microarray procedure and data analysis
Total RNA was isolated from whole blood using the PAXgene Blood RNA System (PreAnalytiX). The RNA from patients with ITP and controls, along with Human Universal Reference RNA (Stratagene), were linearly amplified, labeled, and hybridized to human cDNA microarrays (Stanford Functional Genomics Facility) using a previously published protocol.11 The data were then submitted to the Stanford Microarray Database for further analysis (www.smd.stanford.edu). The microarray data of this study have been deposited in Gene Expression Omnibus (National Center for Biotechnology Information) and are accessible through GEO Series accession number GSE23754 (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE23754).
The microarray data were retrieved from the Stanford Microarray Database, and the array elements with good quality were selected (the detailed filter criteria were described in the supplemental Data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Two-class unpaired significance analysis of microarrays (SAM) was performed to identify genes, which were differentially expressed in chronic ITP compared with self-limited acute ITP. To generate unsupervised clustering images using differentially expressed genes, the gene expression results of patients with self-limited acute ITP, patients with chronic ITP, and normal controls were retrieved by IMAGE clone ID (including only putative genes) at a q value (false positive rate) of 0, and the images were created with the Gene Tree View program (Version 1.60, November 2002, University of California).
Pathway analysis
Pathway analysis of transcripts with elevated expression in chronic ITP was performed using Ingenuity Pathways Analysis (IPA; Version 8.5; www.ingenuity.com) and aberrant functional networks and canonical pathways were recognized. The P value associated with a function or a pathway is a measure of the likelihood that the association between a set of focus genes in the experiment and a given process or pathway is the result of random chance; in general, a P value (calculated using the right-tailed Fisher exact test) < .05 indicates a statistically significant, nonrandom association. In this analysis, the Ingenuity Pathways Knowledge Base (genes + endogenous chemicals) was chosen as the reference set. Both direct and indirect relationships were included, and only molecules of human species were considered.
Quantitative real-time PCR validation
Unamplified RNA samples isolated from whole blood were reverse-transcribed to cDNA, real-time PCR using TaqMan gene expression assays was performed on a 7900HT real-time PCR System. The relative quantification method was used per the manufacturer's instructions (www.appliedbiosystems.com; document no. 040980), and standards were prepared from human bone marrow, brain, or testes RNA (Clontech Laboratories). Detailed information on TaqMan assays and the corresponding standards used in each experiment are listed in Table 2. Samples were run in triplicate and then normalized to the housekeeping gene GAPDH.
For the validation of differentially expressed genes between self-limited acute ITP and chronic ITP, predeveloped TaqMan assays targeting 6 genes (RAPGEF2, NCOA1, SORL1, ACOX1, GNAQ, and DDEF1) were performed in 18 samples for which sufficient amounts of RNA were available (5 self-limited acute ITP and 13 chronic ITP samples). For the validation of differentially expressed genes between self-limited acute ITP and chronic ITP during the acute phase (referred to as “progression to chronic ITP” in the tables and figures), predeveloped TaqMan assays targeting VNN1 and AVIL (Advillin) were used in 8 self-limited acute ITP and 7 progression to chronic ITP samples. VNN1 expression was also validated with the same TaqMan assay in 8 self-limited acute ITP and 6 patients with treatment-resistant chronic ITP.
Expression distribution of VNN1 in subsets of human blood cells
CD15+ granulocytes were sorted from 2 blood donors by magnetic-activated cell sorting with CD15 microbeads (Miltenyi Biotec). CD20+ B cells, CD14+ monocytes, CD3+CD4+ T cells, and CD3+CD8+ T cells were sorted from 3 blood donors by fluorescence-activated cell sorting. Platelets (> 99.9% pure) from 10 blood donors were obtained from the Stanford Blood Center. Total RNA was isolated and reverse-transcribed to cDNA. Real-time quantitative PCR was performed as described in “Quantitative real-time PCR validation” using predeveloped VNN1 and GAPDH TaqMan assays (Applied Biosystems), followed by normalization of VNN1 expression values to GAPDH.
VNN1 and PPARγ expression changes after oxidative stressor treatments in human PBMCs in vitro
Buffy coats from healthy blood donors were obtained from the Stanford Blood Center, and PBMCs were isolated by Ficoll-Paque PLUS gradient centrifugation (GE Healthcare). The fresh PBMCs were treated with sodium arsenite (Sigma-Aldrich) at the final concentration of 5μM or lipopolysaccharide (LPS, Sigma-Aldrich) at the final concentration of 10 ng/mL and cultured in RPMI 1640 medium supplemented with 10% normal human serum, 100 U/mL penicillin, 100 mg/mL streptomycin and l-glutamine in a 37°C humidified incubator with 5% CO2. The treated cells and the nontreated control cells were harvested 12 hours after treatment. The total RNA was extracted and reverse-transcribed to cDNA. Real-time quantitative PCR was performed as described “Quantitative real-time PCR validation” using predeveloped VNN1, PPARγ, and GAPDH TaqMan assays (Applied Biosystems); VNN1 and PPARγ expression values were normalized to GAPDH.
Glutathione level measurement in patients with ITP and controls
The levels of glutathione (GSH, reduced form) and glutathione disulfide (GSSG, oxidized form) in the whole blood of each subject were determined using a liquid chromatography–tandem mass spectrometry–based procedure modified from that of Norris et al.12
Statistical analysis
SAM was conducted for analysis of microarray data to identify differentially expressed genes. The Mann-Whitney test was used in the analysis of real-time PCR validation data. Unpaired t test was conducted for the comparison of glutathione levels in patients with ITP and controls, and a Bonferroni correction was performed to obtain an overall significance level < .05. Paired t test was carried out for the analysis of differences in GSH/GSSG ratios in cells with or without oxidative stressor treatment.
Results
Identification of differentially expressed genes between acute and chronic ITP
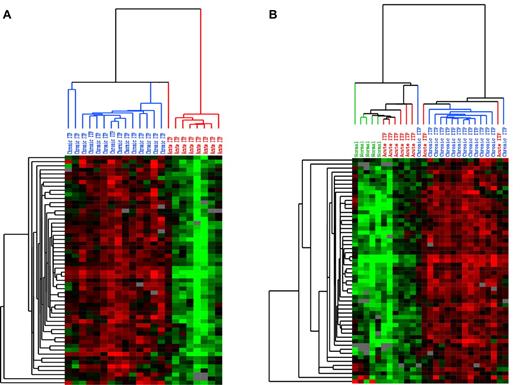
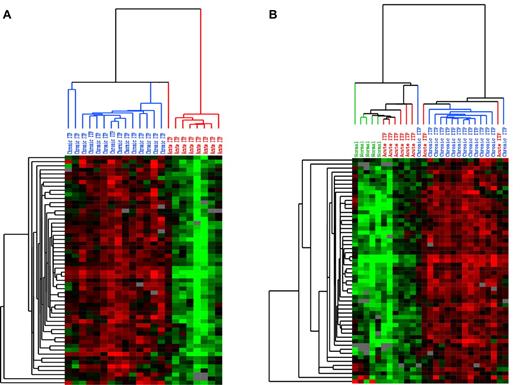
Whole blood from patients with acute and chronic ITP was subjected to cDNA microarray analysis. At a q value of < 5%, 535 transcripts were revealed to be overexpressed and 2 transcripts were underexpressed in chronic ITP (Supplemental data). At the highest significance level of q value at 0, 69 transcripts were overexpressed in chronic ITP; screened with the filter setting described in the Supplemental data, 57 biosequence IDs remained for generating unsupervised clustering image files. The clustering results are shown as heat maps in Figure 1. Figure 1A shows the hierarchical clustering of self-limited acute ITP and chronic ITP: the selected transcripts separate into 2 distinct subgroups. To learn whether either expression pattern is similar to that of healthy persons, we added a healthy control group and used the same set of transcripts for unsupervised clustering. As shown in Figure 1B, 2 expression clusters were revealed. The cluster on the left includes all of the healthy controls and the majority of patients with self-limited acute ITP, whereas the cluster on the right contains mostly patients with chronic ITP. The expression levels of these transcripts are lowest in normal controls, highest in patients with chronic ITP, and slightly elevated in patients with self-limited acute ITP.
Unsupervised hierarchical clustering pattern of expression data. Each row represents a single transcript, and each column represents a single sample. Red represents greater expression; green, lower expression; and gray, missing data. In the sample dendrogram, red represents self-limited acute ITP samples; blue, chronic ITP samples; and green, normal controls. (A) Unsupervised clustering of self-limited acute and chronic ITP samples using the transcripts with significantly elevated expression in chronic ITP at the SAM q value of 0 (using clones corresponding to putative genes and > 80% good data, 57 biosequences passed the filters). Two distinct clusters of samples are revealed: the one on the left contains predominantly chronic ITP samples, whereas the one on the right contains only self-limited acute ITP samples. (B) Unsupervised clustering of self-limited acute ITP, chronic ITP, and healthy controls using the same set of transcripts and the same filter settings (57 biosequences passed filters). The expression level of these transcripts presented a low-to-high gradual transition from normal to chronic ITP. While positioned in the middle, the expression pattern of self-limited acute ITP has greater similarity to that of healthy controls.
Unsupervised hierarchical clustering pattern of expression data. Each row represents a single transcript, and each column represents a single sample. Red represents greater expression; green, lower expression; and gray, missing data. In the sample dendrogram, red represents self-limited acute ITP samples; blue, chronic ITP samples; and green, normal controls. (A) Unsupervised clustering of self-limited acute and chronic ITP samples using the transcripts with significantly elevated expression in chronic ITP at the SAM q value of 0 (using clones corresponding to putative genes and > 80% good data, 57 biosequences passed the filters). Two distinct clusters of samples are revealed: the one on the left contains predominantly chronic ITP samples, whereas the one on the right contains only self-limited acute ITP samples. (B) Unsupervised clustering of self-limited acute ITP, chronic ITP, and healthy controls using the same set of transcripts and the same filter settings (57 biosequences passed filters). The expression level of these transcripts presented a low-to-high gradual transition from normal to chronic ITP. While positioned in the middle, the expression pattern of self-limited acute ITP has greater similarity to that of healthy controls.
Functional network and canonical pathway analysis of overexpressed genes associated with chronic ITP
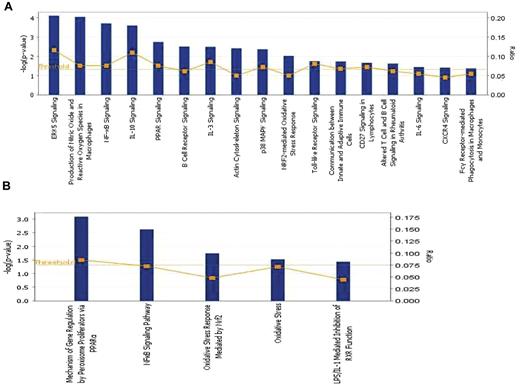
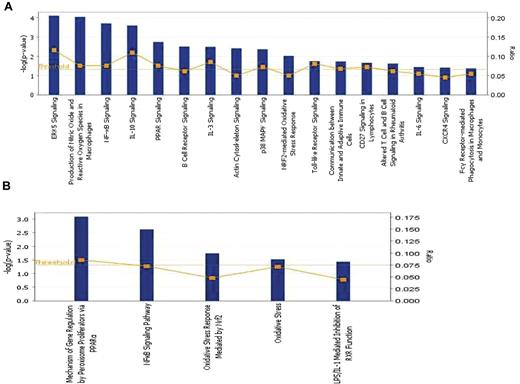
The overexpressed genes (q < 5%) associated with chronic ITP were analyzed using IPA, Version 8.5 (Ingenuity Systems). The bar chart in Figure 2A shows the statistically significant canonical pathways with biologic relevance, including 2 direct oxidative stress–related pathways: production of nitric oxide and ROS in macrophages and NRF2-mediated oxidative stress response. Other signaling pathways with high statistical significance include extracellular signal-regulated kinase 5, nuclear factor κ-B (NF-κB) light-chain-enhancer of activated B cells, interleukin-10, and PPAR. The significantly activated canonical pathways and focus genes are listed in Table 3 and classified into 8 major categories: intracellular and second messenger signaling, cellular immune response, cytokine signaling, humoral immune response, nuclear receptor signaling, organismal growth and development, apoptosis, and cellular stress and injury. The majority of the pathways fall into either the cellular or the humoral immune response category, indicating a predominant involvement of the immune system in the disease process. The overexpressed genes participating in the 2 oxidative stress–related pathways as well as 4 other highly significant canonical pathways were used to create the pathway graph shown in supplemental Figure 1; the connections between the molecules are represented by lines. Toxicity lists are lists of molecules known to be involved in a particular type of toxicity, and IPA scored the dataset against the known lists. As shown in Figure 2B, among the 5 toxicity lists that were significantly associated with chronic ITP, 2 were oxidative stress–related sets, in addition to PPAR and NF-κB signaling pathways.
Pathway analysis by IPA. (A) Significantly altered canonical pathways associated with chronic ITP compared with self-limited acute ITP. A total of 535 transcripts had a q value less than 5% by SAM analysis. These transcripts were mapped to 338 gene IDs in the IPA database and then analyzed by the IPA software to identify the most significantly perturbed canonical pathways. The canonical pathways included in this analysis are shown along the x-axis of the bar chart. The y-axis indicates the statistical significance on the left. Calculated using the right-tailed Fisher exact test, the P value indicates which biologic annotations are significantly associated with the input molecules relative to all functionally characterized mammalian molecules. The yellow threshold line represents the default significance cutoff at P = .05. (B) Significantly altered toxicity lists associated with chronic ITP. These lists have been grouped based on critical biologic processes and toxicologic responses. Only 5 toxicology lists reached statistical significance: PPAR, NF-κB, and oxidative stress pathways are predominant.
Pathway analysis by IPA. (A) Significantly altered canonical pathways associated with chronic ITP compared with self-limited acute ITP. A total of 535 transcripts had a q value less than 5% by SAM analysis. These transcripts were mapped to 338 gene IDs in the IPA database and then analyzed by the IPA software to identify the most significantly perturbed canonical pathways. The canonical pathways included in this analysis are shown along the x-axis of the bar chart. The y-axis indicates the statistical significance on the left. Calculated using the right-tailed Fisher exact test, the P value indicates which biologic annotations are significantly associated with the input molecules relative to all functionally characterized mammalian molecules. The yellow threshold line represents the default significance cutoff at P = .05. (B) Significantly altered toxicity lists associated with chronic ITP. These lists have been grouped based on critical biologic processes and toxicologic responses. Only 5 toxicology lists reached statistical significance: PPAR, NF-κB, and oxidative stress pathways are predominant.
Association of VNN1 overexpression with disease progression during the acute stage of chronic ITP and treatment resistance in chronic ITP
When we used 2-class unpaired SAM to analyze the expression profiles of patients with self-limited acute ITP and patients with chronic ITP during the acute phase, 2 overexpressed genes were revealed at the q value of 0: VNN1 (up-regulated 3.88-fold) and AVIL (up-regulated 2.15-fold). When SAM was applied to the expression profiles of self-limited acute ITP and treatment-resistant chronic ITP samples, VNN1 expression was elevated again in the latter group with a q value of less than 5%. Based on this dataset, VNN1 is the only gene that was detected to be overexpressed in both patients with progression to chronic ITP and patients with treatment-resistant chronic ITP.
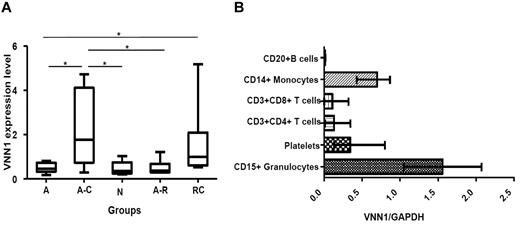
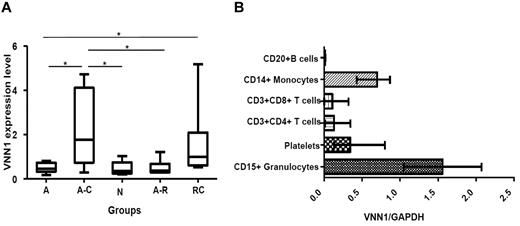
Next, we used quantitative real-time PCR to measure VNN1 expression in the original 3 patient groups (self-limited acute ITP, progression to chronic ITP, and treatment-resistant chronic ITP) as well as 2 additional groups (healthy controls and resolved acute ITP). The results, presented in Figure 3A, show that the increased expression of VNN1 in the progression to chronic ITP group (P = .0093) and treatment-resistant chronic ITP group (P = .0127) was validated by real-time PCR. Interestingly, VNN1 expression in the progression to chronic ITP group was also significantly higher than the normal control or resolved acute ITP groups, whereas the VNN1 expression in the latter 2 groups was comparable with that of the self-limited acute ITP group.
VNN1 expression. (A) Real-time PCR validation of VNN1 expression in different ITP groups and healthy controls. Five groups of samples were included in the validation: self-limited acute ITP (A, n = 8), chronic ITP during the acute phase (A-C, n = 7), healthy control (N, n = 5), resolved acute ITP (A-R, n = 6), and chronic ITP resistant to multiple treatments (RC, n = 6). The nonparametric Mann-Whitney 2-tailed test was performed in the statistical analysis. At the transcriptional level, VNN1 expression in the A-C group is significantly higher compared with the A (P = .0093), N (P = .0177), and A-R (P = .0221) groups; VNN1 expression in the RC group is significantly higher than the A group (P = .0127). The upper and lower limits of each box represent the 75th and 25th percentiles, respectively; the horizontal lines inside the box represent medians; the whiskers represent extreme measurements. (B) Expression distribution of VNN1 in subsets of human blood cells. Purified CD15+ granulocytes, CD20+ B cells, CD14+ monocytes, CD3+CD4+ T cells, CD3+CD8+ T cells, and platelets were obtained from blood donors as described in “Expression distribution of VNN1 in subsets of human blood cells.” The relative expression level of VNN1 determined by real-time PCR in normal human adults is high in granulocytes and monocytes and moderate in platelets. VNN1 expression is low in CD4+ T cells, CD8+ T cells, and B cells. The range and mean of normalized VNN1 expression value in each cell subset are shown.
VNN1 expression. (A) Real-time PCR validation of VNN1 expression in different ITP groups and healthy controls. Five groups of samples were included in the validation: self-limited acute ITP (A, n = 8), chronic ITP during the acute phase (A-C, n = 7), healthy control (N, n = 5), resolved acute ITP (A-R, n = 6), and chronic ITP resistant to multiple treatments (RC, n = 6). The nonparametric Mann-Whitney 2-tailed test was performed in the statistical analysis. At the transcriptional level, VNN1 expression in the A-C group is significantly higher compared with the A (P = .0093), N (P = .0177), and A-R (P = .0221) groups; VNN1 expression in the RC group is significantly higher than the A group (P = .0127). The upper and lower limits of each box represent the 75th and 25th percentiles, respectively; the horizontal lines inside the box represent medians; the whiskers represent extreme measurements. (B) Expression distribution of VNN1 in subsets of human blood cells. Purified CD15+ granulocytes, CD20+ B cells, CD14+ monocytes, CD3+CD4+ T cells, CD3+CD8+ T cells, and platelets were obtained from blood donors as described in “Expression distribution of VNN1 in subsets of human blood cells.” The relative expression level of VNN1 determined by real-time PCR in normal human adults is high in granulocytes and monocytes and moderate in platelets. VNN1 expression is low in CD4+ T cells, CD8+ T cells, and B cells. The range and mean of normalized VNN1 expression value in each cell subset are shown.
Expression of VNN1 in human blood cells and the reciprocal expression changes of VNN1 and PPARγ in response to oxidative stress inducers
Because little is known about the expression and function of VNN1 in human blood cells, we examined its expression at the transcription level in platelets and the major white blood cell subsets. As shown in Figure 3B, the relative expression level of VNN1 is high in CD15+ granulocytes and CD14+ monocytes, moderate in platelets, and low in CD3+CD8+ T cells, CD3+CD4+ T cells, and CD20+ B cells.
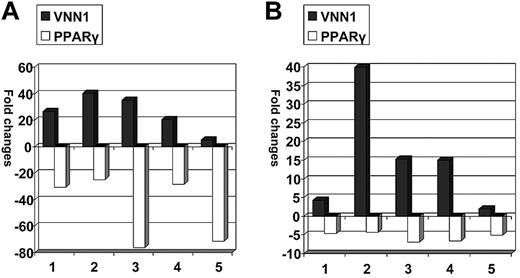
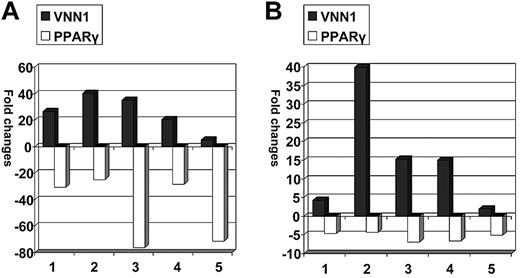
We subsequently asked whether VNN1 overexpression also correlates with oxidative stress in human blood cells. Low doses of LPS (10 ng/mL) and sodium arsenite (5μM), which are both oxidative stress inducers,13-15 were used to treat human PBMCs. The transcriptional expression fold changes of VNN1 and PPARγ in treated cells compared with nontreated cells after 12 hours of treatment are shown in Figure 4. In LPS-treated cells, VNN1 expression increased 5.1- to 40.2-fold, whereas PPARγ expression decreased 24.8- to 71.6-fold; in sodium arsenite-treated cells, VNN1 expression increased 1.9- to 39.8-fold, whereas PPARγ expression decreased 4.3- to 6.9-fold. To ensure that oxidative stress was indeed present after treatment, the glutathione (reduced form) to glutathione disulfide (oxidized form) ratio (GSH/GSSG), a parameter of cellular redox status, was measured by the highly sensitive and specific liquid chromatography-tandem mass spectrometry method in 4 other PBMC samples treated in the same way. There was a statistically significant decrease of the GSH/GSSG ratio in cells treated with either LPS (P = .04) or sodium arsenite (P = .01) compared with untreated cells at 12 hours, indicating the presence of treatment-induced oxidative stress.
Expression changes of VNN1 and PPARγ in response to oxidative stress inducers. PBMC samples were treated with LPS or sodium arsenite and cultured for 12 hours, after which the cells were harvested and VNN1 and PPARγ expression was measured by real-time PCR. The expression fold changes were calculated by dividing the normalized VNN1 and PPARγ expression values in treated cells by values in nontreated cells. (A) After LPS treatment, VNN1 increased 5- to 40-fold, whereas PPARγ decreased 25- to 76-fold. (B) After sodium arsenite treatment, VNN1 increased 2- to 40-fold, whereas PPARγ decreased 4- to 7-fold.
Expression changes of VNN1 and PPARγ in response to oxidative stress inducers. PBMC samples were treated with LPS or sodium arsenite and cultured for 12 hours, after which the cells were harvested and VNN1 and PPARγ expression was measured by real-time PCR. The expression fold changes were calculated by dividing the normalized VNN1 and PPARγ expression values in treated cells by values in nontreated cells. (A) After LPS treatment, VNN1 increased 5- to 40-fold, whereas PPARγ decreased 25- to 76-fold. (B) After sodium arsenite treatment, VNN1 increased 2- to 40-fold, whereas PPARγ decreased 4- to 7-fold.
Real-time PCR validation of genes associated with chronic ITP
Real-time PCR validation of the expression of 6 genes (RAPGEF2, NCOA1, SORL1, ACOX1, GNAQ, and DDEF1) in 18 specimens demonstrated statistically significant P values in all cases, as shown in Table 2.
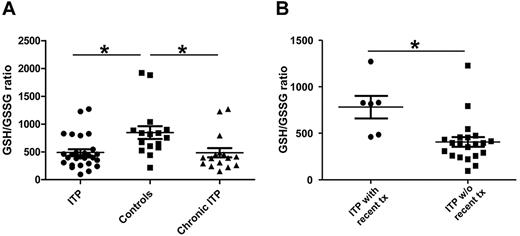
Unbalanced redox state in patients with ITP compared with control subjects
The ratio of reduced (GSH) to oxidized (GSSG) glutathione is an important parameter of redox status. GSH depletion, as indicated by a low GSH/GSSG ratio, is a hallmark of oxidative stress.16 The GSH/GSSG ratio was calculated for each sample. As shown in Figure 5A, the whole blood GSH/GSSG ratios of the ITP group (both patients with acute ITP and patients with chronic ITP, P < .020) as well as the chronic ITP group (P < .020) were both significantly lower than that of the healthy controls, and the overall Bonferroni-corrected P value < .05, indicating a higher oxidative stress state. The difference between the self-limited acute ITP and control group did not reach statistical significance (P = .0545). Another interesting finding, as shown in Figure 5B, is that patients with ITP with recent treatment (within one month of sample collection) had significantly higher GSH/GSSG ratio compared with those without recent treatment (P = .0035). Thus, evidence of increased oxidative stress is exhibited in patients with ITP in general and patients with chronic ITP in particular; patients with ITP with recent treatment (majority were treated with steroids) had ameliorated oxidative stress level.
GSH/GSSG ratio in patients with ITP and controls. The concentrations of GSH and GSSG in whole blood were measured in patients with ITP and pediatric healthy controls; thereafter, the GSH/GSSG ratio was calculated for each sample and the unpaired t test was performed to compare the GSH/GSSG ratio between groups (mean ± SEM). (A) The whole blood GSH/GSSG ratio is significantly lower in patients with ITP in general (P < .02), indicating a higher oxidative stress state compared with healthy controls. The GSH/GSSG ratio is also significantly lower in chronic patients with ITP (P < .02) compared with healthy controls. The overall Bonferroni-corrected P value is < .05. (B) The GSH/GSSG is significantly higher in patients with recent treatments (within 1 month of sample collection) compared with patients without recent treatments (P = .0035).
GSH/GSSG ratio in patients with ITP and controls. The concentrations of GSH and GSSG in whole blood were measured in patients with ITP and pediatric healthy controls; thereafter, the GSH/GSSG ratio was calculated for each sample and the unpaired t test was performed to compare the GSH/GSSG ratio between groups (mean ± SEM). (A) The whole blood GSH/GSSG ratio is significantly lower in patients with ITP in general (P < .02), indicating a higher oxidative stress state compared with healthy controls. The GSH/GSSG ratio is also significantly lower in chronic patients with ITP (P < .02) compared with healthy controls. The overall Bonferroni-corrected P value is < .05. (B) The GSH/GSSG is significantly higher in patients with recent treatments (within 1 month of sample collection) compared with patients without recent treatments (P = .0035).
Discussion
Primary ITP of childhood is often associated with a preceding viral illness or environmental trigger, has a seasonal pattern of presentation, and is a self-limited disease in the majority of patients.17 However, an unpredictable subset of patients with ITP will have a prolonged course of thrombocytopenia, often requiring immunosuppression and even splenectomy. The differences between patients with acute and chronic ITP at the transcriptomic level have not been studied, nor is the pathogenic mechanism underlying the predisposition for chronic disease clearly understood. In the hopes of addressing this question at the molecular level, we analyzed the whole blood gene expression profiles of patients with self-limited acute ITP and patients with chronic ITP. In doing so, we identified signaling and metabolic pathways that were highly associated with chronic ITP; those pathways related to oxidative stress were particularly prominent. Although the findings need to be confirmed in an expanded number of pediatric patients with ITP and possibly in adult patients with ITP as well, the present data provide some new perspectives on the biologic processes of chronic ITP.
Oxidative modification of proteins has been documented to elicit antibodies in a variety of autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, and systemic sclerosis.5 Luchi et al6 recently evaluated the connection between ROS and autoimmune hemolytic anemia in a murine model and implicated oxidative stress as a cause of autoimmune hemolytic anemia. Griffiths et al7 speculated that autoimmune conditions are associated with increased activation of immune effector cells and production of free radical species via NADPH oxidases and nitric oxide synthases. Although low levels of oxidants are important as signaling molecules, overproduction in the absence of adequate antioxidant defense (thereby exceeding the capacity for radical scavenging by antioxidant enzymes or small inhibitors) may cause irreversible changes to biomolecules and the generation of neo-antigenic determinants. This may ultimately contribute to epitope spreading and the abrogation of self-tolerance.7 Lipid peroxidation occurs as a consequence of increased oxidative stress from the disruption of the pro-oxidant/antioxidant balance; during this process, aldehydic products (primarily 4-hydroxy-2-alkenals) are released, which can form adducts with free amino groups of lysine and other amino acids. These aldehyde-modified proteins are highly immunogenic.18
To date, there have been few investigations specifically targeting the role of oxidative stress and damage in ITP. However, the results of previous studies are supportive of a role for oxidative stress in the pathogenesis of chronic ITP.19-22 As infection and inflammation are known triggers of oxidative stress, some frequent findings in the history of children with newly diagnosed ITP are post- or para-infectious events, which are mostly viral. Many studies in adult patients have also demonstrated a strong association between Helicobacter pylori and ITP; furthermore, H pylori eradication is linked with platelet recovery in multiple studies and thus has been suggested as a treatment option for ITP.19-22 In the review by Izzotti et al,22 of the interaction between H pylori and genetic polymorphisms related to noncancer diseases (including ITP), one of the major factors playing a pathogenic role in H pylori–related noncancer diseases is host polymorphisms in genes involved in inflammation and protection against oxidative damage.
Oxidative stress occurs as a result of increased activity of free radical–producing enzymes, decreased activity of free radical–removing enzymes, and insufficient levels of antioxidants. Lipids are the most sensitive molecules to oxidation; the oxidation of cell membrane lipids leads to the loss of cell membrane elasticity, increased cell fragility, and a shortened cellular life span. Polat et al23 measured plasma and erythrocyte malondialdehyde (a marker for lipid peroxidation), erythrocyte glutathione (GSH, reduced form), and ascorbic acid levels in adult patients with ITP and controls. They detected statistically significant differences in the levels of all of these markers in patients with ITP versus controls, further implicating oxidative stress and oxidative damage in the pathogenesis of ITP.
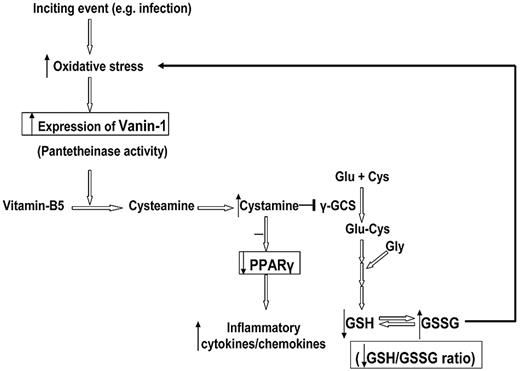
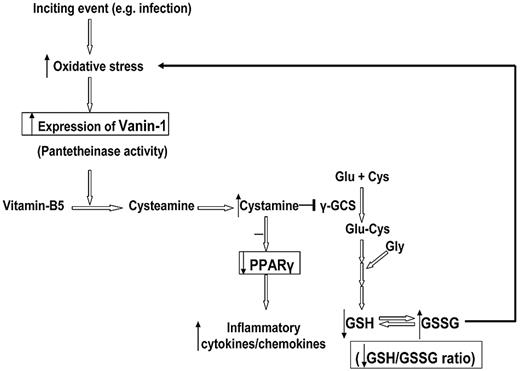
Our findings indicate that vanin-1 is an important target for further investigation. It is an oxidative stress sensor and also acts as a proinflammatory molecule by inhibiting PPARγ in epithelial cells.8,24 The association of overexpressed VNN1 in blood with progression to chronic ITP, as well as treatment-resistant chronic ITP, has not been reported previously. In the Symatlas database,25 VNN1 expression in blood monocytes is strikingly high compared with other tissues, which is in accord with our findings. Vanin-1 is a glycosylphosphatidylinositol-anchored surface molecule with pantetheinase activity.26,27 By hydrolyzing pantetheine, vanin-1 provides cysteamine to tissues.28 In epithelial cells, vanin-1 has been proven to be an oxidative stress sensor, which regulates endogenous glutathione levels, influences the redox status, and licenses the production of inflammatory mediators by modulating both the expression and the ligand-induced activation of PPARγ.8,24 A schematic presentation of the postulated vanin-1 pathway is shown in Figure 6. In the presence of oxidative stress, antioxidant response-like elements within the promoter region of VNN1 act as stress-regulated targets and enhance VNN1 expression. More cysteamine is produced from hydrolysis of pantetheine; cysteamine is then converted to cystamine, which is an inhibitor of γ-glutamylcysteine synthetase, the rate-limiting enzyme of glutathione synthesis. Berruyer et al24 demonstrated that vanin-1−/− mice had elevated endogenous stores of glutathione, the most potent cellular antioxidant, thereby exhibiting resistance to oxidative injury and reduced inflammatory responses to ROS inducers (ionizing irradiation).
Schematic representation of the postulated vanin-1 pathway in human blood cells in response to oxidative stress. This figure summarizes our hypothesis based on the work of Berruyer et al8,24 and our findings. The steps with experimental data support are highlighted in the box. An inciting event (eg, infection) induces generation of free radical species, whereas ROS has a positive modulatory role in immune activation and eradication of viral infections, excessive ROS, or inadequate capability of antioxidant scavengers leads to an oxidative stress state. In the presence of oxidative stress, antioxidant response-like elements within the promoter region of VNN1 act as stress-regulated targets and enhance VNN1 expression. More cysteamine is produced from hydrolysis of pantetheine; cysteamine is then converted to cystamine, which is an inhibitor of γ-glutamylcysteine synthetase (γ-GCS), the rate-limiting enzyme of glutathione synthesis. Thus, the glutathione store as well as the GSH/GSSG ratio decrease, which subsequently intensifies the oxidative stress. On the other hand, the anti-inflammatory checkpoint PPARγ is also antagonized by cystamine; and as a result, more inflammatory cytokines and chemokines are produced.
Schematic representation of the postulated vanin-1 pathway in human blood cells in response to oxidative stress. This figure summarizes our hypothesis based on the work of Berruyer et al8,24 and our findings. The steps with experimental data support are highlighted in the box. An inciting event (eg, infection) induces generation of free radical species, whereas ROS has a positive modulatory role in immune activation and eradication of viral infections, excessive ROS, or inadequate capability of antioxidant scavengers leads to an oxidative stress state. In the presence of oxidative stress, antioxidant response-like elements within the promoter region of VNN1 act as stress-regulated targets and enhance VNN1 expression. More cysteamine is produced from hydrolysis of pantetheine; cysteamine is then converted to cystamine, which is an inhibitor of γ-glutamylcysteine synthetase (γ-GCS), the rate-limiting enzyme of glutathione synthesis. Thus, the glutathione store as well as the GSH/GSSG ratio decrease, which subsequently intensifies the oxidative stress. On the other hand, the anti-inflammatory checkpoint PPARγ is also antagonized by cystamine; and as a result, more inflammatory cytokines and chemokines are produced.
The currently available functional studies of VNN1 are limited to epithelial cells. We were interested in whether overexpression of VNN1 is associated with oxidative stress in human blood cells. In response to oxidative stress induced by LPS and sodium arsenite, VNN1 expression in all human PBMC samples increased significantly. The overexpression of VNN1 coexisted with decreased GSH/GSSG ratios in treated PBMCs. Concurrently, a dramatically decreased expression of PPARγ was also observed, suggesting a possible inhibitory effect of VNN1 on PPARγ in blood cells similar to that seen in epithelial cells. However, a more definitive and precise relationship between VNN1 and PPARγ in specific blood cell subsets (particularly monocytes and platelets) remains to be further investigated.
In our cohort of chronic patients with ITP, both the PPAR and NF-κB pathways were perturbed. PPARγ is widely expressed in immune cells and encodes a member of the PPAR subfamily of nuclear receptors, which form heterodimers with retinoid X receptors and regulate transcription of various genes. PPARγ in monocytes/macrophages exerts anti-inflammatory effects by inhibiting other transcription factors, such as NF-κB and AP-1 (activator protein 1).9,29,30 PPARγ activation skews human monocytes toward M2 macrophages with anti-inflammatory properties.31 Kasono et al reported that thiazolidinedione, a PPARγ agonist, increased platelet counts in an ITP mouse model.32 PPARγ is also expressed in megakaryocytes and platelets.33 O'Brien et al reported their findings from an in vitro study that platelet production from megakaryocytes can be enhanced by 15d-PGJ2, a potent PPARγ ligand.34 On this basis, PPARγ should be considered as a potential therapeutic target in chronic ITP. Based on the current information, we hypothesize that oxidative stress and damage play a role in the pathogenesis of chronic pediatric ITP. An inciting event (eg, infection) induces generation of free radical species and release of pro-oxidant cytokines (ie, tumor necrosis factor and interleukin-1). Although ROS has a beneficial role in immune activation and eradication of viral infections, excessive ROS relative to the activity of antioxidant scavengers leads to an oxidative stress state, resulting in cell injury and tissue damage. Under this condition, peroxidation of lipids and proteins contributes to the generation of immunogenic neo-epitopes. Oxidatively modified proteins are rarely specific, which helps to explain the presence of antibodies against multiple platelet surface glycoproteins in patients with ITP. The extent of oxidative stress and the capability to regain redox balance vary in different persons; those who fail to regain redox balance have persistent oxidative damage, activation of the NF-κB pathway, dominant proinflammatory cytokine production, and a prolonged disease course. During the early disease stage, elevated VNN1 expression in those who later progress to chronic ITP may reflect a state of high underlying oxidative damage. The role of vanin-1 as an inhibitor of GSH production and PPARγ activity in human blood cells warrants further study. Regardless, we think it is a potentially important molecule that acts not only as an oxidative stress response gene but also functionally influences downstream pathways.
Given the abundance of supporting evidence for a role of oxidative stress in the pathogenesis of ITP, using well-designed clinical trials to evaluate the effectiveness of relatively nontoxic antioxidants (eg, N-acetylcysteine) seems attractive. In addition, because oxidative stress may be elevated early in the disease course, administration of antioxidants during the acute phase may help prevent patients from developing chronic ITP.
In conclusion, several oxidative stress–related signaling pathways are perturbed in chronic ITP compared with self-limited acute ITP, suggesting a role for oxidative stress in the pathogenesis of chronic ITP. Agents that ameliorate oxidative stress (especially during the early phase of the disease) or modulate the PPAR pathway should be considered as novel treatment options. As for directions in the future, the study will be continued with higher number of patients with ITP in heterogeneous groups, and possibly in other autoimmune diseases as well.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kokil Bakshi for coordinating the patient sample collection, Stanford Blood Center for providing buffy coats and platelets, and Dr Andrew Beck and Dr Raymond Balise for statistical consults.
This work was supported by the Lucile Packard Children's Health Research Program (award) and the Stanford Departments of Pediatrics and Pathology.
Authorship
Contribution: B.Z., C.L., L.S., R.S., C.J., K.C.-O., and S.P.-S. performed research; B.Z. and C.L. analyzed the data; C.L., W.W., and M.J. provided patient samples; B.Z., T.C., E.G.E., and J.Z. designed research; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James L. Zehnder, Department of Pathology, Rm L-235, Stanford University Medical Center, 300 Pasteur Dr, Stanford, CA 94305-5112; e-mail: zehnder@stanford.edu.