The coagulation system is regulated by several anticoagulant mechanisms.1 The Protein C system provides important control of coagulation via the capacity of activated protein C (APC) to proteolytically inactivate the coagulation cofactors Va and VIIIa.
Protein C is converted into APC by thrombin bound to thrombomodulin, a receptor present on the vascular endothelium, in a process strongly augmented by the presence of the endothelial protein C receptor (EPCR). The factor V Leiden (FVL) mutation is a missense mutation in FV gene replacing arginine at position 506 with glutamine, which results in resistance of activated FV to inactivation by APC and causes increased thrombin formation and, thereby, an elevated risk of (mainly venous) thrombotic events.2 The high prevalence of this mutation (4%-6% in Caucasians) despite its prothrombotic effects has prompted speculation that the mutation might be subject to positive selection pressure.3 In the current issue of Blood, Wang et al report that one such evolutionary advantage of the FVL mutation may be protection against diabetic nephropathy (see figure).4
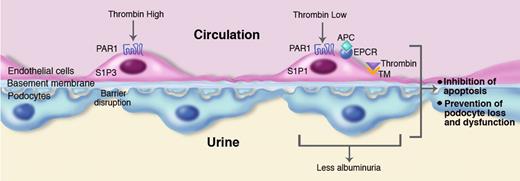
Role of thrombin in diabetic nephropathy. Sustained hyperglycemia results in diabetic nephropathy characterized by apoptosis of glomerular cells and loss and dysfunction of podocytes and albuminuria. Left side: High thrombin concentrations can induce disruption of the physiologic vascular barrier by an effect on protease activated receptor (PAR)1-dependent signaling via sphingosine 1 phosphate S1P receptor 3 (S1P3). High thrombin levels can also cause enhanced apoptosis of podocytes (not depicted in the figure). Right side: Low thrombin concentrations and the presence of activated protein C (APC) cause barrier protective effects via PAR1-dependent activation of another S1P receptor, S1P1. Both low thrombin levels and APC inhibit apoptosis of endothelial cells, as well as apoptosis and loss and dysfunction of podocytes, resulting in reduced albuminuria. Thrombin bound to thrombomodulin (TM) is essential for APC generation, a process augmented by the presence of the endothelial cell protein C receptor (EPCR). In this of Blood, Wang and colleagues4 show that the Factor V Leiden mutation, which is associated with modestly elevated thrombin generation, protects against diabetic nephropathy by mechanisms indicated in the right panel of the figure. Professional illustration by Marie Dauenheimer.
Role of thrombin in diabetic nephropathy. Sustained hyperglycemia results in diabetic nephropathy characterized by apoptosis of glomerular cells and loss and dysfunction of podocytes and albuminuria. Left side: High thrombin concentrations can induce disruption of the physiologic vascular barrier by an effect on protease activated receptor (PAR)1-dependent signaling via sphingosine 1 phosphate S1P receptor 3 (S1P3). High thrombin levels can also cause enhanced apoptosis of podocytes (not depicted in the figure). Right side: Low thrombin concentrations and the presence of activated protein C (APC) cause barrier protective effects via PAR1-dependent activation of another S1P receptor, S1P1. Both low thrombin levels and APC inhibit apoptosis of endothelial cells, as well as apoptosis and loss and dysfunction of podocytes, resulting in reduced albuminuria. Thrombin bound to thrombomodulin (TM) is essential for APC generation, a process augmented by the presence of the endothelial cell protein C receptor (EPCR). In this of Blood, Wang and colleagues4 show that the Factor V Leiden mutation, which is associated with modestly elevated thrombin generation, protects against diabetic nephropathy by mechanisms indicated in the right panel of the figure. Professional illustration by Marie Dauenheimer.
Diabetic nephropathy is a leading cause of end-stage renal failure in the developed world, with albuminuria, a key abnormality, caused by loss and dysfunction of podocytes. Wang and colleagues established that the FVL mutation reduces the extent of diabetic nephropathy using an approach that elegantly combines mouse and human studies.4 Relative to normal wild-type mice, heterozygous and homozygous FVL mice displayed reduced albuminuria after 26 weeks of persistent hyperglycemia induced by intraperitoneal administration of streptozotocin. Other manifestations of diabetic nephropathy were also attenuated in FVL mice, including extracellular matrix accumulation, the number of apoptotic cells in glomeruli, and podocyte loss and dysfunction. These findings were corroborated by the observation that type 1 and type 2 diabetes patients heterozygous for the FVL mutation showed reduced albuminuria compared with diabetes patients without FVL who were similar with regard to other risk factors for diabetic nephropathy such as glucose control and hypertension. Both experimental and clinical data generated by Wang and colleagues point to a decisive role for low but sustained thrombin generation in the protective phenotype of FVL carriers. Indeed, hirudin (a specific thrombin inhibitor) largely prevented the beneficial effect of the FVL mutation on the damage of diabetic nephropathy in mice, whereas diabetic patients carrying the 4G/5G polymorphism in the gene encoding plasminogen activator inhibitor type 1 (which results in increased fibrin but not thrombin formation) did not show reduced albuminuria compared with diabetes patients without this polymorphism. In vitro experiments further established that low-dose thrombin inhibited podocyte apoptosis induced by high glucose concentrations, a protective effect that was reversed at higher thrombin levels.
The current paper4 should be placed in the context of a recent report from the same group that examined the role of APC in diabetic nephropathy.5 Using 2 genetically modified mouse strains with opposite effects on the APC generation capacity, they found that decreasing APC exacerbated experimental diabetic nephropathy, whereas increasing APC prevented it. Notably, the protective effects of APC did not rely on its anticoagulant properties but rather on prevention of apoptosis of glomerular endothelial cells and podocytes. These cytoprotective effects of APC were dependent on the presence of protease activated receptor (PAR)1, a receptor expressed by multiple cell types that can be activated by various proteases including APC. This previous study5 taken together with the current work 4 suggests that low thrombin levels can protect against diabetic nephropathy by more than one mechanism. First, thrombin may cause enhanced APC generation after binding to thrombomodulin, resulting in PAR1-dependent cytoprotective effects on endothelium and inhibition of apoptosis of renal endothelial cells and podocytes. Second, thrombin may exert direct cytoprotective effects as indicated by the in vitro studies reported here by Wang and coworkers using purified podocytes.4 Although not addressed by Wang et al, PAR1 may also at least partially mediate these cytoprotective effects induced by low thrombin concentrations. Indeed, activation of PAR1 by thrombin can have a bimodal effect in endothelial cell permeability depending on the dose of thrombin administered, with low concentrations eliciting a barrier protective effect and high levels producing a barrier disruptive effect.6 Furthermore, at high thrombin levels, APC potently inhibits thrombin-induced vascular hyperpermeability by a mechanism dependent on trans-activation of the sphingosine 1 phosphate receptor 1 (S1P1),7 whereas high thrombin levels induce vascular hyperpermeability dependent on another S1P receptor, S1P3.8 Thus, PAR1 signaling contributes to setting the vascular S1P1/S1P3 balance in inflammatory disorders, whereby high thrombin levels mediate S1P3-dependent disruption of the vascular integrity and APC preserves vascular barrier function via S1P1. This dual role in mediating APC and thrombin effect makes PAR1 a difficult therapeutic target in inflammatory conditions, including in diabetic nephropathy.
The pathogenesis of diabetic nephropathy remains poorly understood. The recent publications by the group led by Isermann4,5 provide novel and interesting insight in how the coagulation system and in particular APC and thrombin may impact the development of this feared complication. Moreover, these comprehensive studies point to an evolutionary benefit conveyed by the mild prothrombotic state associated with the FVL mutation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■