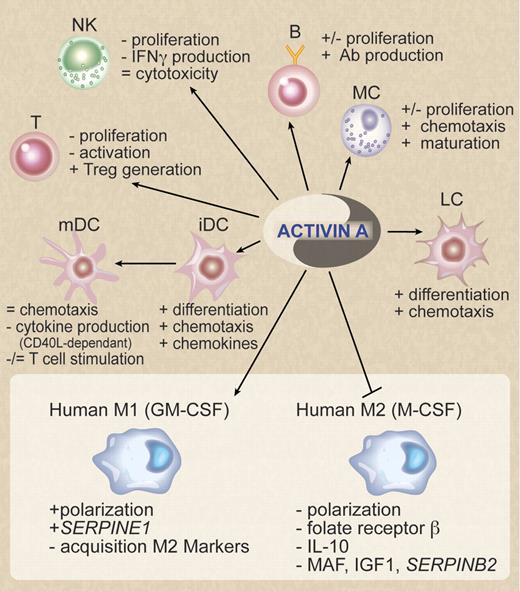
In this issue of Blood, Sierra-Filardi and colleagues shed new light on the still poorly understood proinflammatory role of Activin A in macrophage polarization.1
Activin A is a member of the TGFβ family first identified in late 1980s as an inducer of follicle-stimulating hormone. Similarly to other members of the family, Activin A is highly conserved in evolution and throughout the animal kingdom and regulates a variety of biologic processes including cell proliferation, hematopoiesis, wound healing, and fibrosis. Activin A signals through the activin type I (Alk2, 4, or 7) and type II (ActRII or ActRIIB) receptors and shares with TGFβ the activation of the Smad cascade.2,3 In the past few years the crucial role of TGFβ in the regulation of both innate and adaptive immunity has been thoroughly investigated. In contrast, the role of other members of the TGFβ family in immunity is more elusive and only recently Activin A has attracted the attention of immunologists for the ability to modulate innate and adaptive immune responses.
A role in inflammation is supported by the observation that Activin A is rapidly induced after intravenous injection of LPS with a kinetics that precedes those of primary proinflammatory cytokines, like TNFα, IL-1β, and IL-6. Increased circulating levels of Activin A were reported in certain inflammatory conditions, such as inflammatory bowel disease and rheumatoid arthritis and in humans during bacterial septicemia, hepatitis C infection, and trauma conditions. The role of Activin A in inflammation is further supported by the finding that the administration of follistatin, a natural receptor antagonist, inhibits the onset of the inflammatory cytokine cascade. However, the pure proinflammatory nature of Activin A was challenged in other pathologic situations. In atherosclerosis, Activin A behaves more like an anti-inflammatory mediator being able to inhibit the formation of foam cells. Similarly, in lung hypersensitivity, the systemic administration of Activin A resulted in a protective effect; although in the same experimental model, it acted as a proinflammatory mediator when administered locally.2,3
Many leukocytes, including monocytes, macrophages, dendritic cells, Th2, and B lymphocytes, produce Activin A, with Toll-like receptors being a main pathway of activation.2,4,5 Consistent with a positive action on immune response, Activin A was shown to stimulate the production of proinflammatory cytokines, iNOS, and MMP-2 in myeloid cells. Activin A also promoted in vitro and ex vivo the differentiation of Langerhans cells and the migration of myeloid dendritic cells, Langerhans cells, and mast cells.6-8 Finally, Activin A promoted IgG, and indirectly, IgE production by B cells. In contrast to these proinflammatory effects, Activin A was also reported to inhibit cytokine production in human monocytes/macrophages and to attenuate, in an autocrine manner, cytokine production by CD40L-, but not LPS- or Escherichia coli–activated dendritic cells.4 In the mouse, Activin A was also shown to inhibit phagocytosis and NO production in LPS-stimulated macrophages.2,3 The dichotomy between the pro- and anti-inflammatory actions of this cytokine is further underscored by the finding that Activin A can control Th1 and Th2 responses through the induction of antigen-specific regulatory T cells.9 Finally, Activin A was reported to suppress cytokine (IFNγ IL-6, TNFα, GM-CSF, and IL-1β) and chemokine (MIP-1β, MIP-1α, IL-8, and IP-10) production by activated NK cells, with no effect on their cytotoxic activity.10
In this issue of Blood, Sierra-Filardi and colleagues1 contribute new data to the complex scenario of Activin A regulation of the immune response. Using an in vitro protocol of human blood monocyte polarization to M1 and M2 macrophages, the authors found that M1 cell supernatant is able to inhibit the acquisition of M2 markers by M-CSF–stimulated cells (see figure). Searching for the factor responsible for this effect, the authors identified Activin A as the responsible molecule. Activin A was exclusively released by macrophages during M1 polarization and the presence of Activin A blocking antibodies inhibited the acquisition of M1 markers by GM-CSF–stimulated cells. M1 and M2 macrophages represent the extremes of a broad range of macrophage functional states. M1 macrophages release high levels of IL-12 and IL-23, are characterized by a high production of reactive toxic oxygen species, and mediate resistance against intracellular pathogens. Conversely, M2 macrophages are characterized by the low production of proinflammatory cytokines and play a role in resistance against parasites and in tissue remodeling.11 As discussed by Sierra-Filardi and colleagues, this report identifies a role for Activin A in the regulation of the transcriptome of M1 versus M2 macrophages and thus in the acquisition of the macrophage proinflammatory phenotype.
Schematic representation of the pleiotropic effects of Activin A on immune cells. At the bottom of the figure (in the box) the data on macrophage polarization reported by Sierra-Filardi et al are summarized.1 LC indicates Langerhans cells; iDC, immature dendritic cells; mDC, mature dendritic cells; MC, mast cells; +, stimulation; −, inhibition; and =, no effect. Professional illustration by Debra Dartez.
Schematic representation of the pleiotropic effects of Activin A on immune cells. At the bottom of the figure (in the box) the data on macrophage polarization reported by Sierra-Filardi et al are summarized.1 LC indicates Langerhans cells; iDC, immature dendritic cells; mDC, mature dendritic cells; MC, mast cells; +, stimulation; −, inhibition; and =, no effect. Professional illustration by Debra Dartez.
This report opens new perspectives but also raises new questions. Macrophages undergo different forms of M1 and M2 polarization according to their in vitro cytokine milieu. For instance, different types of M2 polarization are obtained using IL-4/IL-13, IL-10/glucocorticoids, or LPS/immunocomplexis.11 At the moment it is unclear whether the action of Activin A observed here in M-CSF–induced M2 macrophages might also be extended to other types of M2 macrophages. A second open issue is the analogy between human and mouse macrophages. Using mouse macrophages, Ogawa et al reported that Activin A increased the expression of arginase, a marker of mouse but not human M2 macrophages, and inhibited IFNγ-induced expression of iNOS, that is again a marker of mouse but not human M1 macrophages.8 Although both studies highlight a role for Activin A in macrophage polarization, the reason for this apparent discrepancy is unclear. It is possible that the different actions of Activin A should be interpreted in light of the high degree of plasticity that this cytokine shows in relation to the nature of the target cell, the nature of the agonist, and the duration and intensity of the response.8 In this context it is interesting to note that plasticity and multifaced responses seem to be common characteristics of other highly evolutionary conserved proteins, such as pentraxins12 and TGFβ itself, and possibly reflect a strategy to finely tune immune functions with a limited number of effector proteins.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■