Abstract
Human natural killer (NK)–cell repertoires are biased toward more frequent expression of inhibitory killer cell Ig-like receptor (KIR) receptors for self-human leukocyte antigen (HLA) class I. Moreover, only those NK cells that express cognate receptors for self are fully functional in terms of cytotoxicity and cytokine production. It is so far unknown whether functional education and structural adaptation to HLA class I are implemented during NK-cell development and whether both processes are mechanistically connected. Here we show that NK-cell repertoires in cord blood are not yet shaped toward increased clonal frequencies of KIR for self-HLA class I as determined for the 3 major KIR ligands C1, C2, and Bw4. Nonetheless, neonatal NK cells expressing cognate KIR exhibited enhanced effector function on the level of degranulation and cytokine production. The study suggests that functional education of cognate KIR by self-HLA class I precedes structural adaptation of KIR repertoires and that both processes are not directly linked to each other.
Introduction
Human natural killer (NK) cells express inhibitory receptors with specificity for MHC class I, encoded by the killer cell immunoglobulin-like receptor (KIR) family.1 A characteristic feature of KIR, similar to the functionally related Ly-49 family in rodents, is the clonally distributed expression mode, which creates a functionally diverse repertoire of NK cells that differ in the kind and number of receptors expressed on each given NK cell.2,3 The factors that govern formation of these NK-cell repertoires are incompletely understood but involve a strong stochastic component.4,5 In addition, the extensive polymorphisms of major histocompatibility complex (MHC) class I and KIR genes represent additional parameters that influence repertoire and function of NK cells.6 In humans, analogous to mice, structural adaptation of NK-cell repertoires to the highly polymorphic human leukocyte antigen (HLA) class I genes involves increase in frequency of NK cells expressing cognate inhibitory receptors.7-9 Moreover, the presence of MHC class I-encoded ligands also influences NK-cell function: NK cells expressing an inhibitory receptor for self-MHC class I exhibit increased effector functions compared with NK cells lacking a cognate inhibitory receptor.10-12 Together, functional education and structural adaptation increase the frequency of self-specific, functionally competent NK cells, which constitutes an efficient strategy to deal with the polymorphic nature of KIR and HLA class I genes.
It is so far unknown how and when functional and structural adaptation of NK cells to autologous MHC class I molecules occurs and whether this process is an integral part of NK-cell development or whether it rather acts on the stage of mature NK cells. In the latter case, differences in cognate receptor expression would be expected between naive and experienced NK-cell repertoires. In this regard, umbilical cord blood (CB) constitutes a source of neonatal NK cells that typically were not yet exposed to a broad range of pathogens, including viral infections and thus provides a correlate of naive NK cells. In this study, a thorough analysis of KIR and NKG2A repertoires was performed in NK cells from CB to better understand how naive NK cells are educated by self-HLA class I.
Methods
Cell isolation, KIR, and HLA class I genotyping
CB samples were kindly provided by the José Carreras Stem Cell Bank, Düsseldorf. Data of peripheral blood (PB) samples were extracted from Schönberg et al.9 HLA-C frequencies were similar between the CB and PB cohorts. The KIR haplotype distribution was slightly different with haplotype group A/A = 45.3%, A/B = 44.0%, and BB = 10.7% in PB versus haplotype group A/A = 34.5%, A/B = 52.2%, and B/B = 13.3% in CB. NK cells were isolated using the RosetteSep method (Stem Cell Technologies). KIR and HLA class I genotyping was performed by PCR–sequence-specific primer as reported previously.9,13
Flow cytometry
The following mouse anti–human monoclonal antibodies were used as previously described: CD56-PC5, CD159a-phycoerythrin (NKG2A), CD158a-allophycocyanin (KIR2DL1/S1), CD158e-ECD (KIR3DL1), CD158b-allophycocyanin-Cy7 (KIR2DL2/3/S2) (Beckman Coulter), and CD3–Pacific Blue (BD Biosciences). The CD158k-specific monoclonal antibody Q66 (KIR3DL2, kindly provided by A. Moretta) was used in combination with anti–mouse-IgM–fluorescein isothiocyanate (Beckman Coulter).
The CD107 degranulation assay was done as previously described using CD107a–fluorescein isothiocyanate (BD Biosciences).9 NK cells were cocultured with K562 cells at a 1:1 ratio for 6 hours. For measurement of intracellular IFN-γ, cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences), and intracellular IFN-γ production was measured using anti–IFN-γ fluorescein isothiocyanate monoclonal antibody (Invitrogen).
Results and discussion
We have previously shown that adaptation to self-HLA class I is a subtle process that requires conjoint consideration of all relevant inhibitory receptors on the clonal level.9 A similar approach was taken here for the analyses of neonatal NK-cell repertoires: KIR2DL1, KIR2DL2/3, and KIR3DL1, specific for the 3 major HLA class I–encoded ligands C2, C1, and Bw4, respectively, were measured together with the A3/A11-specific KIR3DL2 and the HLA-E–specific NKG2A by multicolor flow cytometry on CD56+CD3− NK cells.
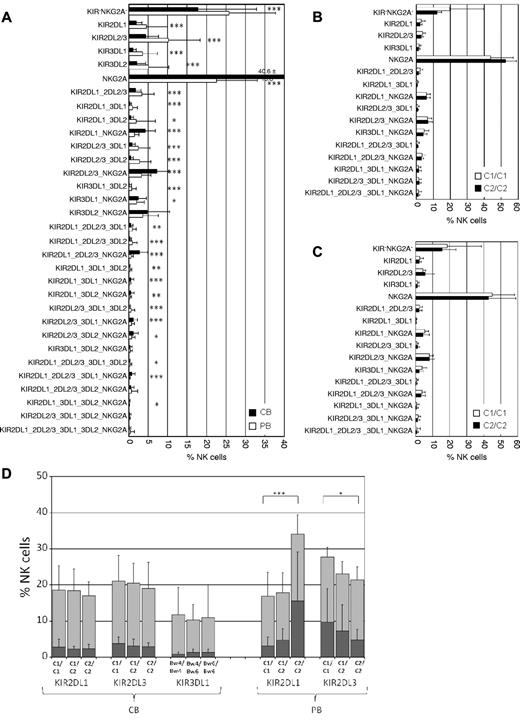
In the absence of published data on clonal NK-cell repertoires in CB, we first analyzed the distribution of the 32 clonal populations (ie, clonotypes) of inhibitory receptors compared with NK cells from adult PB. Similar to PB, all 32 receptor combinations were expressed in the NK-cell repertoires from CB (Figure 1A).14 Also in concordance with previous studies, NKG2A-expressing NK cells were much more abundant in CB than in PB (overall frequency: 67.9% vs 37.4%).15,16 Clonal analysis revealed that single-NKG2A+ as well as clonotypes coexpressing NKG2A together with one or more KIR were significantly more abundant in CB (Figure 1A). On the other hand, a significant decrease was seen in clonotypes expressing single KIR or multiple KIR without NKG2A. Finally, NKG2A−KIR− NK cells, which were previously shown to be hyporesponsive in peripheral blood, were significantly less abundant in CB.10
The KIR repertoires of neonatal NK cells are diverse but not biased toward recognition of cognate HLA class I. (A) The frequencies of all 32 KIR and NKG2A receptor combinations (clonotypes) that can be distinguished by the present flow cytometry approach are shown for CB (black bars, n = 90) and PB (white bars, n = 150) as mean with error bars representing SD. Clonotypes are ordered according to the number of expressed receptors starting with no receptor (KIR−NKG2A−), 1 receptor (either a single KIR or NKG2A), 2 receptors (ie, KIR2DL1_3DL1 refers to a clone expressing KIR2DL1 and KIR3DL1 and no other KIR or NKG2A), and so forth. Designation of clonotypes does not include cross-reactivity with stimulatory KIR. (B) Inhibitory receptor repertoires of CB donors with group A/A KIR haplotypes. The order of clonotypes was similar to that in panel A but without consideration of KIR3DL2. Donors were divided into subgroups possessing C1/C1 (white bars, n = 19) and C2/C2 (black bars, n = 11) ligands. (C) Inhibitory receptor repertoires of CB donors with group A/B and B/B KIR haplotypes. Donors were divided into subgroups possessing C1/C1 (white bars, n = 31) and C2/C2 (black bars, n = 22) ligands. (D) Frequency of CB- and PB-derived NK cells expressing KIR2DL1, KIR2DL3, or KIR3DL1 in donors with group A/A KIR haplotypes. For each given KIR, donors were stratified according to the indicated HLA class I ligands. Results are shown as stapled bars with overall frequency of NK cells expressing a given KIR (light gray) and the corresponding fraction of single-KIR+ NK cells (dark gray). Sample distribution was: C1/C1 (n = 19/38 for CB/PB), C1/C2 (n = 20/30), and C2/C2 (n = 11/10) as well as Bw4/Bw4 (n = 5 for CB), Bw4/Bw6 (n = 16), and Bw6/Bw6 (n = 9). Results are shown as mean with error bars representing SD. Data for PB were calculated from the cohort analyzed in Schönberg et al.9 Statistical significance was determined by 2-tailed t test: *P < .05, **P < .01, and ***P < .001.
The KIR repertoires of neonatal NK cells are diverse but not biased toward recognition of cognate HLA class I. (A) The frequencies of all 32 KIR and NKG2A receptor combinations (clonotypes) that can be distinguished by the present flow cytometry approach are shown for CB (black bars, n = 90) and PB (white bars, n = 150) as mean with error bars representing SD. Clonotypes are ordered according to the number of expressed receptors starting with no receptor (KIR−NKG2A−), 1 receptor (either a single KIR or NKG2A), 2 receptors (ie, KIR2DL1_3DL1 refers to a clone expressing KIR2DL1 and KIR3DL1 and no other KIR or NKG2A), and so forth. Designation of clonotypes does not include cross-reactivity with stimulatory KIR. (B) Inhibitory receptor repertoires of CB donors with group A/A KIR haplotypes. The order of clonotypes was similar to that in panel A but without consideration of KIR3DL2. Donors were divided into subgroups possessing C1/C1 (white bars, n = 19) and C2/C2 (black bars, n = 11) ligands. (C) Inhibitory receptor repertoires of CB donors with group A/B and B/B KIR haplotypes. Donors were divided into subgroups possessing C1/C1 (white bars, n = 31) and C2/C2 (black bars, n = 22) ligands. (D) Frequency of CB- and PB-derived NK cells expressing KIR2DL1, KIR2DL3, or KIR3DL1 in donors with group A/A KIR haplotypes. For each given KIR, donors were stratified according to the indicated HLA class I ligands. Results are shown as stapled bars with overall frequency of NK cells expressing a given KIR (light gray) and the corresponding fraction of single-KIR+ NK cells (dark gray). Sample distribution was: C1/C1 (n = 19/38 for CB/PB), C1/C2 (n = 20/30), and C2/C2 (n = 11/10) as well as Bw4/Bw4 (n = 5 for CB), Bw4/Bw6 (n = 16), and Bw6/Bw6 (n = 9). Results are shown as mean with error bars representing SD. Data for PB were calculated from the cohort analyzed in Schönberg et al.9 Statistical significance was determined by 2-tailed t test: *P < .05, **P < .01, and ***P < .001.
To explore the influence of HLA class I-encoded ligands on shaping of inhibitory KIR repertoires, the cohort was stratified according to the presence of C1 and C2 epitopes. Because previous analyses of NK cells from PB have shown that the dependence on HLA class I ligands was most easily detected in donors that were homozygous for the KIR haplotype group A, which encodes for a defined set of 6 KIR genes, the CB cohort was accordingly broken down.9,17 Surprisingly, no bias toward recognition of self-HLA class I could be detected in donors with group A/A or group B/B haplotypes (Figure 1B-C): in C2/C2 donors, clonotypes with cognate KIR2DL1 were not found at higher frequencies than in C1/C1 donors and, similarly, KIR2DL3 frequencies were not increased in C1/C1 donors. Moreover, neither the frequency of single-KIR nor the cumulative frequency of all NK cells expressing a given cognate KIR was biased toward self-ligands (Figure 1D). Whereas in PB we had previously observed a 4-fold difference in single-KIR2DL1+ NK cells between C1/C1 and C2/C2 donors, not even a tendency toward a similar bias was detected in CB.9 Analysis of statistical power revealed a high probability that the lack of bias was not because of insufficient sampling (β = 0.92 calculated with GPower Version 3.1). Similar results were obtained for KIR3DL1, which did not show a frequency bias toward the Bw4 epitope (Figure 1D). Finally, there was also no correlation found for KIR3DL2 and the presence of A3 and A11 epitopes (data not shown).
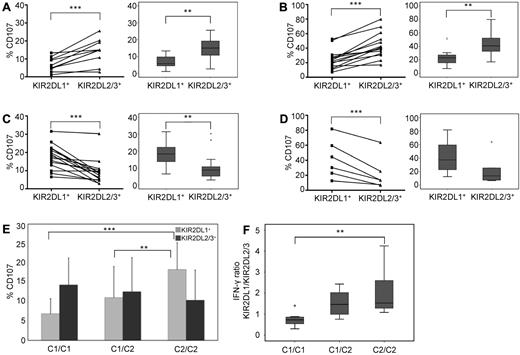
We next asked whether the lack of structural adaptation of KIR repertoires in CB was accompanied by a lack of functional education. To this end, we compared the degranulation responses of C1- and C2-specific NK cells against the class I–negative target cell line K562. The overall effector responses were generally lower in CB (Figure 2A,C) than in PB (Figure 2B,D), which is in agreement with previous literature.18 However, the educational impact of cognate ligands was comparable and highly significant in both sources. In C1/C1 donors, single-KIR2DL2/3+ NK cells exhibited higher effector responses than single-KIR2DL1+ NK cells (Figure 2A-B). Vice versa, single-KIR2DL1+ NK cells from C2/C2 donors exhibited higher effector responses than single-KIR2DL2/3+ NK cells from the same donors (Figure 2C-D). Direct comparison of donors according to the presence or absence of HLA-C ligands shows a dose-dependent and highly significant increase of CD107-producing single-KIR2DL1+ NK cells in the presence of cognate ligands (Figure 2E). As expected, no difference in CD107 mobilization was seen between the 2 clonotypes in C1/C2 donors (Figure 2E). Finally, an educational impact was also seen on IFN-γ production. As shown in Figure 2F, the ratio of single-KIR2DL1+ to single-KIR2DL2/3+ IFN-γ–producing cells did significantly increase in the presence of cognate C2 ligands in a dose-dependent manner, again in concordance with previous observations in PB.10
Neonatal NK cells are functionally educated by autologous HLA class I ligands. CD107 mobilization against K562 was measured in single-KIR2DL1+ and single-KIR2DL2/3+ NK cells from CB of C1/C1 (A) and C2/C2 (C) donors and similarly in PB of C1/C1 (B) and C2/C2 (D) donors. In each case, data are shown as individual frequencies with statistical significance calculated for the ratio of KIR2DL1 to KIR2DL2/3 (left side) and as box plots (right side). Boxes represent the median and 25th/75th percentiles; and whiskers, the lowest and highest data points without outliers (stars, open circles). Statistical significance was determined by 2-tailed t test: *P < .05, **P < .01, and ***P < .001. The sample distribution was C1/C1 (n = 10) and C2/C2 (n = 15) for CB and C1/C1 (n = 14) and C2/C2 (n = 6) for PB. (E) Changes in frequency of CD107-mobilizing single-KIR2DL1+ (light gray) and single-KIR2DL2/3+ (dark gray) NK cells of CB donors. Donors were the same as in panels A and C but including C1/C2 (n = 25) donors. Throughout the figure, functional analysis was done without further subdivision into KIR haplotype groups. Values represent the mean, and error bars represent the SD. (F) Production of IFN-γ in NK cells from CB after 6-hour coculture with K562. Donors were divided into C1/C1 (n = 8), C1/C2 (n = 25), and C2/C2 (n = 11) subgroups. Box plots represent the ratio between IFN-γ-producing single-KIR2DL1+ and single-KIR2DL2/3+ NK cells. P values were calculated by analysis of variance: *P < .05, **P < .01, and ***P < .001.
Neonatal NK cells are functionally educated by autologous HLA class I ligands. CD107 mobilization against K562 was measured in single-KIR2DL1+ and single-KIR2DL2/3+ NK cells from CB of C1/C1 (A) and C2/C2 (C) donors and similarly in PB of C1/C1 (B) and C2/C2 (D) donors. In each case, data are shown as individual frequencies with statistical significance calculated for the ratio of KIR2DL1 to KIR2DL2/3 (left side) and as box plots (right side). Boxes represent the median and 25th/75th percentiles; and whiskers, the lowest and highest data points without outliers (stars, open circles). Statistical significance was determined by 2-tailed t test: *P < .05, **P < .01, and ***P < .001. The sample distribution was C1/C1 (n = 10) and C2/C2 (n = 15) for CB and C1/C1 (n = 14) and C2/C2 (n = 6) for PB. (E) Changes in frequency of CD107-mobilizing single-KIR2DL1+ (light gray) and single-KIR2DL2/3+ (dark gray) NK cells of CB donors. Donors were the same as in panels A and C but including C1/C2 (n = 25) donors. Throughout the figure, functional analysis was done without further subdivision into KIR haplotype groups. Values represent the mean, and error bars represent the SD. (F) Production of IFN-γ in NK cells from CB after 6-hour coculture with K562. Donors were divided into C1/C1 (n = 8), C1/C2 (n = 25), and C2/C2 (n = 11) subgroups. Box plots represent the ratio between IFN-γ-producing single-KIR2DL1+ and single-KIR2DL2/3+ NK cells. P values were calculated by analysis of variance: *P < .05, **P < .01, and ***P < .001.
This is the first study to address the influence of HLA class I on KIR expression in CB. The present data suggest that HLA class I has no effect on initial KIR repertoire formation. The fact that NK-cell repertoires from CB are not biased toward expression of cognate inhibitory KIR suggests that adaptation of KIR expression toward self-HLA class I ligands is not an integral part of NK-cell development. In contrast, functional education, also referred to as licensing, is already in place in neonatal NK cells.12 Thus, the structural adaptation of adult KIR repertoires toward self-ligands is mechanistically uncoupled from the “tuning” of cognate receptors toward increased effector function. Importantly, in the light of the present data in neonatal blood, the original model of a ligand-instructed KIR repertoire development has to be modified.9 Indeed, our data suggest that the “instruction” process is somehow ineffective at the neonatal stage and is initiated only postnatally and in an antigen-experienced environment. An important question arising from this work is what triggers the change from an unbiased neonatal to a biased adult KIR repertoire. A plausible explanation would be that the frequency of NK cells expressing cognate KIR for self-HLA class I is affected by the individual immunologic experience that donors accumulate throughout life. In this regard, it was recently shown that viral infection can lead to specific and long-lasting expansion of NK-cell clones that express single cognate KIR.19 We thus consider the possibility that specific expansion of functionally educated NK cells in response to certain pathogens successively shapes naive NK-cell repertoires toward recognition of self-HLA class I. The accompanied substantial decrease in NKG2A frequency from birth to adulthood would be part of this structural transition from a more generic NK-cell repertoire that is able to accommodate a broad range of KIR and HLA class I polymorphisms ab initio to a more specific, KIR-dominated repertoire. The present work suggests that NK-cell repertoires go through major structural changes from birth to adulthood and raises the question of how this process influences clinical susceptibility to infection and disease. In this regard, it remains to be determined how unbiased neonatal NK-cell repertoires change during childhood and which role the individual infection history plays in this process.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all donors who volunteered for blood donation and Dr A. Moretta for the CD158k-specific antibody (Q66, KIR3DL2).
This work was supported by the Deutsche Forschungsgemeinschaft (grant UH91/5-1; M.U.).
Authorship
Contribution: K.S. designed the project, performed the experiments, and wrote the manuscript; J.C.F. designed the project and performed statistical analysis; G.K. organized the cord blood sampling workflow; and M.U. designed the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Uhrberg, University Clinic of Düsseldorf, Institute for Transplantation Diagnostics and Cell Therapeutics, Bldg 14.80, Moorenstrasse 5, D-40225 Düsseldorf, Germany; e-mail: uhrberg@itz.uni-duesseldorf.de.