Abstract
The enhancement of fibrinolysis constitutes a promising approach to treat thrombotic diseases. Activated thrombin activatable fibrinolysis inhibitor (TAFIa) attenuates fibrinolysis and is an attractive target to develop profibrinolytic drugs. TAFI can be activated by thrombin, thrombin/thrombomodulin, or plasmin, but the in vivo physiologic TAFI activator(s) are unknown. Here, we generated and characterized MA-TCK26D6, a monoclonal antibody raised against human TAFI, and examined its profibrinolytic properties in vitro and in vivo. In vitro, MA-TCK26D6 showed a strong profibrinolytic effect caused by inhibition of the plasmin-mediated TAFI activation. In vivo, MA-TCK26D6 significantly decreased fibrin deposition in the lungs of thromboembolism-induced mice. Moreover, in the presence of MA-TCK26D6, plasmin-α2-antiplasmin complexes in plasma of thromboembolism-induced mice were significantly increased compared with a control antibody, indicative of an acceleration of fibrinolysis through MA-TCK26D6. In this study, we show that plasmin is an important TAFI activator that hampers in vitro clot lysis. Furthermore, this is the first report on an anti-TAFI monoclonal antibody that demonstrates a strong profibrinolytic effect in a mouse thromboembolism model.
Introduction
The primary focus of the fibrinolytic system is the dissolution of blood clots to maintain blood fluidity within the vascular system. During fibrinolysis, tissue-type plasminogen activator (tPA) converts plasminogen into plasmin, resulting in the partial degradation of fibrin into fibrin degradation products, thereby exposing C-terminal lysine residues. Specific interactions between lysines in fibrin and lysine-binding sites in plasminogen and tPA enhance the plasmin formation. Activated thrombin activatable fibrinolysis inhibitor (TAFIa) can attenuate fibrinolysis by removing the lysine residues from partially degraded fibrin, which results in a limited plasmin formation.1,2
TAFIa acts as a metallocarboxypeptidase, is synthesized in the liver, and is released in the circulation as a 56-kDa glycosylated zymogen. The proenzyme TAFI is cleaved at Arg92 by trypsin-like enzymes, such as thrombin, plasmin, or the thrombin/thrombomodulin (T/TM) complex, generating TAFIa.3 The latter shows an intrinsic temperature-dependent instability (ie, half-life of human TAFIa, 8-15 minutes at 37°C). Recently, a stable TAFI variant (ie, TAFI-A147-C305-I325-I329-Y333-Q335, TAFI-ACIIYQ) has been reported with a 180-fold increased half-life and an 18-fold increased antifibrinolytic potential.4 Inactivation of TAFIa is based on its conformational instability resulting in a nonactive form (TAFIai), which is further degraded by proteolytic cleavage.5,6
To date, no physiologic inhibitors of TAFIa are known. Nonetheless, TAFIa is sensitive to inhibition by chelating agents, by compounds that disrupt disulfide bridges, by small synthetic substrate analog inhibitors and by naturally occurring metallocarboxypeptidase inhibitors.7,8 In vivo evidence for a role of TAFI in fibrinolysis is obtained in several animal models (eg, the efficiency of tPA-mediated thrombolysis is increased by inhibition of TAFIa through potato tuber carboxypeptidase inhibitor (PTCI).9-11 However, the applicability of all these inhibitors is restricted given their lack in specificity and safety. Hence, monoclonal antibodies (MAs) raised toward TAFI can circumvent these difficulties. The MAs can either directly interfere with TAFIa activity or can hamper TAFI activation. Two studies reported MAs that inhibit human or rat TAFIa activity directly (MA-T9H1112 and MA-RT30D8,13 respectively). Gils et al12 reported the reduction of TAFIa levels by MAs that hamper the activation of human TAFI through T/TM and plasmin or T/TM alone (eg, MA-T94H3 and MA-T12D11, respectively).12 Unfortunately, the latter MAs raised toward human TAFI revealed no cross-reactivity with mouse or rat TAFI and consequently could not be tested in mice nor in rats. Obviously, in vivo studies are necessary to further unravel the relative contribution of the different TAFI activators. To the best of our knowledge, only one study in baboons reported inhibition of TAFIa generation during Escherichia coli–induced sepsis through an MA specifically inhibiting T/TM-dependent activation of human TAFI (ie, MA-TAFI/TM#16).14 However, it cannot be ruled out that other TAFI activators (eg, plasmin) play an essential role in thrombotic diseases.
The amino acid sequence of mouse TAFI reveals 85% identity with its human counterpart, including conserved residues involved in substrate binding and specificity, glycosylation, and zinc binding.15 TAFI from these 2 species have similar biochemical characteristics with respect to their activatability by T/TM and their antifibrinolytic effect during in vitro clot lysis.15,16 Both TAFI pathways are thus conserved in human and mouse, so that the results of the evaluation of anti-TAFI MAs in mouse models can be extrapolated to humans.17
The first objective of the current study is to unravel the relative contribution of plasmin as TAFI activator. Second, this study evaluates the profibrinolytic effect of TAFI inhibition by an anti-TAFI MA in a mouse thromboembolism model.
Methods
Materials
All materials are provided in the supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The ethical committee of the KU Leuven approved the thromboembolism model.
Production and purification of recombinant TAFI variants
Throughout this study, the following TAFI variants were used: the human wild-type isoform (TAFI-TI), the stable human variant (TAFI-ACIIYQ4 ), mouse TAFI, and a TAFI variant that is activatable by thrombin or T/TM but lacks activation by plasmin (TAFI-TI-K133A). More details on the production and purification of recombinant TAFI variants are provided in the supplemental Data.
Production of monoclonal antibodies
The MA-TCK26D6 used throughout this study was raised toward human TAFI-ACIIYQ in TAFI-deficient mice18 according to the Galfrè and Milstein procedure.19 MA-Tom1-41B2,20 directed toward tomato pectin methylesterase and showing no cross-reactivity with human or mouse TAFI, was used as irrelevant control antibody in in vitro assays. MA-T30E5A2,12 directed toward human TAFI-TI and showing no cross-reactivity with mouse TAFI, was used as negative control antibody in in vivo assays.
MA-TCK26D6
Cross-reactivity.
Cross-reactivity of MA-TCK26D6 was evaluated immunologically using a one-side enzyme-linked immunosorbent assay (ELISA) system, as described previously.12 Briefly, human TAFI-TI, human TAFI-ACIIYQ, and mouse TAFI were coated at a final concentration of 2 μg/mL to compare the reactivity of MA-TCK26D6. Serial 4-fold dilutions of the MAs, starting from 100 μg/mL, were applied followed by incubation for 18 hours at 4°C. For detection of bound MA, RAM-IgG-horseradish peroxidase antibody was used.
Affinity measurements.
Affinity constants for the binding between MA-TCK26D6 and TAFI variants were determined using surface plasmon resonance analysis with the Biacore 3000 analytical system (Biacore) equipped with a CM-5 sensor chip as described earlier with some minor modifications.12 MA-TCK26D6, at a concentration of 5 μg/mL in acetate buffer, pH 4.5, was covalently coupled to approximately 1200 resonance units. Purified TAFI variants were diluted in HBS-EP buffer (Biacore) to concentrations various between 10 and 200nM and injected at a flow rate of 30 μL/min. After each cycle, the chip was regenerated with 10 μL of 10mM glycine solution, pH 1.5. Affinity analysis was performed using Biacore 3000 software Version 4.1.2. For the evaluation of the cross-reactivity of MA-TCK26D6, kinetics were determined via Langmuir binding (local fit) and the equilibrium association constant (KA) was calculated via the association rate constant (ka) divided by the dissociation rate constant (kd). For the interactions obtained with the TAFI-TI mutants used to unravel the molecular mechanism of TAFI inhibition by MA-TCK26D6, the steady-state affinity (general fit) model was applied.
Overall inhibitory effect of MA-TCK26D6 on TAFI activation and TAFIa activity (chromogenic assay).
To determine the inhibitory effect of MA-TCK26D6 on TAFI activation, a chromogenic assay was used as described earlier12 with some modifications. Human TAFI-TI, human TAFI-ACIIYQ, or mouse TAFI (45nM, concentration at activation step) in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 137mM NaCl, 3.5mM KCl, 3mM CaCl2 plus 0.1% bovine serum albumin, pH 7.4) was incubated for 10 minutes at 37°C with either buffer or MA (concentrations ranging from 0.062- to 16-fold molar ratio of MA over TAFI, ie, 11.25-2880nM) before activation by thrombin (20nM), thrombomodulin (5nM), and CaCl2 (5mM) or plasmin (500nM; concentrations at activation step) and CaCl2 during 10 minutes at 22°C. The T/TM- or plasmin-mediated activation of TAFI variants was terminated by the addition of PPACK (30μM) or aprotinin (1μM, concentrations to stop activation), respectively. Then, hippuryl-L-arginine (4mM, concentration during substrate conversion) was added and substrate conversion was allowed to proceed for 10 minutes (T/TM-mediated TAFI activation) or 30 minutes (plasmin-mediated TAFI activation) at 22°C. A colorimetric reaction was performed to quantify the formed hippuric acid.12
The activation of TAFI by thrombin was performed as for T/TM, except for the higher thrombin and PPACK concentrations (100nM and 150μM, respectively), a longer activation time (2 hours at 22°C), and the restriction to use the human stable variant TAFI-ACIIYQ because of the instability of TAFIa. So far, no stable variant of mouse TAFI is available; thus, the effect of MA-TCK26D6 on the thrombin-mediated activation of mouse TAFI could not be tested in this chromogenic assay.
In these assays, MA-Tom1-41B2 was included as irrelevant control antibody at a concentration of 2880nM.
The inhibiting capacity of the antibodies was calculated relative to the amount of activated TAFI in the absence of MA. Reduced TAFIa activity as detected in this experimental setup can be caused either by interference with the activation mechanism or by direct interference with the TAFIa enzymatic activity.
Effect of MA-TCK26D6 on TAFI activation (SDS-PAGE analysis).
SDS-PAGE analysis was performed to verify whether MA-TCK26D6 inhibits TAFI activation. Therefore, purified TAFI-TI or TAFI-TI-K133A (concentrations at activation step: 0.56μM in 20mM Tris, 100mM NaCl, pH 7.4) was incubated with either buffer or a 3-fold molar ratio of MA over TAFI for 10 minutes at 37°C before activation by either thrombin (20nM) and thrombomodulin (5nM) or by plasmin (660nM) for 10 minutes at 37°C or by thrombin alone (500nM) for 2 hours at 37°C. Each activation mixture contained CaCl2 (5mM; concentrations at activation step). The T(/TM) or plasmin activation of TAFI was stopped by adding PPACK (30/750μM) or aprotinin (1μM), respectively. SDS (1%; final concentrations) was added to the reaction mixtures followed by heating for 30 seconds at 100°C. The generated TAFI fragments were separated by SDS-PAGE and visualized by silver staining.
Direct inhibitory effect of MA-TCK26D6 on TAFIa activity (chromogenic assay).
To determine whether MA-TCK26D6 directly interferes with the TAFIa activity, TAFI (90nM in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, concentration at activation step) was activated (10 minutes at 22°C) by thrombin (20nM), thrombomodulin (5nM), and CaCl2 (5mM; concentrations at activation step) before incubation with either buffer or MA (8-fold molar ratio over TAFI, ie, 1.44μM) for 10 minutes at 22°C. Quantification of the residual TAFIa activity was determined using the substrate hippuryl-L-arginine followed by a colorimetric reaction, as described.12
TAFIa activity.
The specific activity (expressed as units per milligram) of TAFIa was evaluated at 22°C using the chromogenic assay and a standard of hippuric acid, spanning a concentration range from 16μM to 2mM to quantify the amount of hippuric acid formed during the substrate reaction.21
In vitro study: clot lysis assay
Clot lysis assays were performed as described previously with some modifications.22 Pooled human plasma (final concentration, 30%) was mixed with either buffer, MA-TCK26D6 (concentrations ranging from 11.25 to 2880nM) or PTCI (4.76μM) in a 96-well microtiter plate. After incubation for 10 minutes at 37°C, tPA was added to the human plasma mixtures to reach a final concentration of 120pM. Either buffer or extra thrombomodulin (1nM; final concentration) was added and CaCl2 (10.6mM) was used to induce clot formation. The plate was incubated at 37°C and read at 405 nm at 2-minute intervals to determine the 50% clot lysis time. The 50% clot lysis time is defined as the time between the maximum turbidity and the midpoint of the maximum turbid to clear transition. Reduction of clot lysis time was calculated relative to the reduction of clot lysis time in the absence (0% reduction) and the presence of PTCI (100% reduction).
A similar method was applied for the evaluation of the effect of TAFI-TI-K133A during clot lysis, except for (1) the use of TAFI-deficient human plasma instead of normal human plasma, (2) the addition of TAFI-TI versus TAFI-TI-K133A (final concentrations of 179nM in plasma), and (3) the use of MA-Tom1-41B2 as irrelevant control antibody.
In vivo study: mouse thromboembolism model
General protocol.
MA-TCK26D6 or MA-T30E5A212 (negative control antibody) was injected intravenously in the tail vein in nonanesthetized Swiss mice. Both MAs were used in a 24-fold molar ratio over TAFI (26 mg/kg), assuming a mouse TAFI concentration of 71nM in plasma and a blood volume of 2 mL in Swiss mice weighing approximately 20 g. After 5 minutes, a suboptimal concentration of tPA (0.1 mg/kg, intravenously) was administered followed by thromboplastin (2.5 μg/kg, intravenously) injection within 20 seconds, the latter to induce thromboembolism in the lungs. Reference mice received only tPA and thromboplastin injections. Swiss mice that received only saline injections were represented as mice under baseline conditions.
Percentage of mice with normal physical activity.
The mice were evaluated at 15 and 30 minutes after thromboplastin injection, and the percentage of mice displaying normal physical activity was calculated. Abnormal physical activity was defined as paralysis of the limbs or death.
Histology.
Fifteen minutes after thromboembolism induction, anesthetized mice (Nembutal; 60 mg/kg, intraperitoneally) were perfused through cardiac puncture with 0.9% NaCl followed by 1% paraformaldehyde in phosphate-buffered saline (PBS). After ligation of the left lung, the right lung was fixed in 4% paraformaldehyde via tracheal instillation. Right lungs were postfixed in the same fixative for 60 hours and embedded in paraffin, whereas left lungs were snap-frozen for subsequent fibrin quantification. Sections of lung tissues were examined microscopically (Leitz DMRB with objectives 10×/0.30 NA PH1 for Figures 3A through C and 40×/0.70 NA for Figure 3D, Axiocam MRc 5 camera and Axiovision 4.7 software; Leica; Microsystems) after Martius Scarlet Blue staining of paraffin slides, allowing identification of red blood cells (yellow-brown), platelets (gray), fibrin (pink), and connective tissue (blue), as described by Lendrum et al.23 Adobe Photoshop CS2 Version 9.0.2 was used to merge the different micrographs.
Fibrin quantification.
Mice were anesthetized with pentobarbital (Nembutal; 60 mg/kg intraperitoneally) 10 minutes after tPA and thromboplastin injections. To prevent postmortem coagulation, heparin (heparin LEO; 500 IU) was injected in the vena cava 20 minutes after thromboembolism induction. After 3 minutes, lungs were perfused with saline containing heparin (10 IU/mL). Isolated left and right lungs were snap-frozen in liquid nitrogen and stored (−80°C) until homogenization. For homogenization, 4 mL of phosphate-buffered saline per gram of tissue was added to the frozen lungs. Subsequently, the lung tissues were homogenized with a tissue homogenizer (Ribolyzer Fast Prep 24 System, MP Biomedicals) and centrifuged. After resuspension of the pellet containing insoluble fibrin, microplasmin (2μM) was added to convert insoluble fibrin into soluble fibrin degradation products. Subsequent to incubation of the samples for 4 hours at 37°C, the samples were centrifuged. The supernatants were collected, and fibrinolysis was stopped by adding aprotinin (4μM). Fibrin degradation product concentrations and thus corresponding fibrin concentrations of the samples were determined using an in-house ELISA. Polystyrene microtiter plates were coated overnight with 200 μL of the RAM antifibrinogen antibody (4 μg/mL diluted in PBS) in each well. Plates were blocked with bovine serum albumin (30 g/L) for 1 hour. Mouse fibrinogen as a standard (2.5-0.039 μg/mL) or samples (1:30 and 1:90 dilutions) were diluted in PBS containing human albumin (10 g/L), Tween 80 (0.002%), Na2-ethylenediaminetetraacetic acid (5mM), and Triton X-100 (0.1%) and were applied to the wells for 2 hours at room temperature. After washing the plates with PBS containing 0.002% Tween 80, goat anti–human antifibrinogen–horseradish peroxidase antibody (2.5 μg/mL) was incubated in the wells for 1 hour at room temperature. Plates were washed again, and the peroxidase reaction was performed by addition of a 0.1M citrate-0.2M sodium phosphate buffer, pH 5.0, containing 400 μg/mL o-phenylenediamine and 0.003% hydrogen peroxide. After the reaction was terminated with 4M H2SO4, the absorbance was measured at 492 nm with an EL808 Ultra Microplate Reader (Bio-Tek instruments).
Coagulation and fibrinolysis markers.
After anesthesia (isoflurane; 2.5%), blood was collected via the retro-orbital vein on sodium citrate 3.8% (1:10 volume). Blood platelets were measured on a Cell-Dyn 3500R counter (Abbott). Platelet-poor plasma was prepared by centrifugation of blood at 15 000g for 10 minutes (twice) and was stored at −80°C. Mouse thrombin-antithrombin (TAT) complexes in plasma were determined with a commercial ELISA kit (Enzygnost TAT, Siemens). Plasmin-α2-antiplasmin complexes (PAP) in plasma were measured by ELISA as described elsewhere24 using polyclonal antibodies against mouse α2-antiplasmin and mouse plasminogen raised in rabbits. Coagulation assays were performed on the BCS-XP coagulation analyzer (Siemens) using reagents from the manufacturer and adapted procedures for mouse coagulation testing.25 Mouse fibrinogen concentrations were measured by the functional Clauss method, using diluted thrombin reagent, calibrated with standard human plasma. Activated partial thromboplastin time and prothrombin time were measured by addition of CaCl2/contact activator (Actin FS) mixture or thromboplastin (Innovin), respectively. Plasma levels of factors VII and VIII were assayed using commercially available human plasma deficient in the respective coagulation factor. A calibration curve for factors VII and VIII activity was obtained by dilution of pooled plasma from Swiss mice.
Statistical analysis
Quantitative data were summarized by mean ± SD. Statistical analysis, data plotting, and curve fitting were performed with GraphPad Prism Version 5 and GraphPad Statmate Version 2 (GraphPad Software). Dose-response curves were fitted to one-site binding hyperbolas. A 2-tailed paired t test was used to compare in vitro 50% clot lysis times in plasma. Differences in maximal inhibition of plasmin-mediated TAFI activation by MA-TCK26D6 for the different TAFI variants were evaluated by a 2-tailed unpaired t test assuming equal variances. A Fisher exact test was applied to compare the difference in percentage of mice showing normal physical activity in the mouse thromboembolism model with and without addition of MA. To determine the amount of mice needed in every group to detect an acceptable difference (25%) in the mouse thromboembolism model, the 80% power after each experiment was calculated until the power dropped to less than 0.25. The difference in percentage of fibrin in the lungs of thromboembolism-induced mice in the presence and absence of MA was assessed using a 2-tailed unpaired t test assuming equal variances. Statistical significance between groups in the thromboembolism model for coagulation and fibrinolytic parameters was analyzed using a 2-tailed unpaired t test assuming equal variances.
Results
(Cross-)reactivity of MA-TCK26D6 toward human and mouse TAFI
One-side ELISA experiments revealed that MA-TCK26D6 (cross-)reacts with human TAFI-TI, TAFI-ACIIYQ, and mouse TAFI (data not shown). These data were confirmed using surface plasmon resonance analysis, in which MA-TCK26D6 strongly binds to human TAFI-TI (KA = 9.8 ± 6.8 × 108 L/mol), TAFI-ACIIYQ (KA = 7.5 ± 2.3 × 108 L/mol), and mouse TAFI (KA = 4.2 ± 0.6 × 108 L/mol).
Inhibitory effect of MA-TCK26D6 on TAFI activation
To determine the effect of MA-TCK26D6 on TAFI activation, an inhibition assay was performed in which MA-TCK26D6 was added before TAFI activation.
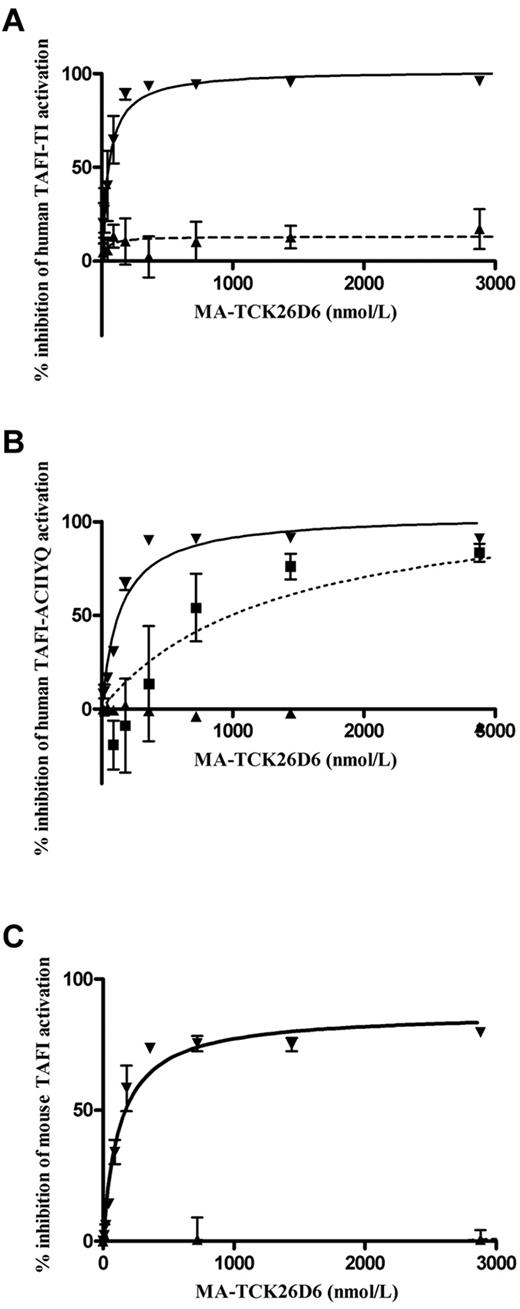
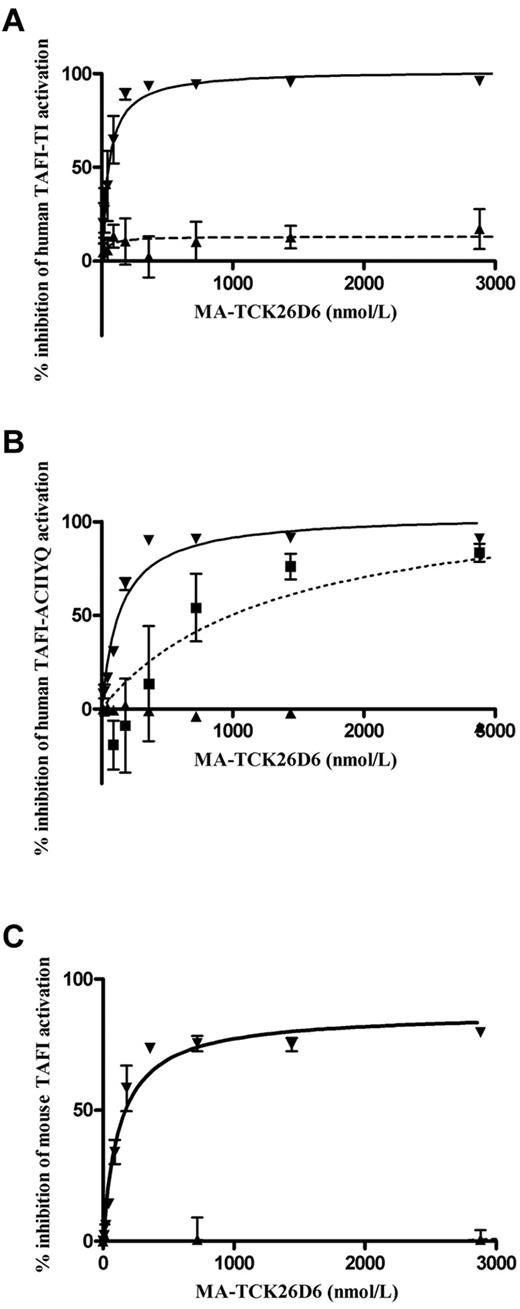
A dose-dependent inhibition for plasmin-mediated human TAFI-TI and human TAFI-ACIIYQ activation by MA-TCK26D6 was observed with a maximum inhibition of 102% ± 5% and 104% ± 4%, respectively, and corresponding 50% inhibitory concentration (IC50) values of 53 ± 12 and 138 ± 21nM (Figure 1A-B). Accordingly, a dose-dependent maximum inhibition of 87% ± 3% was reached for the inhibition of plasmin-mediated mouse TAFI activation with a corresponding IC50 value of 128 ± 20nM (Figure 1C). The inhibitory effect of MA-TCK26D6 on plasmin-mediated activation of human TAFI-TI and mouse TAFI was confirmed by SDS-PAGE analysis (supplemental Figure 1A).
Dose-dependent inhibition of TAFI. Human TAFI-TI (A), human TAFI-ACIIYQ (B), or mouse TAFI (C) activation mediated through plasmin (▾), thrombin (■), or the T/TM complex (▴) by MA-TCK26D6.
Dose-dependent inhibition of TAFI. Human TAFI-TI (A), human TAFI-ACIIYQ (B), or mouse TAFI (C) activation mediated through plasmin (▾), thrombin (■), or the T/TM complex (▴) by MA-TCK26D6.
Chromogenic assays also revealed a dose-dependent inhibition for thrombin-mediated human TAFI-ACIIYQ activation by MA-TCK26D6 with a maximum inhibition of 117% ± 31% and a corresponding IC50 value of 1.3 ± 0.9μM (Figure 1B). The inhibitory effect of MA-TCK26D6 on thrombin-mediated activation of human TAFI-TI and mouse TAFI was evaluated by SDS-PAGE analysis and revealed a clear inhibition (supplemental Figure 1B).
Virtually no inhibition was observed for T/TM-mediated activation of human TAFI-TI, human TAFI-ACIIYQ, and mouse TAFI, with a maximum inhibition of 17% ± 21%, < 2%, and < 2%, respectively. SDS-PAGE analysis confirmed this observation (supplemental Figure 1C).
Human and mouse TAFI activation through plasmin, thrombin, or T/TM was not hampered (ie, a maximum inhibition of < 2%) in the presence of the irrelevant control antibody MA-Tom1-41B2.
Hence, these results demonstrated that MA-TCK26D6 strongly interferes with plasmin-mediated human and mouse TAFI activation and, to a lesser extent, with thrombin-mediated human and mouse TAFI activation.
Direct inhibitory effect of MA-TCK26D6 on TAFIa activity
To exclude the direct effect of MA-TCK26D6 on TAFIa activity, an inhibition assay was performed in which MA-TCK26D6 was added after TAFI activation. No direct TAFIa inhibitory effect was observed (ie, < 2%, at 1.44μM). Thus, MA-TCK26D6 was not able to reduce TAFIa activity.
Effect of MA-TCK26D6 during in vitro clot lysis
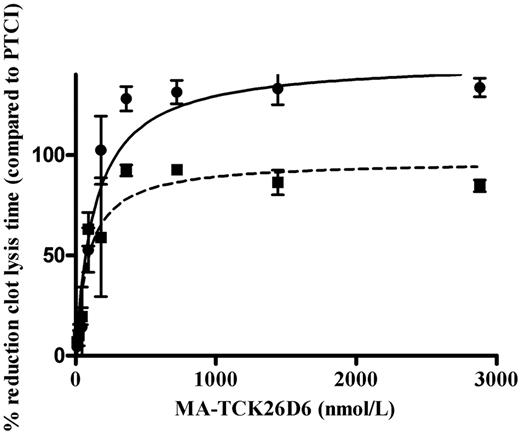
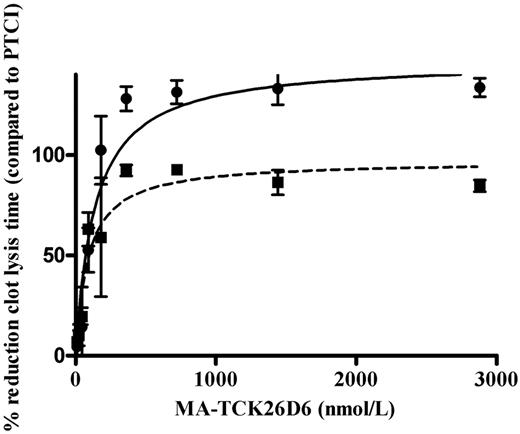
In the absence of thrombomodulin, the clot lysis time was 75 ± 10 minutes in human plasma. In the presence of PTCI, clot lysis time was shortened to 40 ± 3 minutes. A significant (P < .01) dose-dependent profibrinolytic effect of MA-TCK26D6 was observed (Figure 2). The reduction in 50% clot lysis time (expressed as percentage relative to reduction by PTCI) reached a plateau value of 128% ± 11% (at 360nM) and a corresponding IC50 value of 137 ± 33nM.
Dose-dependent reduction of 50% clot lysis time in the presence of MA-TCK26D6 in human plasma with 1nM exogenous thrombomodulin (■) or without additional thrombomodulin (●). Clot lysis times are expressed as percentages relative to reduction of clot lysis time by PTCI (4.76μM).
Dose-dependent reduction of 50% clot lysis time in the presence of MA-TCK26D6 in human plasma with 1nM exogenous thrombomodulin (■) or without additional thrombomodulin (●). Clot lysis times are expressed as percentages relative to reduction of clot lysis time by PTCI (4.76μM).
In the presence of 1nM thrombomodulin, the clot lysis time was 140 ± 9 minutes in human plasma. In the presence of PTCI, clot lysis time was shortened to 70 ± 9 minutes. A significant (P < .01) dose-dependent profibrinolytic effect of MA-TCK26D6 was detected (Figure 2). The reduction in 50% clot lysis time reached a plateau value of 92% ± 5% (at 360nM) and an IC50 value of 89 ± 30nM.
Biochemical properties of TAFI-TI-K133A and its effect during in vitro clot lysis
One of the TAFI-TI mutants (ie, TAFI-TI-K133A) showed interesting biochemical properties. TAFI-TI-K133A appears to be activatable by T/TM (14.7 ± 1.6 vs 17.9 ± 5.1 U/mg for TAFI-TI-K133A and TAFI-TI, respectively) but shows a 33-fold reduced activatability by plasmin (0.2 ± 0.2 vs 6.5 ± 0.8 U/mg for TAFI-TI-K133A and TAFI-TI, respectively). SDS-PAGE analysis confirmed the lack of activation of TAFI-TI-K133A by plasmin and revealed also that TAFI-TI-K133A is activatable by thrombin alone (data not shown).
To evaluate the antifibrinolytic properties of this TAFI variant that lacks activation by plasmin, 179nM of either TAFI-TI-K133A or TAFI-TI was added to TAFI-deficient plasma. TAFI-TI-K133A revealed a significantly (P < .01) smaller prolongation of the 50% clot lysis time (ie, 39 ± 8 minutes vs 160 ± 24 minutes for TAFI-TI-K133A and TAFI-TI, respectively; Table 1). Addition of MA-TCK26D6 to TAFI-deficient plasma supplemented with TAFI-TI significantly (P < .01) reduced clot lysis times (ie, 26 ± 4 minutes vs 160 ± 24 minutes, no MA). The reduction in clot lysis time was the result of the anti-TAFI properties of MA-TCK26D6 and not of the antibody as such, as indicated by the absence of any effect with the irrelevant control antibody (ie, MA-Tom1-41B2, Table 1). On the other hand, in TAFI-deficient plasma supplemented with TAFI-TI-K133A, no significant reduction of clot lysis time was observed with MA-TCK26D6 (ie, 36 ± 4 minutes vs 39 ± 8 minutes, no MA). These data show that the addition of a TAFI variant that lacks activation by plasmin has a similar effect in in vitro clot lysis as MA-TCK26D6 in combination with TAFI-TI and that the simultaneous addition of TAFI-TI-K133A and MA-TCK26D6 does not alter this effect.
Evaluation of the effect of MA-TCK26D6 in a mouse thromboembolism model
The effect of MA-TCK26D6 and the control antibody MA-T30E5A2 on the percentage of mice showing physical activity was evaluated in a mouse thromboembolism model and compared with the effect in the absence of antibody (reference mice; Table 2). At 15 and 30 minutes after thromboplastin injection, MA-TCK26D6 (at 26 mg/kg) significantly increased the percentage of mice showing normal physical activity compared with the reference mice. Mice injected with MA-T30E5A2 demonstrated similar results as the reference mice.
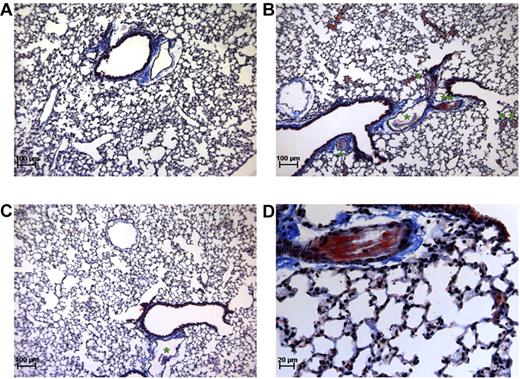
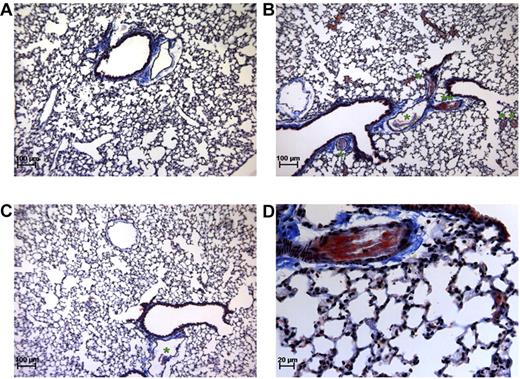
The composition and amount of thrombi formed in this mouse thromboembolism model were assessed by histologic analysis. Martius Scarlet Blue staining of lung sections taken from thromboembolism-induced mice showed that large vessels were obstructed by platelet-fibrin thrombi (Figure 3B-D). However, almost no thrombi were detected in the lungs of MA-TCK26D6-treated mice (Figure 3C). No obstructed vessels were observed in lungs of mice under baseline conditions (Figure 3A).
Martius Scarlet Blue staining of lung sections. Mice under baseline conditions (A) compared with thromboembolism-induced mice on MA-T30E5A2 (B,D) or MA-TCK26D6 (C) treatment. Pink represents fibrin stain; yellow-brown, red blood cells; gray, platelets; and blue, connective tissue. *The presence of a platelet-fibrin thrombus. **Platelet-fibrin thrombus of panel B is magnified in panel D.
Martius Scarlet Blue staining of lung sections. Mice under baseline conditions (A) compared with thromboembolism-induced mice on MA-T30E5A2 (B,D) or MA-TCK26D6 (C) treatment. Pink represents fibrin stain; yellow-brown, red blood cells; gray, platelets; and blue, connective tissue. *The presence of a platelet-fibrin thrombus. **Platelet-fibrin thrombus of panel B is magnified in panel D.
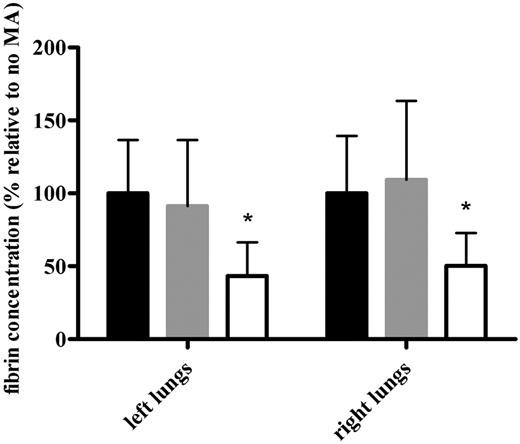
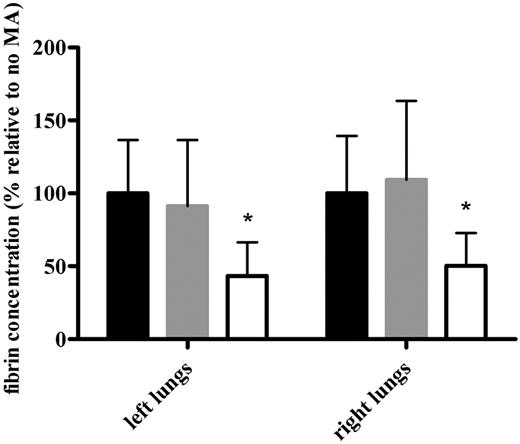
To further quantify the profibrinolytic effect of MA-TCK26D6 on mouse thromboembolism, fibrin was measured in both the left and right lungs of the thromboembolism-induced mice (Figure 4). The percentage of fibrin in the presence of MA-TCK26D6 relative to that in the absence of MA was significantly lower (ie, 43% ± 23% vs 100% ± 36%, P < .001; and 50% ± 23% vs 100% ± 39%, P < .01) in left and right lungs, respectively. Using the control antibody MA-T30E5A2, no significant difference was observed between the relative percentage of fibrin compared with the reference mice (ie, 91% ± 45% vs 100% ± 36%, P = .636; and 109% ± 54% vs 100% ± 39%, P = .670) in left and right lungs, respectively. Furthermore, the profibrinolytic effect of MA-TCK26D6 in the mouse thromboembolism model is independent of the time of administration of the suboptimal tPA concentration (ie, before or directly after thromboplastin injection; data not shown).
The effect of MA-TCK26D6 on the fibrin concentration of both lungs in thromboembolism-induced mice. The fibrin concentrations are expressed as percentage relative to the percentage obtained in the absence of MA. Black bars represent values in the absence of MA; gray bars, in the presence of negative control antibody MA-T30E5A2; and white bars, in the presence of MA-TCK26D6 (26 mg/kg). Ten mice were evaluated in each group. *Significantly different from respective reference mice without MA injection (P < .001).
The effect of MA-TCK26D6 on the fibrin concentration of both lungs in thromboembolism-induced mice. The fibrin concentrations are expressed as percentage relative to the percentage obtained in the absence of MA. Black bars represent values in the absence of MA; gray bars, in the presence of negative control antibody MA-T30E5A2; and white bars, in the presence of MA-TCK26D6 (26 mg/kg). Ten mice were evaluated in each group. *Significantly different from respective reference mice without MA injection (P < .001).
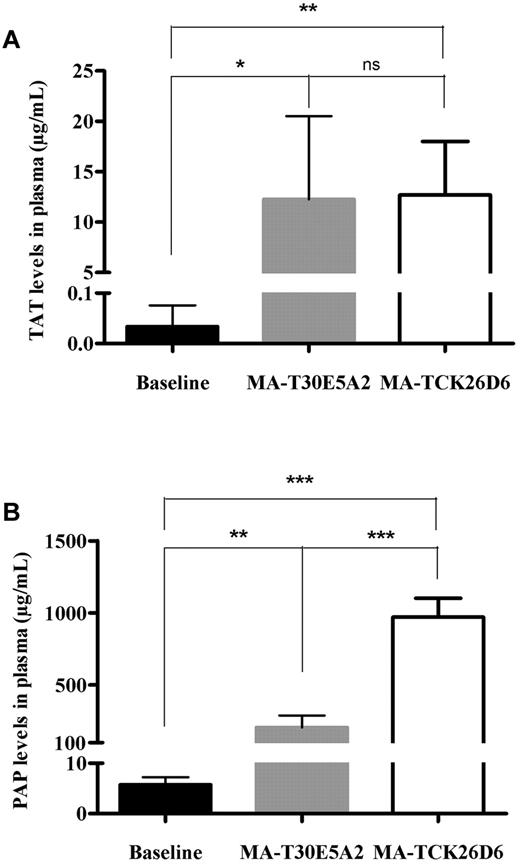
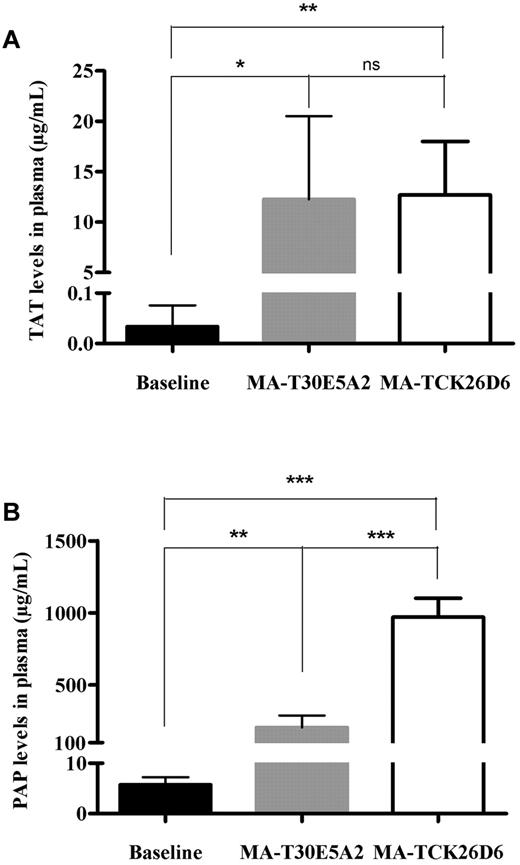
To further assess the effect of MA-TCK26D6 on coagulation and fibrinolysis, levels of TAT, PAP, blood platelets, fibrinogen, coagulation factors VII and VIII, activated partial thromboplastin time, and prothrombin time were determined in plasma of thromboembolism-induced mice (Figure 5; Table 3). Injection of thromboplastin led to an increase in TAT levels in both MA-TCK26D6– and MA-T30E5A2–treated mice compared with baseline levels (385- and 372-fold increase; P < .01 and P < .05, respectively), reflecting in vivo thrombin generation. Thromboplastin injection also resulted in an increase in PAP levels in both MA-TCK26D6– and MA-T30E5A2–treated mice compared with baseline levels (162- and 34-fold increase; P < .001 and P < .01, respectively), pointing to in vivo plasmin formation. Platelet count was significantly decreased in mice receiving thromboplastin with either MA-TCK26D6 or MA-T30E5A2 administration compared with baseline levels (5- and 8-fold decrease; both P < .0001), indicating platelet consumption resulting from thrombus formation. Moreover, thromboplastin injection led to such a massive systemic activation of coagulation that mouse fibrinogen, activated partial thromboplastin time, and prothrombin time could not be determined anymore. FVII and FVIII assays revealed that coagulation factor consumption was mostly determined by a strong decrease in FVIII levels, whereas FVII levels remained largely constant. In mice treated with MA-TCK26D6, PAP levels were significantly higher than in mice treated with control antibody MA-T30E5A2 (973 ± 129 μg/mL vs 206 ± 84 μg/mL; P < .001, respectively), indicating an acceleration of fibrinolysis through impairment of TAFI activation by MA-TCK26D6.
The effect of MA-TCK26D6 on plasma thrombin-antithrombin complexes (A) and plasmin-antiplasmin complexes (B) in thromboembolism-induced mice. Black bars represent baseline values; gray, values on injection of thromboplastin after MA-T30E5A2 (26 mg/kg; negative control antibody); and white bars, values on injection of thromboplastin after MA-TCK26D6 administration (26 mg/kg). Five mice were evaluated in each group. *P < .05; **P < .01; ***P < .001; ns, not significant.
The effect of MA-TCK26D6 on plasma thrombin-antithrombin complexes (A) and plasmin-antiplasmin complexes (B) in thromboembolism-induced mice. Black bars represent baseline values; gray, values on injection of thromboplastin after MA-T30E5A2 (26 mg/kg; negative control antibody); and white bars, values on injection of thromboplastin after MA-TCK26D6 administration (26 mg/kg). Five mice were evaluated in each group. *P < .05; **P < .01; ***P < .001; ns, not significant.
Molecular mechanism of TAFI inhibition by MA-TCK26D6
To elucidate the residue(s) that possibly contribute to the molecular mechanism of TAFI inhibition by MA-TCK26D6, the inhibitory capacity of the antibody was evaluated on a variety of TAFI-TI mutants (Supplemental data). These results suggest a significant contribution of both Asp87 and Thr88 (supplemental Figures 2-3).
Discussion
TAFIa can be generated from TAFI by various activators, such as thrombin, T/TM, plasmin, elastase, and trypsin.3,26-28 To gain more insight into TAFI activation during fibrinolysis, Leurs et al29 determined the TAFIa activity during in vitro clot lysis experiments in human plasma. A biphasic pattern of TAFIa activity was demonstrated in which TAFI was activated during coagulation by thrombin and during fibrinolysis by plasmin. Moreover, the latter study provided evidence that thrombin in complex with thrombomodulin is a major physiologic activator of TAFI. Kinetics support these observations only partly. The catalytic efficiency (kcat/Km) of the T/TM complex for TAFI activation (1.2 L μmol−1 s−1) is approximately 10- and 150-fold greater than that of plasmin with and without heparin as cofactor, respectively (0.13 and 0.008 L μmol−1 s−1).1,26 However, the Km values for TAFI activation by plasmin (55nM) and plasmin-heparin (20nM) are less than the plasma concentration of TAFI (75-250nM), in contrast to the Km value for activation by T/TM (1010nM).1,26 This suggests that also plasmin can play a contributing role in the activation of TAFI.
To further assess the relative contribution of plasmin as TAFI activator, we evaluated TAFI activation in an in vitro clot lysis assay using (1) a MA that inhibits thrombin- and plasmin-mediated activation of human and mouse TAFI (ie, MA-TCK26D6) and (2) a TAFI variant that is activatable by thrombin and T/TM but lacks activation by plasmin (ie, TAFI-TI-K133A). These in vitro clot lysis experiments revealed that MA-TCK26D6 accelerates the clot lysis almost as effective in the presence as in the absence of exogenous TM, which strongly suggests a significant role of plasmin-mediated TAFI activation during clot lysis. A similar conclusion was drawn by Buelens et al30 using inhibitory nanobodies that interfere with the plasmin- and thrombin-mediated TAFI activation. Furthermore, addition of a TAFI variant that lacks activation by plasmin (TAFI-TI-K133A) showed a similar effect in clot lysis as MA-TCK26D6. Moreover, simultaneous addition of MA-TCK26D6 and TAFI-TI-K133A did not alter the acceleration of clot lysis. From these in vitro clot lysis experiments, it could be deduced that (1) MA-TCK26D6 inhibits TAFI activation mainly via inhibition of the plasmin-mediated TAFI activation and (2) plasmin-mediated TAFI activation contributes to the prolongation of clot lysis time. Taken together, our data demonstrate that plasmin-mediated TAFI activation also plays an essential role during in vitro clot lysis.
In vivo studies are crucial to further evaluate the profibrinolytic effect of TAFI inhibition by MA-TCK26D6. Therefore, we set up the mouse thromboembolism model. In mouse plasma, thromboplastin injection led to a sharp decrease in platelet count, a complete consumption of mouse fibrinogen, and a strong decrease in coagulation factors, particularly FVIII. In addition, plasma TAT levels were extremely increased, reflecting thrombin formation. Thrombin can cause fibrin deposition but can also activate platelets, which both contribute to thrombus formation. Moreover, the moderate increase in plasma PAP levels demonstrates that the fibrinolytic system was not able to counterbalance the massive systemic activation of coagulation. Indeed, our histologic investigations confirm that lungs of thromboembolism-induced mice are obstructed by platelet-fibrin thrombi. Platelet activation also results in a release of serotonin that can induce pulmonary vasoconstriction, which may contribute to lung failure and death.31 Leon et al32 deduced that mice mortality probably results from lung occlusion and cardiac arrest. Using this thromboembolism model, we demonstrated that MA-TCK26D6 significantly decreased fibrin deposition in both lungs, resulting in a significant increase in the percentage of mice showing normal physical activity. Moreover, in the presence of MA-TCK26D6, PAP levels were significantly higher than in the presence of a control antibody, indicative for an acceleration of fibrinolysis through MA-TCK26D6. Taken together, these results confirm the in vivo profibrinolytic effect of TAFI inhibition. Furthermore, considering the impairment of the plasmin-mediated activation of TAFI by MA-TCK26D6, it is clear that in this mouse thromboembolism model plasmin is an important TAFI activator.
To elucidate the molecular mechanism of TAFI inhibition by MA-TCK26D6, various TAFI-TI mutants were generated and assessed for their binding properties to the antibody. Based on functional analysis, Asp87 and Thr88 seem to have an important role in this inhibition mechanism. As shown in the crystal structure of human TAFI (supplemental Figure 3), these residues are located in the loop connecting the α3-helix of the activation peptide and the α1-helix of the catalytic domain and in close proximity to Arg92-Ala.93 This implies that MA-TCK26D6 exerts its inhibitory effect by blocking the access of “small” TAFI activators (ie, plasmin and thrombin) to the scissile Arg92-Ala93 bond. Absence of impairment of T/TM-mediated activation may be explained by the observation that T/TM-mediated activation of TAFI requires binding of TM to lysines at positions 42, 43, and 44, resulting in a reorientation of the scissile bond to optimally position TAFI as a substrate for thrombin.31 The latter residues are remote from Asp87 and Thr88 and are presumably not blocked by MA-TCK26D6.
As shown, TAFI-TI-K133A appears to be activatable by thrombin and T/TM but lacks activation by plasmin. Mutations of other residues in a 12 Å region of Lys133 (ie H126A, S129A, P135A, Y137A) also revealed TAFI mutants that exhibit a reduced plasmin-mediated TAFI activation. Consequently, we hypothesize that these residues contribute to the binding site of plasmin.
In conclusion, this study reports the generation, characterization, and application of a MA (ie, MA-TCK26D6) that impairs mainly the plasmin-mediated activation of human and mouse TAFI. The obtained data reveal that plasmin-mediated activation of TAFI plays an important role during in vitro prolongation of clot lysis. Moreover, the results demonstrate the strong in vivo profibrinolytic effect of MA-TCK26D6. Accordingly, it is clear that enhancement of the fibrinolytic activity of tPA by impairing selectively the plasmin-mediated TAFI activation constitutes a promising pharmacologic approach to improve thrombolytic therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Alain Rupin and Marie-Odile Vallez (Division of Angiology and Medicinal Chemistry, Servier Research Institute, Suresnes, France) for assistance with the setup of the mouse thromboembolism model, Els Brouwers and Griet Compernolle for excellent technical support, and Katrien Cludts and Matthias Van Hul for assistance with the processing of histologic image files.
This work was supported by the Fund for Scientific Research-Flanders (FWO-Vlaanderen; grant 6.0833.09). E.V. is a PhD fellow of the Agency for the Promotion and Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
Authorship
Contribution: E.V. designed and performed research, analyzed and interpreted the data, performed statistical analysis, and drafted the manuscript; M.P. performed part of the research and analyzed the data; J.E. and M.F.H. contributed vital new analytical tools and reviewed the manuscript; and P.J.D. and A.G. designed research, interpreted the data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann Gils, Laboratory for Pharmaceutical Biology, Faculty of Pharmaceutical Sciences, Katholieke Universiteit Leuven, Campus Gasthuisberg, O&N2, PB 824, Herestraat 49, B-3000 Leuven, Belgium; e-mail: ann.gils@pharm.kuleuven.be.