Abstract
We have developed a tumor vaccine in which patient-derived myeloma cells are chemically fused with autologous dendritic cells (DCs) such that a broad spectrum of myeloma-associated antigens are presented in the context of DC-mediated costimulation. We have completed a phase 1 study in which patients with multiple myeloma underwent serial vaccination with the DC/multiple myeloma fusions in conjunction with granulocyte-macrophage colony-stimulating factor. DCs were generated from adherent mononuclear cells cultured with granulocyte-macrophage colony-stimulating factor, interleukin-4, and tumor necrosis factor-α and fused with myeloma cells obtained from marrow aspirates. Vaccine generation was successful in 17 of 18 patients. Successive cohorts were treated with 1 × 106, 2 × 106, and 4 × 106 fusion cells, respectively, with 10 patients treated at the highest dose level. Vaccination was well tolerated, without evidence of dose-limiting toxicity. Vaccination resulted in the expansion of circulating CD4 and CD8 lymphocytes reactive with autologous myeloma cells in 11 of 15 evaluable patients. Humoral responses were documented by SEREX (Serologic Analysis of Recombinant cDNA Expression Libraries) analysis. A majority of patients with advanced disease demonstrated disease stabilization, with 3 patients showing ongoing stable disease at 12, 25, and 41 months, respectively. Vaccination with DC/multiple myeloma fusions was feasible and well tolerated and resulted in antitumor immune responses and disease stabilization in a majority of patients.
Introduction
Although the discovery of novel biologic agents has improved therapeutic options for patients with multiple myeloma (MM), curative outcomes remain elusive because of the eventual emergence of resistant disease. Similarly, several studies have demonstrated that autologous stem cell transplantation results in improved disease survival compared with standard chemotherapy but ultimately does not prevent disease progression.1 In contrast, the unique efficacy of cellular immunotherapy is supported by the observation that allogeneic hematopoietic stem cell transplantation is curative for a subset of patients due to the graft-versus-disease effect mediated by alloreactive lymphocytes.2,3 However, allogeneic transplantation is associated with significant morbidity and mortality secondary to regimen-related toxicity and the lack of specificity of the alloreactive response, which results in the development of graft-versus-host disease. A major area of investigation is to develop immunotherapeutic strategies to elicit myeloma-specific immune responses that will selectively eliminate malignant cells and eradicate residual disease that persists after biologic therapy and autologous stem cell transplantation. A variety of tumor-associated antigens have been identified in myeloma cells that may be selectively targeted by host immunity, including the clonal idiotype and the epithelial mucin, MUC1.4-6 However, tumor cells evade immune recognition by presenting antigens in the absence of costimulatory molecules, secreting factors that inhibit antigen-presenting and effector cells, and the increased presence of regulatory T cells that inhibit antitumor immune responses.
Dendritic cell (DC)–based tumor vaccines are being explored as a promising strategy to stimulate immune responses that recognize and selectively eliminate malignant cells. DCs represent a complex network of cells characterized by the expression of costimulatory and adhesion molecules necessary to initiate primary immune responses.7,8 DCs in patients with cancer have quantitative and functional deficiencies that contribute to tumor-associated immune tolerance.9 In contrast, functionally active DCs can be generated ex vivo by culture of peripheral blood mononuclear cells (PBMCs) in the presence of cytokines.10,11 DCs loaded with specific tumor antigens by pulsing with peptide or proteins or by the insertion of tumor-specific genes have elicited antigen-specific antitumor responses in preclinical experiments, animal models, and clinical studies.12-17 Single-antigen approaches are limited, however, by the small number of known tumor-specific antigens, their variable immunogenicity, and the potential ability of tumor cells to alter expression of individual antigens to evade immune recognition. Alternatively, DCs may be loaded with antigens derived from whole tumor cells that potentially stimulate a broader antitumor response.14,18-20
We have developed a promising DC-based cancer vaccine involving the fusion of tumor cells with autologous DCs using polyethylene glycol.21,22 DC/tumor fusions present a broad array of antigens in the context of the potent antigen-presenting machinery of the DC fusion partner. DC/tumor fusions uniquely stimulate both helper and cytotoxic T lymphocyte (CTL) responses through the presentation of internalized and newly synthesized antigens, respectively.23 In diverse animal tumor models, including MM, vaccination with DC/tumor fusions is protective against an otherwise lethal challenge of tumor cells and, most significantly, results in the eradication of disease in tumor-bearing animals.21,24,25 In a MUC1 transgenic mouse model, fusion cells effectively induced antitumor immunity against an MUC1-expressing malignancy without the development of autoimmunity.26 In preclinical human studies, fusion cells stimulated autologous antigen-specific CD4 and CD8+ responses and effectively stimulated CTL responses that targeted patient-derived tumor cells.22,27-29 In a hematologic malignancy model, fusion cells were more effective in stimulating antitumor immunity than DCs pulsed with apoptotic bodies or tumor lysate.30 In initial clinical studies of patients with solid malignancies, vaccination with DC/tumor fusions was not associated with significant toxicity, stimulated antitumor immunity in a majority of patients, and induced clinical responses in a subset of patients.31,32 A correlation between immunologic and clinical response was observed.32
We now report on a phase 1 study in which patients with MM underwent vaccination with autologous DC/myeloma fusion cells. Successive cohorts of patients were treated with escalating doses of fusion cells to define treatment-associated toxicity and the maximum tolerated/achievable dose. We demonstrated that vaccination with up to 4 × 106 DC/myeloma fusion cells was feasible and well tolerated, without the induction of autoimmunity. Cellular immune responses against autologous myeloma cells and the MUC1 tumor antigen were induced, and humoral immune responses against novel antigens were detected. Disease stabilization was observed in a majority of patients with advanced disease.
Methods
Patient characteristics
Patients eligible for the study included patients with active disease who had received at least 1 prior treatment regimen. In addition, patients with stage I myeloma who did not require therapy and were otherwise being observed were eligible. Patients must have exhibited at least 20% involvement of the bone marrow with myeloma cells to facilitate vaccine generation. Patients must not have been treated with chemotherapy, steroids, radiation therapy, or immunotherapy within 4 weeks of study enrollment. Patients with a history of clinically significant autoimmune disease or organ dysfunction as measured by a bilirubin level > 2.0 or creatinine > 2.0 were excluded. All patient protocols were approved by the Beth Israel Deaconess Medical Center institutional review board.
Reagents for vaccine characterization and immunologic assays
Purified mouse anti–human monoclonal antibodies against HLA-DR, CD80, CD86, CD40, CD83, CD38, and CD138 were purchased from BD Pharmingen. Phycoerythrin (PE)–conjugated mouse anti–human monoclonal antibodies against CD4 and fluorescein isothiocyanate (FITC)–conjugated anti-CD4 (RPA-T4, immunoglobulin G1 [IgG1]), anti-CD8 (RPA-T8, IgG1), and FITC-, PE-conjugated matching isotype controls IgG1, IgG2a, IgG2b, and purified mouse monoclonal IgG1 (MOPC-21) isotype control were also purchased from BD Pharmingen. Monoclonal antibody DF3 (anti-MUC1 N-ter) has been described previously.33 For intracellular cytokine staining, PE-conjugated anti–human monoclonal antibodies for interferon-γ (IFN-γ; mouse IgG1-B27) and the respective PE-conjugated matching isotype controls (rat IgG1-PE and mouse IgG1-PE) were purchased from Invitrogen. FITC-conjugated goat anti–mouse (IgG1) was purchased from Chemicon International.
Vaccine generation
DC/tumor fusions were generated as described previously.31 Patients underwent aspiration of 20-30 mL of bone marrow, from which mononuclear cells were isolated by Ficoll density gradient centrifugation. Autologous plasma was obtained from the leukapheresis product or by harvesting the supernatant after Ficoll centrifugation of 50 mL of peripheral blood. Bone marrow mononuclear cells were cultured in RPMI 1640 culture media that contained 2mM l-glutamine (Lonza) supplemented with heat-inactivated 10% autologous serum and 10 μg/mL gentamicin (Baxter). In some cases, myeloma cells were cryopreserved in 10% dimethylsulfoxide/90% autologous plasma and later thawed at the time of fusion generation. Myeloma preparations were characterized by fluorescence-activated cell sorter (FACS) analysis to document expression of tumor-associated markers CD38, CD138, and/or MUC1 and the absence of DC-associated markers.
Patient-derived DCs were generated from adherent mononuclear cells isolated from a leukapheresis collection. PBMC collections underwent plastic adherence for 1-2 hours in the presence of 1% autologous plasma, and nonadherent cells were removed. Adherent cells were cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) 1000 U/mL (Berlex) and interleukin-4 500 IU/mL (Cellgenix USA) for 5-7 days. Cells were cultured with 2mM l-glutamine 1% autologous serum and 10 μg/mL gentamicin. DCs then underwent maturation by exposure to tumor necrosis factor-α 25 ng/mL (Cellgenix USA) for 2-3 days. DCs underwent phenotypic analysis with immunohistochemistry and/or flow cytometric analysis to assess expression of major histocompatibility complex class II, costimulatory (CD86, CD40, and/or CD80), and maturation (CD83) molecules and to confirm absence of expression of appropriate tumor-associated markers (MUC-1, CD38, and/or CD138).
DC/tumor fusions were generated by mixing tumor cells and DCs at a 1:3 to 1:10 ratio (depending on cell yields). The cells were then washed extensively, and the cell pellet was resuspended in a 50% solution of polyethylene glycol in phosphate-buffered saline. After a short incubation, the polyethylene glycol was slowly diluted by the addition of media, and after several washing steps, the cells were placed in media that contained 10% autologous plasma and GM-CSF 500 U/mL and incubated at 37°C. DC/tumor fusions were quantified by determining the percentage of cells that coexpressed unique DC and tumor-associated antigens by immunohistochemical analysis. A fusion efficiency of at least 10% based on immunohistochemical staining was required as a release criterion for vaccine administration. An aliquot of the DC, tumor cell, and fusion cell preparations was sent for microbiologic assessment. The fused cells were frozen in autologous plasma (90%) and dimethylsulfoxide (10%) and placed in single-use vials. Fusion cell doses were stored frozen in the vapor phase of liquid nitrogen for subsequent use. Before patient administration, the sterility of the product was confirmed, including mycoplasma, endotoxin, and sterility assays. At the time of vaccine administration, the fused cells were thawed, and a viability assay and Gram stain were performed. The product was irradiated at 30 cGy, drawn into a syringe, and delivered to the clinical site.
Functional assessment of the DC, MM, and DC/MM fusion preparations
As a measure of immunologic potency, the capacity of the fusion cell preparation to stimulate allogeneic T-cell proliferation was assessed and compared with that observed with the unfused DC and myeloma preparations. PBMCs were obtained from leukopak preparations undergoing Ficoll density centrifugation. Nonadherent PBMCs were isolated from a leukopak collection obtained from a normal donor who was not matched with the patient for major histocompatibility complex, and T cells were further enriched by passage through a CD3 column or nylon wool. T cells (1 × 105) were cocultured for 5 days with MM, DC, and fusion preparations at a ratio of 10:1. T-cell proliferation was determined by measuring incorporation of [3H]thymidine after overnight pulsing (1 μCi/well) of triplicate samples.
Vaccine administration
Successive cohorts of at least 3 patients were treated with 1 × 106, 2 × 106, and 4 × 106 fusion cells administered as a subcutaneous injection in the upper thigh at 3-week intervals for a total of 3 doses. The cohort was to be expanded to 6 patients if dose-limiting toxicity was encountered, as defined by vaccine-related grade 3 or 4 toxicity. A total of 10 patients were treated at the highest dose level. GM-CSF (100 μg) was administered at the vaccine site on the day of vaccination and for 3 days thereafter. Patients for whom the targeted dose of fusion cells was not achieved were treated at a lower dose level. Patients were seen weekly during the period of vaccination and then monthly for 6 months after completion of vaccination. Patients underwent serial assessment for evidence of toxicity and autoimmunity by physical examination and laboratory evaluation, including antinuclear antibody and erythrocyte sedimentation rate.
Vaccine induction of tumor-reactive lymphocytes
To measure the immunologic response to vaccination, we determined the percentage of circulating CD4+ and CD8+ T cells that recognized autologous myeloma cells as manifested by the percentage of cells that expressed IFN-γ after ex vivo exposure to autologous tumor lysate. Immunologic assessments were performed before each vaccination and at 1, 3, and 6 months after the last vaccine. The peak response after vaccination was compared with prevaccination levels of tumor-reactive T cells to assess the fold increase in tumor-reactive T cells after vaccination. At each time point, mononuclear cells were isolated from peripheral blood by Ficoll density centrifugation and cryopreserved. After completion of the study, PBMCs were thawed, and 1 × 106 cells were cultured with lysate generated by repeated freeze/thaw cycles of 1 × 105 autologous myeloma cells. As a control, PBMCs were cultured with tetanus toxoid (10 μg/mL) or media alone. After 5 days of coculture, expression of IFN-γ by CD4+ and CD8+ populations was determined by intracellular FACS analysis. Cells were restimulated with tumor lysate for 6 hours and cultured overnight with 1 μg/mL GolgiStop (BD Biosciences). The cells were stained with CD4 or CD8 antibodies conjugated to FITC and permeabilized with Cytofix/Cytoperm Plus (BD Biosciences). Cells were also stained with PE-conjugated anti–human IFN-γ, fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
Vaccine induction of MUC1 antigen–specific responses
In HLA-A2.1 patients, PBMCs were isolated before each vaccination and at 1, 3, and 6 months after completion of vaccination as outlined above. The number of CD8+ T cells binding the MUC1 tetramer was determined by bidimensional FACS analysis with CD8-FITC and MUC1 tetramer–PE antibody.
Phytohemagglutinin and tetanus-induced T-cell proliferation
PBMCs were isolated at serial time points (as outlined above) and cocultured with the phytohemagglutinin mitogen and tetanus toxoid (10 μg/mL) for 3 and 5 days, respectively. T-cell proliferation was measured by determining uptake of [3H]thymidine after overnight pulsing (1 μCi/well) of triplicate samples.
Vaccine-site reactions
Vaccine-site reactions were biopsied in a subset of patients, and the presence of CD4 and CD8 infiltrating cells was determined by immunocytochemical analysis. In addition, recruitment of native DCs to the vaccine site was assessed by immunocytochemical staining of the cell infiltrate for CD1a in the vaccine bed.
Pictures (hematoxylin and eosin and immunohistochemistry) were taken at a total magnification of 150× (10× objective, 10× ocular, and 1.5× telescope), using an Olympus DP70 camera and DP controller software Version 1.2.1.108 (Olympus Optical Co, 2003). Figures were created in PowerPoint for Mac (v 11.3.5).
SEREX assessment
Serologic analysis of Recombinant cDNA Expression library (SEREX) was performed to assess humoral response to vaccination and to identify targeted novel antigens. The cDNA library was constructed as described previously34,35 from total RNA isolated from bone marrow CD138+ cells from a patient with myeloma. The cDNA library was inserted into a recombinant phage vector, transfected into Escherichia coli, and plated on agar at 5 × 104 plaques per 150-mm Petri dish. Expression of recombinant proteins was induced by incubation with isopropyl-β-d-thiogalactoside–treated nitrocellulose membranes for 3.5 hours at 37°C. Filters were subsequently washed in Tris-buffered saline with Tween to remove excess agar and blocked overnight with 1% wt/vol nonfat dry milk in Tris-buffered saline. The filters were incubated overnight with prevaccination and postvaccination patient serum diluted at 1:500 in Tris-buffered saline with Tween. The sera were absorbed against phage lysate and the E coli strain to minimize nonspecific antibody binding. Specific binding of antibody to recombinant proteins expressed on the lytic plaques was detected by incubation with alkaline phosphatase–conjugated goat anti–human IgG antibody diluted at 1:2000 in Tris-buffered saline with Tween. Visualization of the antigen-antibody complexes was accomplished by staining with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium (BCIP/NBT). cDNA inserts from clones that were positive after vaccination were isolated by excision of phagemids and then sequenced with T3 and T7 primers (Dana-Farber Cancer Institute Molecular Biology Core Facility).
Quantification of regulatory T cells
As outlined above, PBMCs were isolated at serial time points, and regulatory T cells were quantified by determining the percentage of CD4/CD25high T cells by bidimensional FACS analysis. Expression of FOXP3 by CD4/CD25 cells was measured in selected patients by intracellular FACS analysis.
Clinical disease assessment
Patients were required to have measurable disease as manifested by an increased M component in the serum or urine or an increase in the serum free light chains. Serial measurements of the M protein were obtained before each vaccination and at 1, 3, and 6 months after vaccination. Bone marrow aspiration and biopsy were performed before vaccination and at 1, 3, and 6 months after vaccination. Skeletal survey and other appropriate radiologic evaluations were performed before vaccination and at 3 and 6 months after vaccination.
Results
Patient characteristics
Eighteen subjects were enrolled after they met eligibility criteria. Seventeen subjects underwent therapy after successful vaccine generation (Table 1). One patient was removed from the study because of inadequate cell yields for vaccine generation. Twelve patients were male, and 6 were female. The mean age was 57 years. Patients had received a mean of 4 prior treatment regimens, and 14 patients had previously undergone high-dose chemotherapy with autologous stem cell transplantation. Two patients had stage I disease and had not received prior therapy. Successive cohorts consisting of a minimum of 3 patients were treated with escalating doses of fusion cells. Three, 4, and 10 patients were vaccinated with 1 × 106, 2 × 106, and 4 × 106 fusion cells, respectively. One patient assigned to the 4 × 106 dose level received 2 × 106 fusion cells because of cell yields. One patient received only a single dose of the vaccine because of the development of an unrelated cardiac event for which the patient was taken off study. No dose-limiting toxicities were observed, and the intended dose escalation was completed.
Vaccine generation
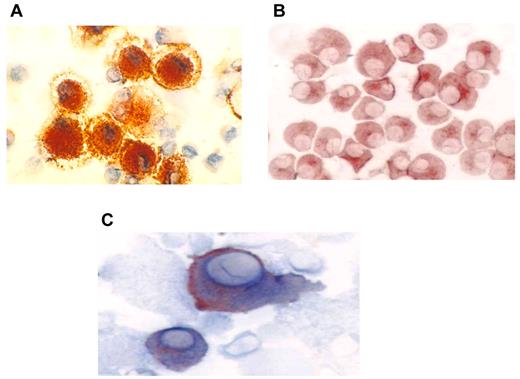
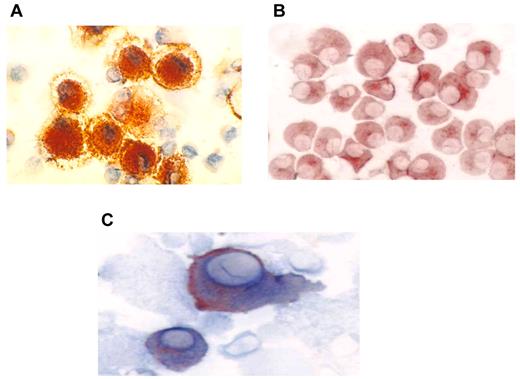
DCs were generated from adherent mononuclear cells obtained from a single leukapheresis collection. The mean yield of mononuclear cells collected was 9.8 × 109. DCs were generated from adherent mononuclear cells cultured for 1 week with GM-CSF and interleukin-4 and then matured for 48 hours with tumor necrosis factor-α. The mean yield of DCs was 1.23 × 108 cells, with a mean viability of 88%. DCs uniformly expressed HLA-DR, CD11c, CD86, and CD83 consistent with a mature phenotype (Figure 1A). Myeloma-specific markers including CD38 and CD138 were absent. Myeloma cells were derived from a single bone marrow aspirate of 20-25 mL and demonstrated a mean yield and viability of 5.7 × 106 cells and 92%, respectively. Myeloma cells uniformly expressed CD38 and/or CD138 (Figure 1B). In contrast, myeloma cells did not express the costimulatory marker CD86 or the DC maturation marker CD83. Fusion cells were quantified by measuring the percentage of cells that coexpressed unique DC (CD86) and myeloma (CD38 or CD138) markers (Figure 1C). The mean fusion percentage was 39% (range, 18%-52%) with a mean viability of 84% (range, 69%-96%). As a measure of their potency as antigen-presenting cells, the capacity of the DC, myeloma, and fusion cells to stimulate proliferation of allogeneic T cells was measured. Mean ± SEM stimulation indices were 9 ± 3, 43 ± 7, and 29 ± 5 for the myeloma, DC, and fusion cell preparations, respectively (Figure 2).
Immunohistochemical staining of DC, tumor, and fusion cells. (A) Autologous DCs were generated from adherent mononuclear cells isolated from a leukapheresis collection. DCs were cultured with GM-CSF and interleukin-4 for 5 days and then with tumor necrosis factorα for 48-72 hours. DC preparations were analyzed for expression of costimulatory molecules. DC expression of CD86 (red) is shown (×60). (B) Patient-derived myeloma cells were cultured in RPMI 1640 complete medium and were analyzed for expression of the tumor-associated antigens CD138 and CD38. Tumor expression of CD38 (red) is shown (60×). (C) Fusion cells were generated by coculture of DCs and myeloma cells in the presence of polyethylene glycol. Fusion cell preparations were analyzed for coexpression of the DC-derived costimulatory molecule CD86 (blue) and tumor-associated antigen CD38 (red).
Immunohistochemical staining of DC, tumor, and fusion cells. (A) Autologous DCs were generated from adherent mononuclear cells isolated from a leukapheresis collection. DCs were cultured with GM-CSF and interleukin-4 for 5 days and then with tumor necrosis factorα for 48-72 hours. DC preparations were analyzed for expression of costimulatory molecules. DC expression of CD86 (red) is shown (×60). (B) Patient-derived myeloma cells were cultured in RPMI 1640 complete medium and were analyzed for expression of the tumor-associated antigens CD138 and CD38. Tumor expression of CD38 (red) is shown (60×). (C) Fusion cells were generated by coculture of DCs and myeloma cells in the presence of polyethylene glycol. Fusion cell preparations were analyzed for coexpression of the DC-derived costimulatory molecule CD86 (blue) and tumor-associated antigen CD38 (red).
Potency of fusion cells in the stimulation of allogeneic T-cell proliferation. Patient-derived DCs, myeloma cells, and fusions were cocultured with T cells from a healthy donor at a T-cell:target ratio of 1:10, 1:30, 1:100, 1:300, and 1:1000. Cells were cocultured for 5 days, and T-cell proliferation was determined by incorporation of [3H]-thymidine (1 μCi/well) after overnight pulsing. Stimulation index (SI) represents counts per minute (CPM) of sample per CPM of unstimulated T cells. Results are presented as mean ± SEM from 16 samples at an antigen-presenting cell:T-cell ratio of 1:10.
Potency of fusion cells in the stimulation of allogeneic T-cell proliferation. Patient-derived DCs, myeloma cells, and fusions were cocultured with T cells from a healthy donor at a T-cell:target ratio of 1:10, 1:30, 1:100, 1:300, and 1:1000. Cells were cocultured for 5 days, and T-cell proliferation was determined by incorporation of [3H]-thymidine (1 μCi/well) after overnight pulsing. Stimulation index (SI) represents counts per minute (CPM) of sample per CPM of unstimulated T cells. Results are presented as mean ± SEM from 16 samples at an antigen-presenting cell:T-cell ratio of 1:10.
Toxicity
Vaccination was well tolerated, without significant evidence of autoimmunity (Table 2). All vaccine-associated events were of mild to moderate intensity (grade 1 or 2) and of transient duration. The most common events were injection-site reactions that consisted of erythema and/or pain that lasted a few days. Other manifestations of vaccine-induced toxicity included transient fever, myalgias, pruritus, and rash. One patient with a history of a prior deep vein thrombosis developed a deep vein thrombosis and pulmonary embolus while on study. This event was unlikely to be related to vaccination, but given that GM-CSF may be associated with an increased risk of thrombosis, patients with a history of clinically significant thromboembolic disease (unrelated to myeloma therapy) were subsequently excluded from the trial.
Vaccine-site reactions
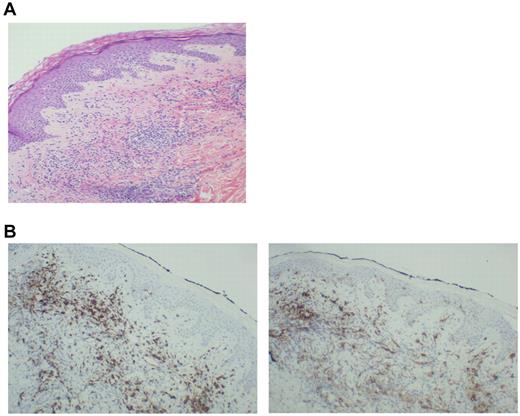
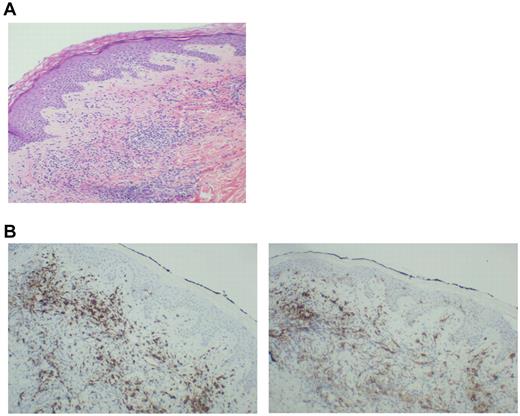
Thirteen patients exhibited injection-site reactions after vaccination. Biopsy of these areas demonstrated a dense mononuclear cell infiltrate (Figure 3A). A majority of cells expressed CD8 (Figure 3B left), which suggested recruitment and education by the DC/MM fusions at the site of vaccination. In addition, the presence of CD1a cells was observed in the vaccine bed (Figure 3B right), which suggested that native Langerhans cells recruited by the presence of GM-CSF might participate in the vaccine response.
Biopsy of a vaccine-site reaction. A biopsy of vaccine-site reactions was obtained, fixed in formalin, and stained with hematoxylin and eosin. To further characterize the nature of the cellular infiltrate, immunohistochemical staining for CD8+ and CD1a was performed. (A) A representative example of a vaccine site reaction biopsy is shown, demonstrating a dense mononuclear cell infiltrate at the vaccine site. (B) A representative example of immunohistochemical staining is shown, demonstrating an infiltrate of CD8+ T cells (left) and CD1a+ immature DCs (right) at the biopsy site.
Biopsy of a vaccine-site reaction. A biopsy of vaccine-site reactions was obtained, fixed in formalin, and stained with hematoxylin and eosin. To further characterize the nature of the cellular infiltrate, immunohistochemical staining for CD8+ and CD1a was performed. (A) A representative example of a vaccine site reaction biopsy is shown, demonstrating a dense mononuclear cell infiltrate at the vaccine site. (B) A representative example of immunohistochemical staining is shown, demonstrating an infiltrate of CD8+ T cells (left) and CD1a+ immature DCs (right) at the biopsy site.
Cellular immunologic response to vaccination
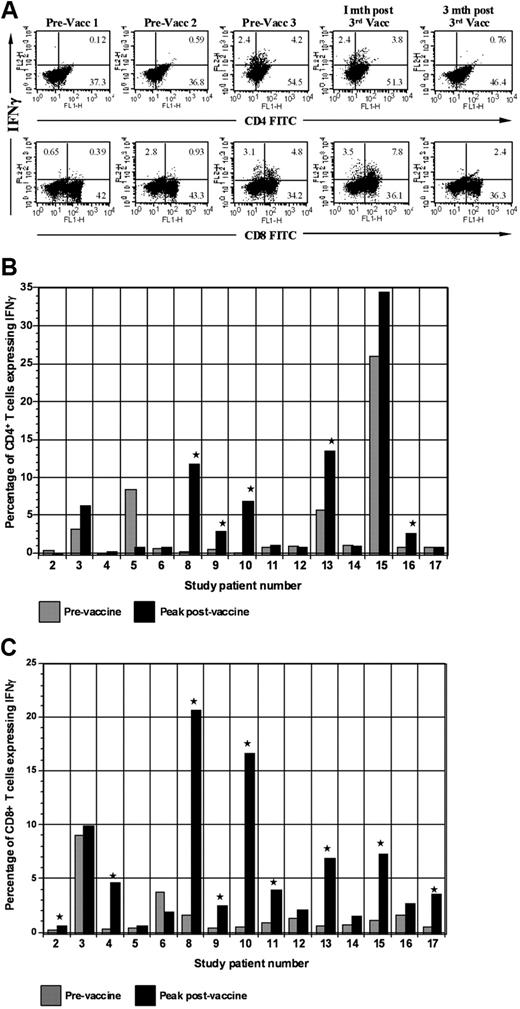
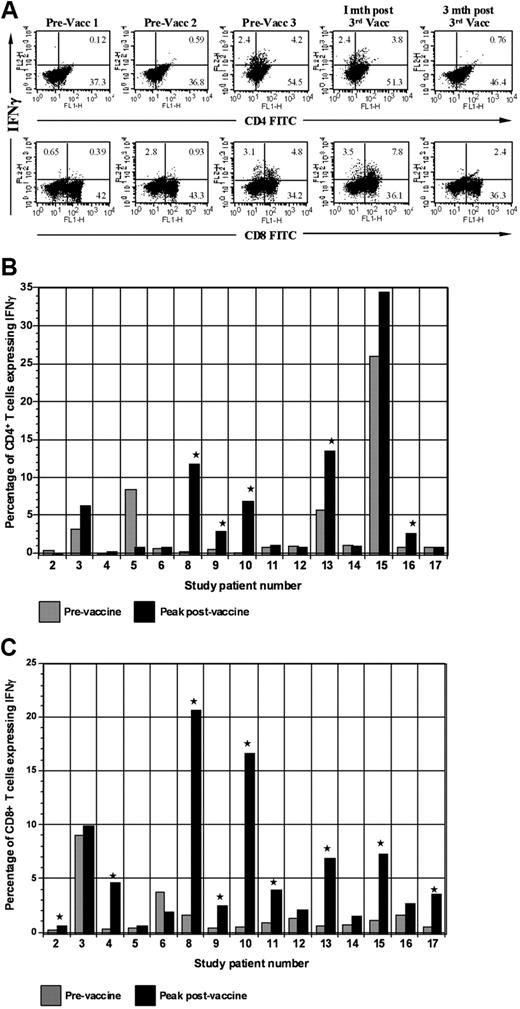
We assessed the impact of vaccination on circulating tumor-reactive lymphocytes by determining the percentage of CD4+ and CD8+ T cells that expressed IFN-γ after ex vivo culture with autologous PBMCs pulsed with autologous tumor lysate (Figure 4A). Of 15 evaluable patients, 11 demonstrated at least a 2-fold increase in the percentage of CD4 or CD8+ tumor-reactive T cells (Figure 4B-C). Mean baseline and peak postvaccine levels of tumor-reactive CD8+ T cells were 0.6% and 2.4%, respectively (P = .01). A rise of mean CD4+ tumor-reactive T cells was also observed with baseline levels of 1.5% and peak levels of 3.2% (P = .02). Among responders, mean baseline and peak levels of CD8+ tumor-reactive T cells were 0.68% and 7.14% (P = .01), respectively (P = .04). Mean baseline and peak CD4+ levels were 1.2% and 6.5%, respectively (P = .04).
Expression of IFN-γ by CD4 and CD8 populations before and after vaccination. (A) Intracellular expression of IFN-γ by CD4 and CD8 populations. PBMCs isolated before each vaccination and at serial time points after vaccination were cocultured with autologous tumor lysate, pulsed with GolgiStop, labeled with FITC-conjugated CD4 or CD8 antibodies, and then permeabilized by incubation in Cytofix/Cytoperm Plus. Cells were then incubated with PE-conjugated anti–IFN-γ or a matched isotype control antibody and fixed in 2% paraformaldehyde. Labeled cells were analyzed by flow cytometry. (B) Vaccine induction of tumor-reactive CD4+ T cells. Percentage of CD4+ T cells expressing IFN-γ after ex vivo exposure to autologous tumor lysate is shown. Percentage of tumor-reactive CD4+ T cells is shown before the initial vaccination and at the peak time after vaccination. (C) Vaccine induction of tumor-reactive CD8+ T cells. Percentage of CD8+ T cells expressing IFN-γ after ex vivo exposure to autologous tumor lysate is shown. Percentage of tumor-reactive CD8+ T cells is shown before the initial vaccination and at the peak time after vaccination. Vacc indicates vaccination; and mth, month.
Expression of IFN-γ by CD4 and CD8 populations before and after vaccination. (A) Intracellular expression of IFN-γ by CD4 and CD8 populations. PBMCs isolated before each vaccination and at serial time points after vaccination were cocultured with autologous tumor lysate, pulsed with GolgiStop, labeled with FITC-conjugated CD4 or CD8 antibodies, and then permeabilized by incubation in Cytofix/Cytoperm Plus. Cells were then incubated with PE-conjugated anti–IFN-γ or a matched isotype control antibody and fixed in 2% paraformaldehyde. Labeled cells were analyzed by flow cytometry. (B) Vaccine induction of tumor-reactive CD4+ T cells. Percentage of CD4+ T cells expressing IFN-γ after ex vivo exposure to autologous tumor lysate is shown. Percentage of tumor-reactive CD4+ T cells is shown before the initial vaccination and at the peak time after vaccination. (C) Vaccine induction of tumor-reactive CD8+ T cells. Percentage of CD8+ T cells expressing IFN-γ after ex vivo exposure to autologous tumor lysate is shown. Percentage of tumor-reactive CD8+ T cells is shown before the initial vaccination and at the peak time after vaccination. Vacc indicates vaccination; and mth, month.
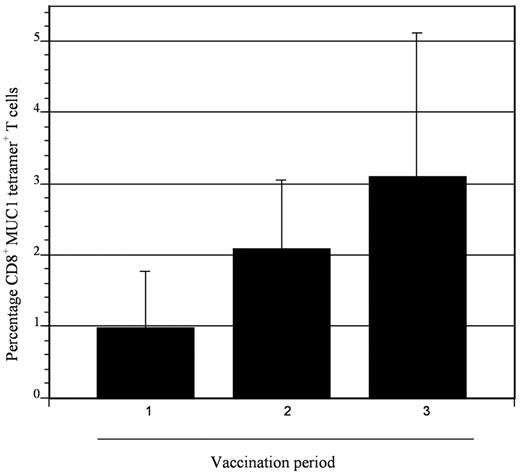
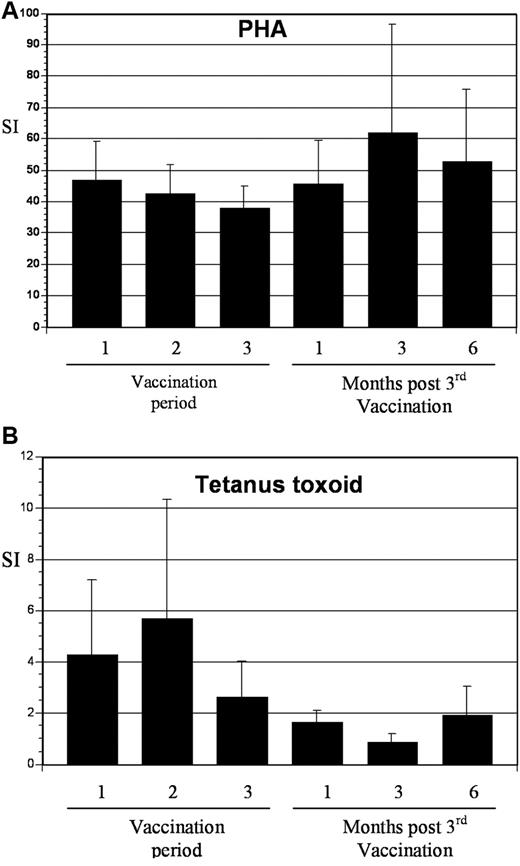
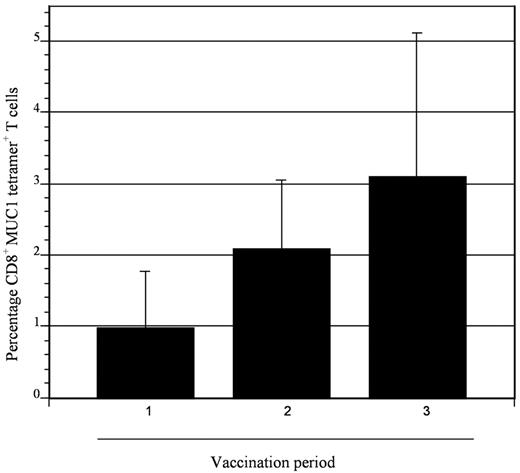
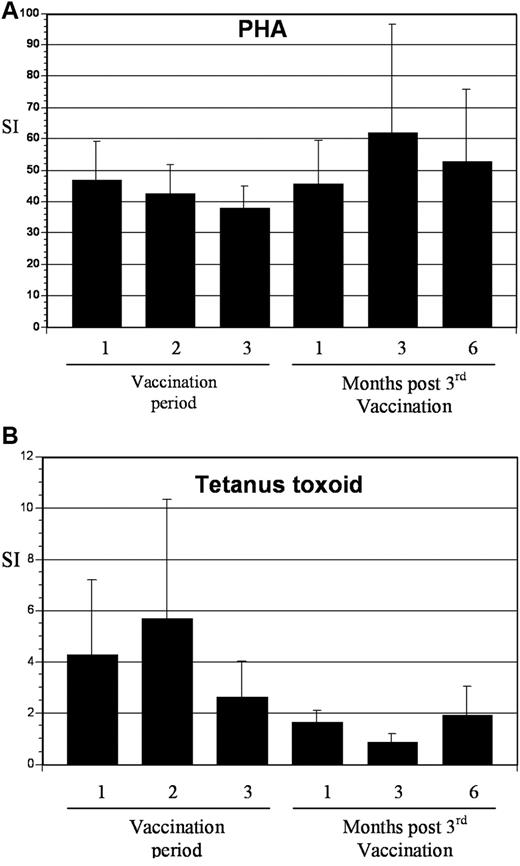
To assess the impact of vaccination on immunologic response directed against MUC1, tetramer analysis was performed on samples derived from 5 patients who were HLA-A2.1 positive. All patients demonstrated at least a 2-fold increase in tetramer-positive cells after vaccination, with mean levels of 0.98% and 4.0% at baseline and peak postvaccination, respectively (Figure 5). To determine whether vaccination resulted in nonspecific immune activation and T-cell expansion, we quantified T-cell response to mitogenic stimulation with phytohemagglutinin and to the tetanus toxoid recall antigen. Response to phytohemagglutinin did not change during the period of vaccination (mean stimulation index 47 and 46 before vaccination and 1 month after completion of vaccination, respectively). Similarly, no increase in tetanus-specific responses was observed after vaccination. In fact, a decrease in tetanus-induced T-cell proliferation was observed, with a mean stimulation index that decreased from 4.3 before vaccination to 1.7 at 1 month after completion of vaccinations (Figure 6). These data suggest that vaccination induced antitumor immunity through the selective expansion of tumor-specific T cells rather than nonspecific T-cell stimulation.
Expansion of MUC1 tetramer–positive cells after vaccination. CD8+ T cells binding the MUC1 tetramer were quantified at serial time points (1, before first vaccination; 2, before second vaccine; and 3, before third vaccination) in patients who were HLA-A2.1. Binding to a control tetramer was quantified in parallel, and the control value was subtracted from that obtained for the MUC1 tetramer. Mean values of 5 patients are presented with associated standard error. An incremental, although not statistically significant, increase in MUC1 tetramer–positive cells was observed after vaccination.
Expansion of MUC1 tetramer–positive cells after vaccination. CD8+ T cells binding the MUC1 tetramer were quantified at serial time points (1, before first vaccination; 2, before second vaccine; and 3, before third vaccination) in patients who were HLA-A2.1. Binding to a control tetramer was quantified in parallel, and the control value was subtracted from that obtained for the MUC1 tetramer. Mean values of 5 patients are presented with associated standard error. An incremental, although not statistically significant, increase in MUC1 tetramer–positive cells was observed after vaccination.
T-cell response to phytohemagglutinin (PHA) and tetanus toxoid before and after vaccination. (A) PBMCs were collected at the indicated time points and incubated with 2 μg/mL of PHA for 3 days. Proliferation was measured by incorporation of tritiated thymidine. Values are presented as mean stimulation index (SI; proliferation of stimulated/unstimulated cells ± SE). (B) PBMCs were collected at the indicated time points and incubated with tetanus toxoid at 10 μg/mL for 5 days. Proliferation was measured by incorporation of tritiated thymidine. Values are presented as mean stimulation index (proliferation of stimulated/unstimulated cells ± SE).
T-cell response to phytohemagglutinin (PHA) and tetanus toxoid before and after vaccination. (A) PBMCs were collected at the indicated time points and incubated with 2 μg/mL of PHA for 3 days. Proliferation was measured by incorporation of tritiated thymidine. Values are presented as mean stimulation index (SI; proliferation of stimulated/unstimulated cells ± SE). (B) PBMCs were collected at the indicated time points and incubated with tetanus toxoid at 10 μg/mL for 5 days. Proliferation was measured by incorporation of tritiated thymidine. Values are presented as mean stimulation index (proliferation of stimulated/unstimulated cells ± SE).
Impact of vaccination on circulating regulatory T cells
Regulatory T cells play a critical role in supporting tumor-mediated immune suppression. Consequently, we determined the levels of circulating regulatory T cells before and after vaccination by quantifying CD4/CD25high cells by bidimensional FACS analysis. No significant change in levels of CD4/CD25high cells was observed, with these cells representing 0.5%, 0.6%, 0.4%, and 0.4% of the mononuclear cell population before vaccine 1, vaccine 2, and vaccine 3 and 1 month after the last vaccination, respectively.
Humoral response to vaccination
SEREX analysis was performed to assess humoral response in patients undergoing vaccination with DC/MM fusions and to identify potential novel myeloma targets. A cDNA expression library was constructed from CD138+ myeloma cells obtained from a patient with an IgG paraprotein. The library was screened with prevaccination and postvaccination serum from 12 patients. Library screening identified 55 clones. After restriction-enzyme digestion and DNA sequencing, 10 of the 55 clones were found to correspond to 3 different gene products that have been described previously (Table 3). Humoral responses directed against regulators of G-protein signaling 19 (RGS19) was detected in 2 of 12 patients after vaccination (Figure 7). The antibody response remained strongly positive at 3 months after vaccination in both patients. Antibody response against the β-subunit of heat shock protein 90 (HSP90β) was detected 1 month after vaccination in 2 of 12 patients. Antibodies against these targets were not present before vaccination. Antibody response against BRCA1-associated protein (BRAP) was detected in prevaccination and postvaccination serum of 1 of 12 patients. Importantly, no antibody response against any of these antigens has been identified in 5 age-matched healthy persons (Table 3).
A representative example demonstrating a humoral immune response against RGS19 in response to vaccination. Sera from patient number 10 was obtained before vaccination (left panel) and 1 month after vaccination (right panel) and incubated with E coli transfected with phage expressing myeloma-derived cDNA. Specific binding of antibody to recombinant proteins expressed on the lytic plaques was detected by incubation with alkaline phosphatase–conjugated goat anti–human IgG antibody. Antigen-antibody complexes were visualized by staining with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium. Vaccination induced an antibody response directed against RGS19.
A representative example demonstrating a humoral immune response against RGS19 in response to vaccination. Sera from patient number 10 was obtained before vaccination (left panel) and 1 month after vaccination (right panel) and incubated with E coli transfected with phage expressing myeloma-derived cDNA. Specific binding of antibody to recombinant proteins expressed on the lytic plaques was detected by incubation with alkaline phosphatase–conjugated goat anti–human IgG antibody. Antigen-antibody complexes were visualized by staining with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium. Vaccination induced an antibody response directed against RGS19.
Clinical response
Disease response was assessed by serial measurement of the serum and urine paraprotein levels and quantification of the level of bone marrow involvement. Of 16 evaluable patients, 11 demonstrated stable disease after vaccination. Three patients have ongoing stable disease after vaccination without evidence of progression at 12, 25, and 41 months, respectively. An additional 8 patients exhibited disease stabilization for 2.5 (4 patients) 3, 4, 4.5, and 5 months after vaccination.
Discussion
MM demonstrates resistance to standard biologic and chemotherapy but may be sensitive to cellular immunotherapy. Allogeneic transplantation results in targeting of myeloma cells by alloreactive lymphocytes and is associated with durable remissions in a subset of patients. However, the lack of tumor specificity results in significant morbidity and mortality. Investigators have pursued tumor vaccine models in an effort to elicit myeloma-specific immunity. Tumor-associated antigens that are selectively expressed on myeloma cells include MUC1 and the idiotype protein.4,6 Vaccination with idiotype protein in conjunction with GM-CSF has been associated with cellular and humoral immune responses with uncertain clinical effects.36,37 Expression of cancer testis antigens such as NY-ESO has been shown to correlate with disease progression, and antigen-specific CTL responses are induced after repetitive stimulation in vitro with peptide-pulsed DCs.38 Humoral responses directed against NY-ESO have been documented after allogeneic transplantation, which suggests that targeting of this antigen may be associated with the graft-versus-disease response.
In an effort to stimulate a broader antitumor immunologic response, investigators have explored the use of whole tumor cells as a source of multiple antigens for vaccination. Patient-derived DCs loaded with autologous tumor lysate induced antitumor immunity after repetitive stimulation in vitro.39 In another approach, DCs were loaded with heat shock protein as a chaperone molecule containing cell-derived proteins. DCs loaded with autologous or pooled allogeneic HSP96 elicited antimyeloma responses in both in vitro and in vivo models.40
We have examined a whole-cell–based vaccine approach in which patient-derived myeloma cells are fused with autologous DCs. In the present study, we examined the feasibility, toxicity, immunologic effects, and clinical response of fusion cell vaccination in patients with MM. For a majority of patients, vaccine production was accomplished with a single leukapheresis collection for DC generation and an aspirate of bone marrow as a source of autologous myeloma cells. The DC/MM fusions expressed the DC-derived costimulatory and maturation markers, as well as the tumor-associated antigens CD38, CD138, and/or CD138. In contrast to myeloma cells, fusion cells potently stimulated allogeneic T-cell proliferation, consistent with their role as potent antigen-presenting cells.
In this phase 1 trial, a majority of the patients had advanced disease and had been treated with a mean of 4 prior regimens. Vaccination was well tolerated, without significant toxicity or evidence of autoimmunity. One patient developed a pulmonary embolus that was thought to be unrelated to the vaccine given the patient's prior history of deep vein thromboses. Dose escalation to the target of 4 × 106 fusion cells was found to be both feasible and safe. The primary toxicity was transient vaccine-site reactions, consistent with recruitment of CD8+ T cells into the vaccine bed. This observation suggests that vaccine-mediated education of T cells did not necessarily require migration to the site of the draining lymph node. Of note, increased levels of Langerhans cells were also observed, consistent with their potential contribution in modulating the immune response.
Immunologic responses were measured by determining the effect of vaccination on the levels of circulating tumor-reactive lymphocytes as measured by the percentage of circulating T cells that expressed IFN-γ in response to autologous tumor lysate. Vaccination resulted in at least a 2-fold increase in the percentage of tumor-reactive CD4 and/or CD8 T cells in 11 of 15 evaluable patients. Autologous tumor lysate was generated from marrow-derived malignant plasma cells. It is possible that an element of the immunologic response was directed against antigens found in normal plasma cells or other marrow elements. As a control to further define the tumor specificity of the immunologic response, it would have been optimal to measure T-cell response to antigens derived from isolated normal hematopoietic elements such as DCs or B cells; however, because of limitations with cell yields, these studies were not performed. Of note, vaccination did not result in signs of autoimmunity or suppression of blood counts. Notably, a rise in T cells specific to the MUC1 tumor antigen was detected after vaccination, which demonstrates the generation of an immune response to a tumor-associated antigen.
Humoral responses were detected by SEREX analysis that targeted the product of the RGS19 and HSP90 genes. RGS proteins are a family of proteins involved in the inhibition of G-protein–coupled receptor signaling. After in vivo activation by antigen, B cells rapidly regulate the expression of RGS molecules, which suggests that antigen-mediated changes of RGS proteins are important for B-cell activation, tolerance, and migration within lymphoid tissues.41,42 RGS19 has been shown to play a role in lymphoproliferative disease; however, it has not been well described in MM. HSP90, also known as gp96, functions as a molecular chaperone, coupling with a variety of antigenic peptides.43 Surface expression of gp96 on tumor cells has been shown to result in DC activation and antitumor immunity.44 Moreover, DCs pulsed with myeloma-derived gp96 induce myeloma-specific CTLs that are able to lyse myeloma cells.45 In 1 patient, an antibody response against BRAP was identified in both prevaccination and postvaccination serum. Interestingly, antibody responses to BRAP have been identified by SEREX in patients with a variety of malignancies (http://www2.licr.org/CancerImmunomeDB).
Although the majority of patients in the present study had been treated with multiple prior regimens, most exhibited stabilization of disease, with several patients still stable between 12 and 41 months after vaccination. Several factors likely modified the clinical effects of the vaccine in patients who demonstrated immunologic response. Tumor-mediated immune suppression remains a major challenge for cellular immunotherapy, particularly in those patients with bulky disease. Of note, a majority of patients in the present trial exhibited a dampening of the immunologic response by 6 months after vaccination, which suggests the down-modulation of antitumor immunity. Vaccination of patients with a lower disease state, such as after cytoreduction with biologic therapy or transplant, will likely increase vaccine response by limiting tumor-mediated immune suppression and may promote longer-term responses. In addition, boosting vaccinations at later time points may result in more durable responses. Heterogeneity of patients in the study may limit interpretation of immunologic and clinical responses. However, all but 2 patients had advanced and heavily pretreated myeloma. The present study lays the foundation for subsequent studies to evaluate the use of the fusion vaccine in a more homogeneous group of patients who will undergo vaccination after cytoreduction in the early post–autologous transplantation setting.
Increased levels of regulatory T cells have been observed in patients with malignancy and are associated with worse outcomes. Regulatory T cells inhibit primary T-cell activation and are paradoxically expanded by tumor vaccines. In preclinical models, we have demonstrated that DC/tumor fusions stimulate the expansion of both activated and suppressor cell populations. In animal models and a clinical trial, depletion of regulatory T cells enhanced vaccine response and tumor eradication.46,47 In the present study, mean levels of regulatory T cells remained stable over the period of vaccination.
Recent phase 3 studies of tumor vaccines have demonstrated a survival benefit in patients with advanced prostate and renal carcinoma without clear evidence of disease regression.48 Of note, in a recently reported study, 27 patients with MM undergoing post–autologous transplantation vaccination with antigen-presenting cells pulsed with autologous plasma as a source of idiotype protein (Mylovenge) demonstrated improved survival compared with 124 sequential patients who had undergone transplantation alone.49 Modulation of antitumor immunity may play a role in determining the pattern of tumor growth and interaction with the host in a manner distinct from that observed with traditional chemotherapeutic agents. In this regard, studies to examine the impact of immunologic response and long-term survival are needed for patients with MM. Areas of further investigation include vaccination in conjunction with regulatory T-cell depletion in the post–autologous transplantation setting and modulation of inhibitory pathways, including CTLA-4 and the PD-1/PDL-1 pathway, as a means of augmenting vaccine response.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr German Pihan for his assistance.
This work was funded in part by National Institutes of Health grant no. 5 PO1 CA078378-10.
National Institutes of Health
Authorship
Contribution: J.R. was involved in research design, patient accrual and assessments, analysis of data, and manuscript preparation; B.V. was involved in research design, running experiments, data analysis, and manuscript preparation; L.U. supervised vaccine preparation; S.B. performed SEREX analysis; C.M. and P.S. were responsible for vaccine generation; Z.W. was responsible for vaccine characterization; R.J., J.D.L., N.M., and P.R. were involved in patient accrual and assessments; D.D. and Y.E.Y. were involved in data collection; K.F. and D.F. were involved in patient assessments; E.W. was involved in data analysis; K.A. was involved in patient accrual and study design; D.K. was involved in study design, data analysis, and manuscript preparation; and D.A. is the principal investigator of the clinical trial and was involved in study design, data analysis, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacalyn Rosenblatt, MD, Beth Israel Deaconess Medical Center, 330 Brookline Ave, KS-121, Boston, MA 02215; e-mail: jrosenb1@bidmc.harvard.edu.
References
Author notes
J.R. and B.V. contributed equally to this study.

![Figure 2. Potency of fusion cells in the stimulation of allogeneic T-cell proliferation. Patient-derived DCs, myeloma cells, and fusions were cocultured with T cells from a healthy donor at a T-cell:target ratio of 1:10, 1:30, 1:100, 1:300, and 1:1000. Cells were cocultured for 5 days, and T-cell proliferation was determined by incorporation of [3H]-thymidine (1 μCi/well) after overnight pulsing. Stimulation index (SI) represents counts per minute (CPM) of sample per CPM of unstimulated T cells. Results are presented as mean ± SEM from 16 samples at an antigen-presenting cell:T-cell ratio of 1:10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/2/10.1182_blood-2010-04-277137/4/m_zh89991064440002.jpeg?Expires=1768457472&Signature=MjZLzWN3MmOlVYoCF7-hMdRaiPexYgJl2FMFa~AyFfeiTcZxTACNFFxmJv5DTsVRfDlE9j8f8bo8yQBgbMx0G0LeLvy9h1zoQ7gn9VStbcII6piC~32TcfL04IY6SFn1iysFB1xaiX~mpe~9vjhk-zo-UbabAjj~Ti2rSKZ0ltpom9AoV06Lna21Pvw3Pm9jCLp2lc~71aQDqtpTW-NmVngS3LxD7Kyt2KbA8yESusNWnQTw41Mqru-fUkZQ-vobsh5uFJR4tTs8VloefPQ3~PDQb3Spk5a4KBul5atggOXigx5A19N6MrCtIiwd0TbNaKRoQKxB-qQxCLbW6bCngg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Potency of fusion cells in the stimulation of allogeneic T-cell proliferation. Patient-derived DCs, myeloma cells, and fusions were cocultured with T cells from a healthy donor at a T-cell:target ratio of 1:10, 1:30, 1:100, 1:300, and 1:1000. Cells were cocultured for 5 days, and T-cell proliferation was determined by incorporation of [3H]-thymidine (1 μCi/well) after overnight pulsing. Stimulation index (SI) represents counts per minute (CPM) of sample per CPM of unstimulated T cells. Results are presented as mean ± SEM from 16 samples at an antigen-presenting cell:T-cell ratio of 1:10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/2/10.1182_blood-2010-04-277137/4/m_zh89991064440002.jpeg?Expires=1768696673&Signature=jjH0vCJSc~cZ21zHEN1nICmuyW2iytXnp3~PuI3s5Q84ea9j9W2WoVl87Q75aEGc9yP3MI2HXDcXPBr-0LrFwqfUeEE2OWjyWP7250vAKOqJB1j~yydM682KRxf0uA4X0ZMOr53nssJIhTgGprPS-7zpenmbOPhoTwezkKLP5O3l2PMd4LVBDPfeOJZn93F3W6QsKRxfDeIZAZ6ATxs1Ix5Jznen7xdlRW8nK6Ti5Ek-Rgl-c1zEPap-J0wMTo0OHhHZYLoQ5c7cESg3~6u5DSwIb05WiF2Q~Ny9EpPgi8gX3Tyfym5yKVt~iP8aetFMT33xtESjchen~V-Yoq6sQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)