Abstract
CD36 plays a critical role in the inhibition of angiogenesis through binding to the type 1 repeats of thrombospondin-1 (TSP-1) and activating Fyn tyrosine kinase and MAPK pathways. Here, we reveal a novel association of CD36 with VEGFR-2 and spleen tyrosine kinase (Syk). We also address the correlation between the expression of CD36 and Syk by demonstrating that overexpression of CD36 in HUVECs up-regulates endogenous Syk expression. We also define a new role for TSP-1 and CD36 in the activation of the VEGFR-2 signaling pathway that requires Syk. Our findings also identify a role for Syk as a stimulator of VEGF-A–induced angiogenesis by increasing phosphorylation of Y1175 in VEGFR-2, which is a major tyrosine for promoting VEGF-A–induced endothelial cell migration. Together, these studies introduce a new signaling pathway for TSP-1, CD36, and Syk, and address the role of these proteins in regulating the angiogenic switch.
Introduction
CD36 is a transmembrane glycoprotein that functions in cell adhesion, angiogenesis, atherogenesis, and the sequestration of Plasmodium falciparum–infected erythrocytes.1,2 As a scavenger receptor, CD36 mediates the uptake of oxidized low-density lipoproteins (LDL) by macrophages and the formation of foam cells during arterial atherogenesis.3,4 CD36 is also a receptor for thrombospondin-1 (TSP-1) and -2, as well as collagen.5,6 The binding of TSP-1 to CD36 inhibits endothelial cell migration and induces apoptosis.6,7 In endothelial cells that express CD36, treatment with TSP-1 decreases VEGF-A–induced phosphorylation of VEGFR-2 and p38 MAPK.8 Although several signaling proteins including Fyn, JNK, p38 MAPK, and caspase 3 have been implicated in TSP-1–induced apoptosis, the precise signaling pathway has not been elucidated.1,9-11 In platelets, CD36 interacts with the integrins αIIbβ3 and α6β1, as well as the tetraspanin CD9.12,13 We have reported that these interactions of CD36 with integrins and CD9 are preserved by Brij 99 and CHAPS, but dissociated by Triton X-100.13 This behavior is consistent with the localization of CD36 to tetraspanin-enriched microdomains (TEMs).14 Disruption of platelets with Triton X-100 followed by sucrose gradient ultracentrifugation yields 2 distinct pools of CD36, one in the low-density lipid raft fraction and the other in the high-density soluble fraction.13 The Src family kinases Fyn, Lyn, and Yes colocalize with CD36 in the low-density lipid fraction of Triton X-100 solubilized platelets and endothelial cells.13,15,16 In melanoma cells, CD36 associates with α3β1 and α6β1 via its extracellular region, and in endothelial cells, it interacts with integrin β1.8,17,18 The N- and C-terminals of CD36 do not contain consensus sequences for the docking of signaling molecules.19 Thus, the association of CD36 with receptors and adaptor proteins within the plane of the membrane may be critical for its function in regulating signaling pathways. In this study, we have identified receptors and signaling proteins that associate with CD36 in human dermal microvascular endothelial cells (HDMECs). The results established the association of CD36 with integrin subunits β1, αV, and α5 in Brij 99-solubilized lysates. We also identified several new partners for CD36 including VEGFR-2, p85 subunit of PI3K, Syk (spleen tyrosine kinase), and Vav. Our results also demonstrated that the association of VEGFR-2 with CD36 and Syk decreased in the absence of TSP-1. Syk promoted VEGF-A–induced endothelial cell migration by up-regulating phosphorylation of Y1175 in the cytoplasmic domain of VEGFR-2. Our data demonstrate that CD36 functions at the cell surface to modulate plasma membrane protein organization and signal transduction, and presents a possible role for the CD36-Syk complex in priming the angiogenic switch.
Methods
Ab-array analysis
The Ab-array assay was performed using a nitrocellulose membrane that is arrayed with high-quality Abs against 400 well-known signaling proteins (Hypromatrix). This assay is especially suitable for screening of interacting proteins from hundreds of possible candidates. To perform this assay, the array membrane was incubated with 5% blocking reagent (Amersham Pharmacia Biotech) in PBST for 1 hour at room temperature. Brij99- or Triton X-100–solublized HDMEC whole-cell lysate were incubated with Ab-arrayed nitrocellulose membrane for 2 hours at room temperature. After washing the membrane twice with PBST, the membrane was blotted with biotinalyted FA6-152 anti-CD36 mAb in 5% blocking reagent in PBST overnight at 4°C. The biotinylated FA6-152 was prepared using EZ-Link (Sulfo-NHS-Biotin kit according to the manufacturer's protocol (Pierce). To detect CD36-associated proteins, the membrane was incubated with HRP-conjugated streptavidin for an hour at room temperature. After developing the blot, the positive spots were analyzed and considered for further confirmation.
Biotin labeling and immunoprecipitation
The membrane surface proteins of confluent HDMECs that were treated with 1μM rosiglitazone for 48 hours were labeled with biotin using the ECL Protein Biotinylation System (RPN2202) according to protocols supplied by the manufacturer (Amersham Pharmacia Biotech). In other cases, cells were trypsinized, and the cell pellets were washed twice with ice-cold PBS, and then, used for protein extraction. The cell extracts were prepared with 1% Brij 99 or 1% Triton X-100 lysis buffer containing (20mM HEPES, pH 7.5, 150mM NaCl, 5mM EDTA, 1% of the detergent, and protease inhibitor cocktail) for 20 minutes at 4°C, and the samples were centrifuged at 20 000g for 30 minutes at 4°C in a Beckman SW55.1 Ti rotor. The cell lysate was either used immediately for immunoprecipitation experiments or stored at −80°C. To determine whether any of the proteins were not completely recovered in the supernatant, the pellet was rinsed twice in lysis buffer and dissolved in 500 μL of sample buffer for SDS-PAGE.
For immunoprecipitation, 600-900 μg of the cell extract was precleared with 3-5 μg of nonimmune IgG and 50 μL (pellet volume) of protein G- or A-agarose beads for 1 hour at 4°C. After removal of the beads by centrifugation, lysates were incubated with one of the following Abs: 5 μg of CD36 Abs (3 μg of FA6-152 and 2 μg of CLB-IVC7), Syk (C-20) Abs, 1:100 dilution of VEGFR-2, or CD9 Ab, and the samples were incubated for 2 hours at 4°C. Fifty microliters of protein G or A beads were added, and the samples were incubated for an additional 1-2 hours at 4°C. For immunoprecipitation of mouse tissues, 3 μg of anti-CD36 mouse mAb (BD PharMingen) and protein L-agarose beads were used. The beads were washed 3 times with lysis buffer, and the precipitated immunocomplex was eluted in 50 μL of 2× SDS-PAGE sample buffer by boiling for 4 minutes. The eluted samples were separated by SDS-PAGE either in the presence or absence of 1% DTT.
To further determine the CD36-tetraspanin interactions, HDMEC were lysed in 1% Brij 96 lysis buffer (20mM HEPES, pH 7.5, 150mM NaCl, with or without 5mM EDTA or MgCl2) for an hour at room temperature. CD36 immunoprecipitation was performed as indicated.
Detection of biotinylated proteins and immunoblotting
After SDS-PAGE, the proteins were transferred to a nitrocellulose membrane (Bio-Rad), and for detection of biotinylated samples, the membrane was blocked in 5% blocking reagent (Amersham Pharmacia Biotech) in PBS (pH 7.4) containing 0.1% Tween 20 (PBST) for 1 hour. The membrane was rinsed twice in PBST and incubated for 1 hour in HRP-conjugated streptavidin solution. After 3 washes in PBST, ECL detection was performed with the ECL Western Blotting Detection reagents (34080) from Pierce. For immunologic detection, the electrophoretic transfer membrane was incubated in 5% nonfat dry milk or 5% BSA in TBST (10mM Tris-HCl [pH 7.4], 150mM NaCl, either 0.1% or 0.05% Tween 20) for 1 hour at room temperature. The primary Abs were diluted in blocking solutions at 1:1000 dilution except for VEGFR-2 (1:500), Syk (1:500; Cell Signaling Technology), and CD36 (1:250; Cayman Chemicals and BD PharMingen). The membrane was incubated either at room temperature for 2 hours or overnight at 4°C with mixing. After 5 washes for 5 minutes each in TBST, the HRP-conjugated secondary Ab was added, and the blot was incubated for 2 hours at room temperature. The membrane was washed 5 times for 5 minutes each in TBST, and the bands were visualized using ECL detection.
Results
CD36 is a component of multiple signaling pathways
To identify the CD36-associated proteins in HDMECs, we used an Ab array assay. HDMECs were grown to confluence and lysed either in 1% Brij 99 or 1% Triton X-100. Because the expression of CD36 varies depending on the passage number and culturing condition of endothelial cells, we treated cells with rosiglitazone for 48 hours to increase the level of CD36 expression, as described in the cell culture section in “Methods.” Analysis of a signal-transduction Ab array membrane revealed the association of CD36 with a diverse group of HDMEC proteins, which we classified based on the intensity of their signals in repeated experiments (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In Brij 99 lysates, we found 15 potential CD36-interacting proteins, while a lower number of proteins were detected in Triton X-100 lysates (supplemental Figure 1B). This can be because of the differential ability of Brij 99 and Triton X-100 to solubilize proteins and to preserve their interactions.20,21 These results suggested that CD36 may be involved in distinct protein complexes in different cell membrane microdomains. For example, integrins, VEGFR-2, Src, Cbl, and the p85 subunit of PI3K were detected in Brij 99 lysates, while caspase 3, Syk, and Vav were detected in Triton X-100 lysates (supplemental Figure 1B). Biotinylated nonimmune IgG was used as a negative control to detect the possible background signals from IgG or biotinylation (supplemental Figure 1A). Among the identified proteins, some were already known to interact with CD36 (Fyn, Yes, and integrin β1) in various cell types, which helped to validate our experimental approach.8,13,15-18 We chose VEGFR-2, integrins, and Syk as potential candidates in regulating CD36-dependent membrane proximal signaling events because VEGFR-2 mediates endothelial cell migration in response to VEGF-A,22 and Syk is important in integrin αIIbβ3-mediated platelet aggregation.23
CD36 associates with integrins and tetraspanins in HDMECs
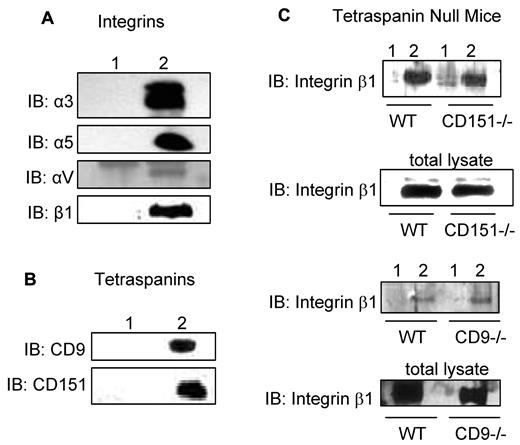
The association of CD36 with integrins αV, α5, and β1 subunits and the tetraspanins CD9 and CD151 was confirmed by CD36 immunoprecipitation from Brij 99-solubilized lysates (Figure 1). To determine whether CD9 or CD151 is required for complex formation, extracts were prepared from lung tissues from CD9- and CD151-null mice. The association of CD36 with integrin β1 is detected in both CD9- and CD151-null tissue extracts suggesting that neither tetraspanin is required (Figure 1C). Extraction of surface-biotinylated HDMECs with various detergents revealed high-molecular-weight bands corresponding to integrin subunits, and lower-molecular-weight bands relating to CD9 and other tetraspanins in Brij 99 and CHAPS CD36 immunoprecipitations (supplemental Figure 2A). These bands were markedly reduced or absent in Triton X-100 lysates, and only the lower-molecular-weight band was detected in Brij 99 with 0.2% Triton X-100–solubilized lysates (supplemental Figure 2A). Addition of 5mM MgCl2 to Brij 96 lysis buffer, which has been reported to maintain specific interactions,24,25 preserved the association of CD36 with integrins and tetraspanins (supplemental Figure 2B). These data indicate that CD36 and integrins are constituents of TEMs.
Association of CD36 with integrins and tetraspanins. HDMECs were lysed in 1% Brij 99 (supplemental Figures 1-2). One milligram of protein was immunoprecipitated with a combination of 3 μg of FA6-152 and 2 μg of CRF-2712 anti-CD36 Abs (lane 2) or 5 μg of nonimmune mouse Ab (lane 1). The immunoprecipitants were Western blotted with Abs against (A) identified integrin subunits in Ab-array assays in supplemental Figure 1B or in platelets and (B) tetraspanin family members. (C) To address the role of CD9 and CD151 as linkers for the association of integrin β1 with CD36, lungs from CD151- and CD9-null mice were isolated, and proteins were extracted, as described in the immunoprecipitation section in “Methods.” Anti-CD36 Abs (lane 2) or nonimmune IgG (lane 1) immunoprecipitants were probed for anti-integrin β1 Abs. The lack of CD9 or CD151 did not abolish the CD36-β1 interaction suggesting that either these 2 tetraspanins can compensate for each other,24 or there is a direct interaction between CD36 and β1, as proposed by others.8,18
Association of CD36 with integrins and tetraspanins. HDMECs were lysed in 1% Brij 99 (supplemental Figures 1-2). One milligram of protein was immunoprecipitated with a combination of 3 μg of FA6-152 and 2 μg of CRF-2712 anti-CD36 Abs (lane 2) or 5 μg of nonimmune mouse Ab (lane 1). The immunoprecipitants were Western blotted with Abs against (A) identified integrin subunits in Ab-array assays in supplemental Figure 1B or in platelets and (B) tetraspanin family members. (C) To address the role of CD9 and CD151 as linkers for the association of integrin β1 with CD36, lungs from CD151- and CD9-null mice were isolated, and proteins were extracted, as described in the immunoprecipitation section in “Methods.” Anti-CD36 Abs (lane 2) or nonimmune IgG (lane 1) immunoprecipitants were probed for anti-integrin β1 Abs. The lack of CD9 or CD151 did not abolish the CD36-β1 interaction suggesting that either these 2 tetraspanins can compensate for each other,24 or there is a direct interaction between CD36 and β1, as proposed by others.8,18
CD36 interacts with Syk and VEGFR-2
We also confirmed the associations of CD36 with Syk, VEGFR-2, Vav, and p85 subunit of PI3K in Brij 99– or Triton X-100–solublized HDMECs (Figure 2A).26 The interactions of CD36 with VEGFR-2 and PI3K were detected in Brij 99 lysates, while associations with Syk and Vav were detected in Triton X-100 lysates (Figure 2A). CD36 was also detected in reciprocal immunoprecipitations performed with anti-Syk and anti-VEGFR-2 Abs (Figure 2B-C). Because both VEGFR-2 and Syk interacted with CD36, we investigated the association of VEGFR-2 with Syk in Brij 99- and Triton X-100–solubilized HDMEC lysates (Figure 2C-D). The results showed higher association of Syk with VEGFR-2 in Brij 99 lysates compared with those made with Triton X-100. The association of VEGFR-2 with CD36 was also diminished in Triton X-100 lysates, but maintained in Brij 99, and Brij 96 with MgCl2 lysates (Figure 2E).26
Interaction of CD36 with Syk, VEGFR2, and their downstream signaling proteins. HDMECs were lysed either in 1% Brij 99 or 1% Triton X-100, and an equal amount of protein was used for immunoprecipitation. (A) Anti-CD36 (lane 2) and nonimmune IgG (lane 1) immunoprecipitants were Western blotted with Abs to Syk, Vav, and p85 PI3K. The p85 subunit of PI3K was detected in 1% Brij 99, while Syk and Vav were present in 1% Triton X-100 immunoprecipitants (supplemental Figure 1B). (B) Triton X-100–solubilized lysates were immunoprecipitated with anti-Syk (lane 2) and nonimmune IgG (lane 1) Abs (supplemental Figure 1B). The nitrocellulose membrane was probed with an anti-CD36 Ab. (C) In reciprocal immunoprecipitations, HDMEC cells were lysed in 1% Brij 99 and immunoprecipitated with anti-VEGFR-2 (lane 2) and nonimmune IgG (lane 1) Abs, and immunoprecipitants were probed with either anti-Syk (left) or anti-CD36 (right) Abs (supplemental Figure 3). (D-E) To determine whether Triton X-100 can disrupt the association of Syk and CD36 with VEGFR-2, HDMECs were lysed in either 1% Brij 99 or 1% Triton X-100 and immunoprecipitated with anti-VEGFR-2 (lane 2) and nonimmune IgG (lane 1) Abs. Immunoprecipitants were probed with anti-Syk, anti-CD36, and anti-VEGFR-2 Abs, as indicated.
Interaction of CD36 with Syk, VEGFR2, and their downstream signaling proteins. HDMECs were lysed either in 1% Brij 99 or 1% Triton X-100, and an equal amount of protein was used for immunoprecipitation. (A) Anti-CD36 (lane 2) and nonimmune IgG (lane 1) immunoprecipitants were Western blotted with Abs to Syk, Vav, and p85 PI3K. The p85 subunit of PI3K was detected in 1% Brij 99, while Syk and Vav were present in 1% Triton X-100 immunoprecipitants (supplemental Figure 1B). (B) Triton X-100–solubilized lysates were immunoprecipitated with anti-Syk (lane 2) and nonimmune IgG (lane 1) Abs (supplemental Figure 1B). The nitrocellulose membrane was probed with an anti-CD36 Ab. (C) In reciprocal immunoprecipitations, HDMEC cells were lysed in 1% Brij 99 and immunoprecipitated with anti-VEGFR-2 (lane 2) and nonimmune IgG (lane 1) Abs, and immunoprecipitants were probed with either anti-Syk (left) or anti-CD36 (right) Abs (supplemental Figure 3). (D-E) To determine whether Triton X-100 can disrupt the association of Syk and CD36 with VEGFR-2, HDMECs were lysed in either 1% Brij 99 or 1% Triton X-100 and immunoprecipitated with anti-VEGFR-2 (lane 2) and nonimmune IgG (lane 1) Abs. Immunoprecipitants were probed with anti-Syk, anti-CD36, and anti-VEGFR-2 Abs, as indicated.
CD36 colocalizes with VEGFR-2 in intact HDMECs
Strong expression of CD36 and VEGFR-2 on HDMECs was detected by immunofluorescence microscropy (supplemental Figure 3A-B). An intensity correlation analysis of these images showed significant colocalization of CD36 and VEGFR-2 predominantly on the cell edges (supplemental Figure 3C). Treatment of HDMECs with VEGF-A results in internalization of VEGFR-2 and activation of downstream signaling proteins to promote endothelial cell migration.27,28 Similar behavior has been reported for CD36 during uptake of oxidized LDL.29 Our data indicate that CD36 and VEGFR-2 colocalize at the cell surface in a protein pool that is initially targeted to the plasma membrane, and the interaction of CD36 with VEGFR-2 occurs before ligand binding and receptor internalization.
CD36 induces Syk expression
To address the role of TSP-1, as a major ligand for CD36 in endothelial cells, in mediating the association of CD36 with integrins, VEGFR-2, and Syk, we investigated these associations in the lung tissues from wild-type and TSP-1–null mice. Western blot analysis of lung tissue, which is a proximately 50% endothelial cells, indicated that the absence of TSP-1 did not significantly affect the expression level of VEGFR-2, but it increased the levels of CD36 and Syk, which suggested the possible correlations between CD36 and Syk expression (supplemental Figure 4A). To further address this correlation, the expression of Syk was examined in CD36-null lung tissues. Whereas no differences were detected in the level of VEGFR-2 expression in CD36-null lung tissue, the level of Syk was markedly reduced. To further address the effect of CD36 on Syk expression, HUVECs, which express low levels of CD36 and Syk, were infected with full-length CD36 expression or control vectors.30 Higher levels of CD36 and Syk were observed in the lysates of CD36-overexpressing HUVECs compared with control cells, but the level of VEGFR-2 remained the same (supplemental Figure 4B). Furthermore, passaging and culturing of endothelial cells can affect the level of CD36 expression (supplemental Figure 5B). Our results demonstrated that the correlation between the levels of Syk and CD36 expression was also detected in various detergent lysates and different HDMEC preparations (supplemental Figure 5A-B). However, more experiments are required to determine the mechanism by which CD36 modulates Syk expression.
TSP-1 affects the association of CD36 with signaling proteins
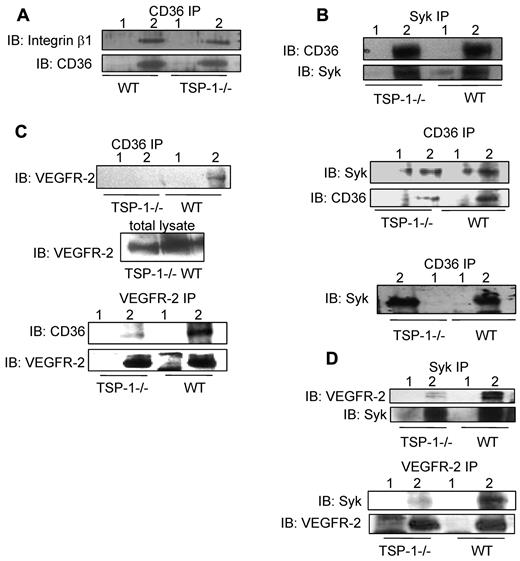
We compared detergent solubilized lung extracts from wild-type and TSP-1–null mice to determine whether TSP-1 affects complex formation. The absence of TSP-1 did not significantly affect the interaction of CD36 with Syk, but it did result in an approximately 20% decrease in the association of CD36 with integrin β1 (Figure 3A-B). The knockout of TSP-1 markedly diminished the CD36-VEGFR-2 and Syk-VEGFR-2 associations (Figure 3C-D). Reciprocal immunoprecipitations performed with an anti-VEGFR-2 Ab demonstrated a similar decrease in the association of VEGFR2 with Syk and CD36 in TSP-1–null lung tissue (Figure 3C-D). These studies suggest that TSP-1 has an important role for maintaining the Syk-CD36-VEGFR-2 complex formation.
Association of CD36 with VEGFR-2, Syk, and integrin β1 subunit in the lungs of wild-type and TSP-1--null mice. (A) Brij 99 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or anti-CD36 Ab (lane 2) and Western blotted with anti-β1 integrin Ab. (B) Top: Triton X-100 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or an anti-Syk Ab (lane 2) and Western blotted with an anti-CD36 Ab. Middle: In parallel experiments, tissue extracts were immunoprecipitated with anti-CD36 Ab (lane 2) and nonimmune IgG (lane 1) and Western blotted with anti-Syk and anti-CD36 Abs, as indicated. Bottom: The high-density fractions of sucrose gradients prepared with Triton X-100 extracts of TSP-1-null and wild-type lung tissues (supplemental Figure 6B) were immunoprecipitated with nonimmune IgG (lane 1) or anti-CD36 Ab (lane 2) and Western blotted with an anti-Syk Ab. (C) Top: Brij 99 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or anti-CD36 Ab (lane 2) and Western blotted with anti-VEGFR2 Ab. In this experiment, the total lysates were used as loading control (middle panel). Bottom: Brij 99 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or anti-VEGFR-2 Ab (lane 2) and Western blotted with anti-CD36 Ab. The immunoprecipitates were also Western blotted with an anti-VEGFR-2 Ab to establish that comparable amounts of VEGFR-2 were immunoprecipitated from the TSP-1–null and wild-type tissue. (D) Top: Brij 99 lysates from TSP-1–null and wild-type lung tissue were immunoprecipitated with nonimmune IgG (lane 1) or anti-Syk Ab (lane 2) and Western blotted with anti-VEGFR-2 or anti-Syk Abs, as indicated. Bottom: Brij 99 lysates from TSP-1–null and wild-type lung tissue were immunoprecipitated with nonimmune IgG (lane 1) or anti-VEGFR-2 Ab (lane 2) and Western blotted with anti-Syk and anti-VEGFR-2 Abs. In these experiments, either total lysates or immunoprecipitants were also Western blotted to establish that comparable amount of proteins were detected or immunoprecipitated from TSP-1–null and wild-type tissues.
Association of CD36 with VEGFR-2, Syk, and integrin β1 subunit in the lungs of wild-type and TSP-1--null mice. (A) Brij 99 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or anti-CD36 Ab (lane 2) and Western blotted with anti-β1 integrin Ab. (B) Top: Triton X-100 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or an anti-Syk Ab (lane 2) and Western blotted with an anti-CD36 Ab. Middle: In parallel experiments, tissue extracts were immunoprecipitated with anti-CD36 Ab (lane 2) and nonimmune IgG (lane 1) and Western blotted with anti-Syk and anti-CD36 Abs, as indicated. Bottom: The high-density fractions of sucrose gradients prepared with Triton X-100 extracts of TSP-1-null and wild-type lung tissues (supplemental Figure 6B) were immunoprecipitated with nonimmune IgG (lane 1) or anti-CD36 Ab (lane 2) and Western blotted with an anti-Syk Ab. (C) Top: Brij 99 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or anti-CD36 Ab (lane 2) and Western blotted with anti-VEGFR2 Ab. In this experiment, the total lysates were used as loading control (middle panel). Bottom: Brij 99 lysates from TSP-1–null and wild-type lung tissues were immunoprecipitated with nonimmune IgG (lane 1) or anti-VEGFR-2 Ab (lane 2) and Western blotted with anti-CD36 Ab. The immunoprecipitates were also Western blotted with an anti-VEGFR-2 Ab to establish that comparable amounts of VEGFR-2 were immunoprecipitated from the TSP-1–null and wild-type tissue. (D) Top: Brij 99 lysates from TSP-1–null and wild-type lung tissue were immunoprecipitated with nonimmune IgG (lane 1) or anti-Syk Ab (lane 2) and Western blotted with anti-VEGFR-2 or anti-Syk Abs, as indicated. Bottom: Brij 99 lysates from TSP-1–null and wild-type lung tissue were immunoprecipitated with nonimmune IgG (lane 1) or anti-VEGFR-2 Ab (lane 2) and Western blotted with anti-Syk and anti-VEGFR-2 Abs. In these experiments, either total lysates or immunoprecipitants were also Western blotted to establish that comparable amount of proteins were detected or immunoprecipitated from TSP-1–null and wild-type tissues.
Sucrose gradient fractionation was performed to determine whether the differential distribution of CD36, VEGFR-2, and Syk affected their association in the wild-type and TSP-1–null lung tissues. VEGFR-2 and Syk were present in high-density fractions of Brij 99 and Triton X-100 sucrose gradients of wild-type and TSP-1–null lysates, while CD36 was detected in both the high- and low-density fractions (supplemental Figure 6A-B). We confirmed the association of CD36 and Syk in the high-density fraction using immunoprecipitation (Figure 3B). We also detected the presence of TSP-1 in the same high-density fractions as VEGFR-2 and Syk (data not shown). Together, our results suggest the presence of 2 CD36 pools in the plane of the cell membrane. One pool of CD36 is colocalized with VEGFR-2 and Syk in TEMs. Another pool of CD36 is distributed in low-density lipid rafts and localized with Src family kinases. The fact that TSP-1 is strongly detected in the high-density fractions, is consistent with the concept that TSP-1 plays an important role in the formation of the VEGFR-2-Syk-CD36 complex, as shown in Figure 3. Further experiments are required to determine whether the activation state of the cells, such as treatment of cells with VEGF-A, can affect the distribution of CD36.
Syk participates in the VEGFR-2 signaling pathway
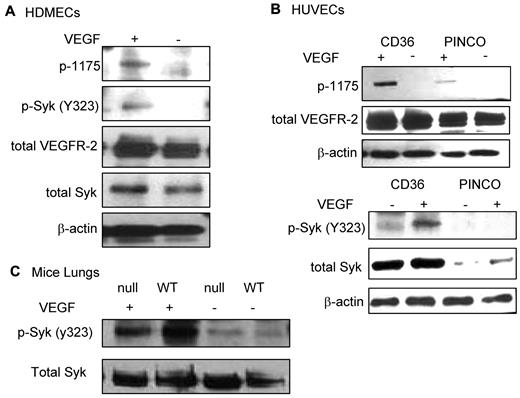
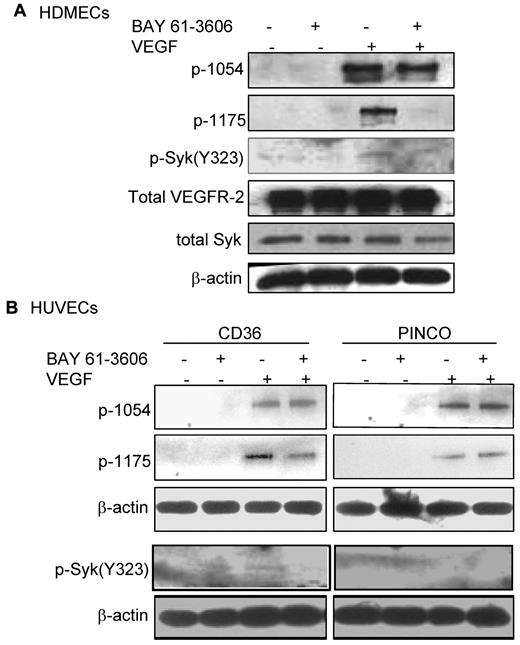
Recent studies have demonstrated that phosphorylation of B-cell receptors on the ITAM sequence results in activation and phosphorylation of Syk.31 Activated Syk, then, phosphorylates neighboring B-cell receptors and other Syk molecules in a positive feedback loop to enhance the B-cell signaling pathway. To determine whether the association of Syk with VEGFR-2 was also sufficient to alter the phosphorylation level of either protein, similar to what has been shown for the B-cell receptor, HDMECs were serum-starved overnight and treated with VEGF-A (50 ng/mL). Phosphorylation of Syk at Y323, one of the major autophosphorylation sites and a docking site for the p85 subunit of PI3K,32,33 was detected in VEGF-A–treated samples (Figure 4A). We also examined the effect of CD36 on the phosphorylation of VEGFR-2 at Y1175. The results demonstrated an increase in Y1175 phosphorylation in HUVECs with overexpression of CD36, and therefore Syk, compared with HUVECs infected with control vector (Figure 4B). VEGF-A induces autophosphorylation of VEGFR-2 at Y951 and Y1054/1059.34-36 Our results also showed that the up-regulation of Syk and CD36 expression did not affect the level of p-Y1054 after VEGF-A treatment (Figure 5), which suggests that the effect of the CD36-Syk complex is specific to Y1175. Recently, we reported that VEGF-A–induced phosphorylation at Y1175 of VEGFR-2 was diminished in TSP-1–null lung tissue.26 To determine whether the absence of TSP-1 also affected the VEGF-A–induced phosphorylation of Syk, lung extracts from VEGF-treated TSP-1–null and wild-type mice were examined with an Ab specific to p-Y323 Syk (Figure 4C). Even though we detected higher expression levels of Syk in TSP-1–null tissue, there was a significant decrease in the level of p-Y323 (Figure 4C). These observations could be explained by a marked reduction in the association of CD36 and Syk with VEGFR-2 in TSP-1–null lung tissues and the fact that TSP-1 was required for the formation of the VEGFR-2-CD36-Syk complex (Figure 3C-D). The low, but detectable, level of Syk and VEGFR-2 phosphorylation in TSP-1–null lung tissue suggests that other kinases, possibly Src, can also phosphorylate VEGFR-2 and Syk, and this effect is independent of TSP-1.34 The effect of Syk on VEGFR-2 phosphorylation was confirmed by treating cells with a Syk inhibitor (BAY 61-3606, Syk inhibitor IV) that inhibits Syk autophosphorylation and activation at a concentration of 4.7μM with no effect on Src family kinases.37 HDMECs and HUVECs were serum-starved overnight with 2% and 0.25% serum, respectively. These cells were first treated with 4.7μM BAY 61-3606 for 30 minutes at 37°C, and then with VEGF-A (50 ng/mL) for an additional 10 minutes. As expected, treatment with BAY 61-3606 decreased p-Y323 (Figure 5).37 Inhibition of Syk did not affect the phosphorylation of VEGFR-2 at Y-1054 in either cell type, but it significantly reduced p-Y1175 (Figure 5). Tyrosine 1175 of VEGFR-2 has an important role in mediating endothelial cell migration.22,38,39 Substitution of Y1175 with phenylalanine in knock-in mice causes an early embryonic lethal phenotype like that seen in VEGFR-2 knockout mice, indicating that phosphorylation at this site is essential for VEGFR-2 function.40 We also investigated the effect of CD36 and Syk on VEGFR-2–induced cell migration in response to VEGF-A. HUVECs with overexpression of CD36 and Syk exhibited a significantly higher level of migration toward VEGF-A relative to control cells (Figure 6B). The migration of HDMECs and HUVECs with overexpression of CD36 was comparable (Figure 6A). Whereas this increase in migration was abrogated by BAY 61-3606 treatment of HDMECs and CD36-expressing cells, treatment of control HUVECs with BAY 61-3606 did not significantly affect their migration (Figure 6A-B). To establish that the effect of BAY 61-3606 was specific to Syk, we also used siRNA to knockdown Syk expression in CD36-expressing HUVECs. Decreased cell migration was also detected after Syk knockdown (supplemental Figure 7A-B).
VEGF-A–induced phosphorylation of Syk (Y323). (A) HDMECs were serum-starved overnight in media containing 2% FBS. Cells were washed and incubated with either 0.5% FBS in PBS or treated with VEGF-A (50 ng/mL) in 0.5% FBS in PBS for 10 minutes at 37°C. Cells were lysed in 1% Triton X-100 lysis buffer, and equal amounts of protein were Western blotted with anti-p-Y323 Syk, total Syk (supplemental Figure 5A), anti-p-Y1175 VEGFR-2, and total VEGFR-2 Abs, as indicated. The results showed an up-regulation of p-Y323 Syk in response to VEGF-A treatment. (B) Parallel studies were performed using HUVECs that were infected with full-length CD36 or empty (PINCO) vector (supplemental Figure 4B). After treatment of cells with VEGF-A, cells were lysed in 1% Triton X-100, and immunoblotting was performed as indicated. A significant increase in the level of p-Y1175 VEGFR-2 and p-Y323 Syk was only detected in HUVECs with overexpression of CD36. Note that Syk expression is significantly higher in the HUVECs that were engineered to express CD36 (bottom panel, total Syk, and supplemental Figure 4B). These experiments were repeated 3 times independently. (C) Wild-type and TSP-1–null mice were injected with 2 μg of VEGF-A in 100 μL of sterile PBS via the tail vein. After 5 minutes, mice were euthanized and lungs were harvested as described in the immunoblotting section in “Methods.” An equal amount of protein was Western blotted with anti-p-Y323 and anti-total Syk. VEGF-A treatment up-regulated the level of Syk phosphorylation as was seen in panel A, but the response was higher in wild-type mice compared with TSP-1–null, even though the wild-type mice express lower levels of total Syk (bottom panel and supplemental Figure 4A). The slight difference in Syk expression (Figure 4A-B) is possibly because of difference in loading, as it is also detected in actin and VEGFR-2 lanes. Because cells were treated only for 10 minutes, it is not sufficient time for VEGF-A to up-regulate Syk expression. In these experiments, we resuspended cells in PBS with BSA to avoid any interference from the media or serum.
VEGF-A–induced phosphorylation of Syk (Y323). (A) HDMECs were serum-starved overnight in media containing 2% FBS. Cells were washed and incubated with either 0.5% FBS in PBS or treated with VEGF-A (50 ng/mL) in 0.5% FBS in PBS for 10 minutes at 37°C. Cells were lysed in 1% Triton X-100 lysis buffer, and equal amounts of protein were Western blotted with anti-p-Y323 Syk, total Syk (supplemental Figure 5A), anti-p-Y1175 VEGFR-2, and total VEGFR-2 Abs, as indicated. The results showed an up-regulation of p-Y323 Syk in response to VEGF-A treatment. (B) Parallel studies were performed using HUVECs that were infected with full-length CD36 or empty (PINCO) vector (supplemental Figure 4B). After treatment of cells with VEGF-A, cells were lysed in 1% Triton X-100, and immunoblotting was performed as indicated. A significant increase in the level of p-Y1175 VEGFR-2 and p-Y323 Syk was only detected in HUVECs with overexpression of CD36. Note that Syk expression is significantly higher in the HUVECs that were engineered to express CD36 (bottom panel, total Syk, and supplemental Figure 4B). These experiments were repeated 3 times independently. (C) Wild-type and TSP-1–null mice were injected with 2 μg of VEGF-A in 100 μL of sterile PBS via the tail vein. After 5 minutes, mice were euthanized and lungs were harvested as described in the immunoblotting section in “Methods.” An equal amount of protein was Western blotted with anti-p-Y323 and anti-total Syk. VEGF-A treatment up-regulated the level of Syk phosphorylation as was seen in panel A, but the response was higher in wild-type mice compared with TSP-1–null, even though the wild-type mice express lower levels of total Syk (bottom panel and supplemental Figure 4A). The slight difference in Syk expression (Figure 4A-B) is possibly because of difference in loading, as it is also detected in actin and VEGFR-2 lanes. Because cells were treated only for 10 minutes, it is not sufficient time for VEGF-A to up-regulate Syk expression. In these experiments, we resuspended cells in PBS with BSA to avoid any interference from the media or serum.
Reduction of VEGF-A–induced p-Y1175 VEGFR-2 by a Syk inhibitor. (A) HDMECs were serum-starved overnight and washed with PBS (supplemental Figure 5A). Cells were incubated with 0.5% FBS in PBS with or without 4.7μM BAY 61-3606 (Syk inhibitor), as described in the endothelial kinase assay section in “Methods.” Cells were lysed in 1% Triton X-100, and an equal amount of protein was used for immunoblotting using Abs against p-Y323 Syk, or p-1054 VEGFR2 or p-Y1175 VEGFR-2. Inhibition of Syk did not affect the autophosphorylation of VEGFR-2 at Y1054, but it decreased phosphorylation of Y1175 in response to VEGF-A. (B) The experiment above was repeated using HUVECs that were infected with either control vector or full-length CD36 vector (supplemental Figure 4B). Cells were treated as described in panel A and phosphorylation of Syk and VEGFR-2 was detected. The results showed that Syk inhibitor reduced phosphorylation of VEGFR-2 at Y1175, but did not affect the level of p-Y1054 in cells with overexpression of CD36. The level of both p-Y1175 and p-Y1054 remained unchanged in control cells. These experiments were done independently 3 times.
Reduction of VEGF-A–induced p-Y1175 VEGFR-2 by a Syk inhibitor. (A) HDMECs were serum-starved overnight and washed with PBS (supplemental Figure 5A). Cells were incubated with 0.5% FBS in PBS with or without 4.7μM BAY 61-3606 (Syk inhibitor), as described in the endothelial kinase assay section in “Methods.” Cells were lysed in 1% Triton X-100, and an equal amount of protein was used for immunoblotting using Abs against p-Y323 Syk, or p-1054 VEGFR2 or p-Y1175 VEGFR-2. Inhibition of Syk did not affect the autophosphorylation of VEGFR-2 at Y1054, but it decreased phosphorylation of Y1175 in response to VEGF-A. (B) The experiment above was repeated using HUVECs that were infected with either control vector or full-length CD36 vector (supplemental Figure 4B). Cells were treated as described in panel A and phosphorylation of Syk and VEGFR-2 was detected. The results showed that Syk inhibitor reduced phosphorylation of VEGFR-2 at Y1175, but did not affect the level of p-Y1054 in cells with overexpression of CD36. The level of both p-Y1175 and p-Y1054 remained unchanged in control cells. These experiments were done independently 3 times.
Participation of Syk in the VEGFR-2 signaling pathway. (A-B) Syk-dependent migration of HUVECs and HDMECs in response to VEGF-A. HUVECs were infected with either control (PINCO) or full-length CD36 vector (supplemental Figure 4B-C). Cells were serum-starved, and some were treated with 4.7μM Syk inhibitor (BAY 61-3606) while others were left untreated, as described in the endothelial kinase assay section in “Methods.” Results are expressed as a percentage of migrated cells (mean ± SE, n = 8) in 2 independent experiments. P values were calculated with an unpaired Student t test against HUVECs or HDMECs with Syk inhibitor or without inhibitor and VEGF-A. In these experiments, we resuspended cells in PBS with BSA to avoid any interference from the media or serum. (C) A proposed model for the TSP-1 regulation of the VEGF-A–induced VEGFR-2 signaling pathway. (a) Binding of TSP-1 to CD36 and/or VEGFR-2, and their associated integrins and tetraspanins (not shown) brings the 2 receptors in close proximity. (b) VEGF-A initiates angiogenesis by binding to VEGFR-2 and inducing its autophosphorylation, which, ultimately promotes Syk phosphorylation directly or through other adaptor proteins. (c) Syk activation further phosphorylates VEGFR-2 at Y1175 and mediates endothelial cell migration. (d) In the absence of VEGF-A, the TSP-1-CD36 complex only promotes Fyn activation, which inhibits endothelial cell migration and returns the angiogenic switch to the “off” position. The dashed lines suggest the possible involvement of other signaling and adaptor proteins.
Participation of Syk in the VEGFR-2 signaling pathway. (A-B) Syk-dependent migration of HUVECs and HDMECs in response to VEGF-A. HUVECs were infected with either control (PINCO) or full-length CD36 vector (supplemental Figure 4B-C). Cells were serum-starved, and some were treated with 4.7μM Syk inhibitor (BAY 61-3606) while others were left untreated, as described in the endothelial kinase assay section in “Methods.” Results are expressed as a percentage of migrated cells (mean ± SE, n = 8) in 2 independent experiments. P values were calculated with an unpaired Student t test against HUVECs or HDMECs with Syk inhibitor or without inhibitor and VEGF-A. In these experiments, we resuspended cells in PBS with BSA to avoid any interference from the media or serum. (C) A proposed model for the TSP-1 regulation of the VEGF-A–induced VEGFR-2 signaling pathway. (a) Binding of TSP-1 to CD36 and/or VEGFR-2, and their associated integrins and tetraspanins (not shown) brings the 2 receptors in close proximity. (b) VEGF-A initiates angiogenesis by binding to VEGFR-2 and inducing its autophosphorylation, which, ultimately promotes Syk phosphorylation directly or through other adaptor proteins. (c) Syk activation further phosphorylates VEGFR-2 at Y1175 and mediates endothelial cell migration. (d) In the absence of VEGF-A, the TSP-1-CD36 complex only promotes Fyn activation, which inhibits endothelial cell migration and returns the angiogenic switch to the “off” position. The dashed lines suggest the possible involvement of other signaling and adaptor proteins.
Discussion
Here, we demonstrate several novel observations regarding the effects of TSP-1 and CD36 on VEGF-A–induced signal transduction. We show that CD36 forms at least 2 distinct multiprotein complexes with other proteins, including VEGFR-2, integrins, tetraspanins, Syk, and Fyn, which can be distinguished biochemically. The interaction of CD36 with Src family kinases occurs in lipid rafts and is preserved in Triton X-100.13,41 This interaction is reportedly mediated by lipids and propagates the anti-angiogenic signal of TSP-1.1,16 In contrast, the associations of CD36 with VEGFR-2, integrins, and tetraspanins are found in the high-density fractions of sucrose gradients and are preserved in Brij 99. Previous studies have reported that CD36, VEGFR-2, and tetraspanins interact with integrins.13,42-44 We have not determined whether these proteins form a single complex or multiple distinct complexes. Large portions of the lipid bilayer are not required to preserve these complexes because they are detected in lysates that have been centrifuged at 100 000g and in the high-density fractions of sucrose gradients. It is worth noting that the protein-protein associations described here can be detected with endogenous levels of protein expression.
We also detected a novel and robust association of CD36 with Syk, which was present in the high-density fractions of sucrose gradients prepared with Triton X-100. The CD36 cytoplasmic domains do not have the ITAM sequence that has been shown to bind Syk, suggesting that adaptor proteins may mediate the CD36-Syk association. Because Triton X-100 disrupts the interaction of CD36 with integrins and tetraspanins,13 these proteins are not required for the association of CD36 with Syk, which is preserved in Triton X-100. Our results also demonstrated that Syk expression is up-regulated by CD36. Recent studies have reported that Syk expression is low in HUVECs, but its expression is induced by VEGF-A.30
CD36 appears to regulate endothelial cell migration through a unique signaling pathway that involves VEGFR-2 and Syk. Our detection of diminished association of Syk and CD36 with VEGFR-2 in lung tissue from TSP-1–null mice suggests that TSP-1 supports the formation of this complex. Recently, we reported decreased VEGF-A–induced phosphorylation of VEGFR-2 at Y1175 in TSP-1–null mice compared with wild-type mice.26 Treatment with the anti-angiogenic domain of TSP-1 (designated 3TSR) also decreased phosphorylation at this site.26 Kaur et al, recently reported that intact TSP-1 has the same effect, which suggested that treatment with these proteins may compete for binding sites for endogenous TSP-1 and disrupt complex formation.45 Indeed, TSP-1 treatment also resulted in the disassociation of VEGFR-2 and another TSP-1 receptor, CD47.45 In our studies, we showed that TSP-1 was able to promote VEGF-A signal transduction in a short-term assay, which was consistent with a decreased permeability response to VEGF-A in TSP-1–null mice as measured by the Miles assay.26 These studies suggest that TSP-1 can prime VEGF-A signal transduction in endothelial cells. We do not know whether this effect persists beyond initial activation but because TSP-1 has antiangiogenic activity in most long-term assays, it appears that its inhibitory effect predominates over time.
We also found that overexpression of CD36, and consequently Syk, resulted in increased VEGF-A–induced phosphorylation of VEGFR2 at Y1175 and increased cell migration. These effects were specifically blocked by a Syk inhibitor (BAY 61-3606) and Syk siRNA. A previous study has shown that Y1175 is phosphorylated by Src, which may mediate the basal level of phosphorylation that we observed in the control HUVECs.34 Because BAY 61-3606 does not inhibit Src activity,37 it is unlikely that the reduced phosphorylation of Y1175 in BAY 61-3606–treated cells is because of inhibition of Src. This conclusion is consistent with the observation that BAY 61-3606 did not affect the VEGF-A–induced migration of HUVECs infected with the control vector. Therefore, these findings suggest that the inhibitory activity of BAY 61-3606 results from direct effects on Syk and not off-target effects on VEGFR-2 or other kinases. This conclusion is further reported by our experiments with Syk siRNA.
Recent studies have shown that the dimerization of C-type lectin receptor CLEC-2 enables it to use a single YXXL motif, designated hemITAM, to activate Syk.31,46,47 BecauseVEGFR-2 has a hemITAM sequence beginning at Y1175, a similar mechanism may enable VEGF-A to activate Syk directly through VEGFR-2 dimerization. Rolli et al,48 have demonstrated that after binding to ITAM sequence, Syk becomes autophosphorylated and phosphorylates other ITAM sequences, which recruit more Syk. Based on this model, we suggest that the presence of the Syk-CD36-VEGFR-2 complex in the TEMs brings the VEGFR-2 hemITAM sequence in close proximity to Syk. On VEGF-A–induced dimerization of VEGFR-2, binding of Syk to the p-hemITAM results in the activation of Syk and the initiation the Syk/p-hemITAM positive feedback loop.
Our findings identify a novel role for TSP-1 in the regulation of the endothelial cell's response to VEGF-A and suggest important implications for the nature of the angiogenic switch. Whereas the basal set point for the angiogenic switch in adult tissue is “off,” it is probably beneficial to have the activation occur with rapidity and sensitivity in response to activators such as VEGF-A. This could be accomplished by maintaining the pro- and anti-angiogenic signals in close balance; however, this might result in an unstable state in which the angiogenic switch would be prone to toggling on and off. Using TSP-1 to prime the VEGF-A signaling pathway, TSP-1 can maintain the angiogenic switch in the “off” position in the absence of VEGF-A, and facilitate the transition to the “on” position when VEGF-A is present (Figure 6B). In our model, we propose that TSP-1 promotes the formation of the CD36-Syk-VEGFR-2 complex in TEMs where β1 integrins may also function as TSP-1 receptors. In the presence of VEGF-A, the angiogenic switch is in “on” position, and VEGF-A induces the autophosphorylation of VEGFR-2, which, directly or indirectly, increases the level of Syk autophosphorylation and activation. Activated Syk phosphorylates VEGFR-2 at Y1175 and promotes endothelial cell migration. This approach may permit angiogenesis to proceed in the presence of TSP-1 until VEGF-A levels decrease and the angiogenic switch returns quickly to the “off” position. In the absence of VEGF-A, the TSP-1-CD36 complex promotes Fyn phosphorylation and activation resulting in the inhibition of endothelial cell migration. Therefore, by using TSP-1 and CD36 to both inhibit and prime endothelial cell responses, the degree of priming is inherently coupled to the extent of inhibition.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr William Luscinskas for providing HUVECs, Dr Xuefeng Zhang for extracts of VEGF-treated lung tissue, and Drs Martin E. Hemler and Alexander R. Kazarov (Dana-Farber Institute, Boston, MA) for providing us with the lung tissues from CD151-null mice. We also thank Drs Sareh Parangi and Vassiliki Boussiotis for helpful suggestions.
This work has been supported by National Cancer Institute grant CA130895. S.K. is supported by NIH institutional training grant T32 HL07893.
National Institutes of Health
Authorship
Contribution: S.K., M.D., R.K.-F., and J.L. designed the experiments and analyzed the results; S.K., M.D., and M.A.R. did the biochemistry and cell biology to establish the CD36 associations with other proteins; C.P. isolated and cultured HDMECs and performed the bead isolation of CD36-expressing HUVECs; S.K. and K.S. performed the in vivo studies; L.P. and F.B. prepared the control and CD36 expression vectors and M.D. prepared the infected cells; M.D. and I.R. performed the localization of CD36 and VEGFR2; and S.K., J.T.L., and J.L. prepared the manuscript with editorial input from all of the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Jack Lawler, PhD, Department of Pathology, Division of Experimental Pathology, Beth Israel Deaconess Medical Center, RN 270-K, 99 Brookline Ave, Boston, MA 02215; e-mail: jlawler@bidmc.harvard.edu.