Abstract
Few published studies characterize early lymphocyte recovery after intensive chemotherapy for acute myelogenous leukemia (AML). To test the hypothesis that lymphocyte recovery mirrors ontogeny, we characterized early lymphocyte recovery in 20 consecutive patients undergoing induction timed sequential chemotherapy for newly diagnosed AML. Recovering T lymphocytes were predominantly CD4+ and included a greatly expanded population of CD3+CD4+CD25+Foxp3+ T cells. Recovering CD3+CD4+CD25+Foxp3+ T cells were phenotypically activated regulatory T cells and showed suppressive activity on cytokine production in a mixed lymphocyte reaction. Despite an initial burst of thymopoiesis, most recovering regulatory T cells were peripherally derived. Furthermore, regulatory T cells showed marked oligoclonal skewing, suggesting that their peripheral expansion was antigen-driven. Overall, lymphocyte recovery after chemotherapy differs from ontogeny, specifically identifying a peripherally expanded oligoclonal population of activated regulatory T lymphocytes. These differences suggest a stereotyped immunologic recovery shared by patients with newly diagnosed AML after induction timed sequential chemotherapy. Further insight into this oligoclonal regulatory T-cell population will be fundamental toward developing effective immunomodulatory techniques to improve survival for patients with AML.
Introduction
Acute myelogenous leukemia (AML) is a heterogeneous disease in terms of its molecular, genetic, and epidemiologic profiles as well as its clinical biology. Overall, survival for AML remains poor; the 5-year survival rate is 40%-50% in younger adult patients with AML and drops off dramatically with age.1
Given the poor response to chemotherapy alone, immunotherapy is being explored as an adjunctive treatment modality. Although immunotherapy trials to date have shown mixed results, immunotherapy remains an attractive modality for several reasons. Leukemic cells are readily accessible to normal immunologic cells. Leukemic cells can express a variety of normal cell surface differentiation antigens, adhesion molecules, and costimulatory molecules, and they are capable of releasing diverse immunomodulatory agents. In selected instances, leukemic cells can present unique antigens, resulting from characteristic chromosomal changes, translocations with resultant antigenic fusion proteins, and certain gene mutations. Finally, cytoreduction and immune reconstitution allow an opportune window to deploy immunotherapies to prime a particular immune response.2
Despite the intense interest in immunotherapy, there is a dearth of studies characterizing early peripheral blood lymphocyte (PBL) recovery after induction chemotherapy for AML. The few identified studies examining lymphocyte recovery within the first month after induction chemotherapy showed that the CD4/CD8 ratio is relatively normal,3-5 the recovering unsorted lymphocytes may be peripherally derived and oligoclonal,6 and exogenous cytokine administration can alter the phenotype of recovering PBLs.3,6
Yet despite a poor understanding of the characteristics of early PBL recovery after induction chemotherapy, there is a plethora of studies showing a relationship between the absolute lymphocyte count (ALC) and survival in a variety of hematologic malignancies. Lymphocyte recovery after cytotoxic chemotherapy is an independent prognostic factor for survival in patients with AML and acute lymphoblastic leukemia.7-9 Similar trends have been shown after autologous peripheral blood stem cell transplantation in AML10 and a variety of other hematologic disorders.11-17 Nevertheless, the mechanism of these clinical associations with lymphocyte recovery is not understood.
Although we hypothesized that lymphocyte recovery after chemotherapy would mirror ontogeny, this question to date had not been answered. Because the nature of lymphocyte recovery after chemotherapy has vital ramifications for immunomodulatory treatments, we sought to more completely characterize early PBL recovery within the first month after AML induction chemotherapy. Further insight into early PBL recovery could guide the design of immunotherapeutic strategies by defining the ideal immunologic setting to best prime antileukemic activity of the reconstituting immune system. Additionally, a more robust understanding of early PBL recovery could clarify the nature of the reported relationship between the ALC and survival.
Given the heterogeneity of AML, we sought to better characterize immunologic recovery phenomena that would be widely applicable to all patients with AML. Therefore, we studied early PBL recovery in 20 consecutive patients with newly diagnosed AML admitted for intensive induction timed sequential therapy (TST). We speculated that some of the heterogeneity among patients with AML might be overcome by a generalized immunologic response to induction chemotherapy.
Methods
Patients
Twenty-six consecutive patients with newly diagnosed AML admitted to The Johns Hopkins Sidney Kimmel Comprehensive Cancer Center from September 2006 to April 2007 for TST as induction regimens were invited to participate in this study. Twenty patients elected to participate. For their induction regimens, 11 received cytosine arabinoside, daunorubicin, and etoposide (AcDVP16)18,19 and 9 received flavopiridol, cytosine arabinoside, and mitoxantrone (FLAM).20
This study was conducted with the prior approval of The Johns Hopkins Medical Institutions Institutional Review Board. Early leukocyte recovery was defined as total white blood cell (WBC) count ≥ 0.2 × 109/L (200 cells/mm3) or ≥ 0.2 × 109/L (200 cells/mm3) above nadir if nadir ≥ 0.2 × 109/L (200 cells/mm3). At the onset of leukocyte recovery, 20-mL blood draws were performed serially every other day for a goal of 5 total draws. During the period between administration of TST and sample collection, none of the patients were exposed to exogenous growth factors, although all patients were on antibiotics either prophylactically or for active treatment of infection, given their profound aplasia after chemotherapy.
Flow cytometry
Peripheral blood mononuclear cells obtained during the initial phase of leukocyte recovery after TST from the 20 patients were isolated by Ficoll-Hypaque density centrifugation and then stained and subjected to multicolor flow cytometric analysis with the use of a FACSCanto (Becton Dickinson Immunocytometry Systems) as previously described.21 Directly conjugated monoclonal antibodies (peridinin chlorophyll protein complex–cyanine 5-5–anti-CD3, fluorescein isothiocyanate (FITC)–anti-CD4, allophycocyanin–cyanine 7–anti-CD8, phycoerythrin (PE)–cyanine 7–anti-CD25, PE–anti-Foxp3, PE–anti-CD45RO, FITC–anti-CD45RA, allophycocyanin–anti-CD45RA, and FITC–anti-Ki67) were purchased from BD Biosciences. Expression of Foxp3 was detected by intracellular staining after cell permeabilization according to the instructions of the manufacturer.22
T-cell receptor excision circle assay
Determination of T-cell receptor excision circles (TRECs) was performed by quantitative polymerase chain reaction (PCR) with the use of an ABI 7500 real-time PCR system (Applied Biosystems Inc [ABI]).23 Real-time PCR reactions were performed with use of the TaqMan assay (ABI). DNA was isolated from peripheral blood lymphocytes. TREC genes were synthesized with the specific primer sequences (Integrated DNA Technologies). Multiplexed quantitative determination was done in triplicate wells with the use of the DNA from 105 cells/well along with the primer/probe sets for TREC (FAM labeled) and RNaseP (VIC labeled) with the standard ABI chemistry and reagents. Threshold cycle during the exponential phase of amplification was determined by real-time monitoring of fluorescent emission after cleavage of sequence-specific probes by nuclease activity of Taq polymerase. Standards with the use of titrated plasmids containing either the RNaseP target sequence or the sjTREC sequence (107 to 1 copy in 10-fold serial dilutions) were used to generate standard curves and to calculate the copy number per 105 cells on the basis of comparison to the known copy number (2) of RNaseP per cell.
Cytokine gene expression by regulatory T cells
mRNA transcripts were isolated from CD3+CD4+CD25+Foxp3+ and CD3+CD4+CD25−Foxp3− sorted lymphocytes (1 × 105) by Proteinase K digestion followed by isolation with the use of TRIzol (Invitrogen; no. 15596-026). The expression of the mRNA was then quantified with real-time PCR to assess levels of mRNA transcripts for transforming growth factor-β, interleukin-10 (IL-10), and interferon-γ (IFN-γ).
Suppression assays
CD4+CD25+ T cells (5 × 104) were isolated from peripheral mononuclear cells by first depleting CD8+ T cells with immunomagnetic beads (Invitrogen) followed by positively selecting CD25+ T cells with anti-CD25 immunomagnetic beads. This selection was done according to the instructions supplied by the manufacturer. The selected CD25+ T cells were washed 3 times before suspension in complete culture medium. Purity of the population was confirmed by flow cytometric analysis with > 95% CD4+CD25+ T cells. These CD4+CD25+ T cells were then added to unfractionated cells (1 × 105) stimulated with irradiated allogeneic lymphocytes (1 × 105) in a mixed lymphocyte reaction (MLR). Two days after initiation of culture, the supernatants were harvested and assessed for inflammatory cytokines (IL-2, IFN-γ, tumor necrosis factor-α [TNF-α]), and macrophage inflammatory protein 1-α [MIP-1α]) with the use of the Bio-Plex assay (see “Bio-Plex”).
In the second type of suppression assay, peripheral blood mononuclear cells were depleted of CD25+ T cells with the use of anti-CD25 immunomagnetic beads. The depleted cells were washed 3 times in tissue culture medium before suspension in complete culture medium. Flow cytometric analysis showed that > 97% of CD25+ T cells were removed after depletion. Inflammatory cytokine production (IL-2, IFN-γ, TNF-α, and MIP-1α) was measured before and after CD25+ T-cell depletion in a MLR as described earlier.
Bio-Plex
Cytokine levels were measured on the Bio-Plex 200 suspension array system (Bio-Rad) with the Bio-Plex Pro Human Cytokine 17-plex Assay (Bio-Rad). The concentrations (in pg/mL) of target cytokines were determined with the use of the vendor-supplied software.
Bio-Plex analysis was conducted with a set of standards supplied by the manufacturer and tested in triplicate. Variation among the triplicates both for the controls and for the patient samples averaged < 2%. The correlation coefficient for the regression analysis of the standard curves was 0.98. Virtually identical levels of cytokines with no significant differences were detected on repeat of patient samples selected at random.
T-cell receptor spectratyping
RNA was isolated from CD3+CD4+CD25+Foxp3+ and CD3+CD4+CD25−Foxp3− sorted lymphocytes (1 × 105) by Proteinase K digestion followed by isolation with TRIzol (Invitrogen; no. 15596-026). Random primers (Invitrogen; no. 58875) and RTG YouPrime beads (General Electric) were used to generate cDNA. The diversity of CDR3 regions for 24 T-cell receptor (TCR) Vβ regions was assessed with the TCRExpress Quantitative Analysis Kit (Biomed Immunotech; no. H0534).24 Expected size distributions for each Vβ were determined by averaging the results from 7 healthy adults. Peaks were considered to be antigen-driven when the observed proportion of a given fragment size exceeded 3 standard deviations from the control population distribution per the manufacturer's protocol.
RNA extraction and analysis
The level of target gene expression was determined by quantitative PCR (in triplicate with multiplexed target and control gene primer/probe sets) with the use of an ABI 7500 prism system with the standard ABI chemistry and reagents. Expression relative to the housekeeping gene GADPH was determined by comparing the differences in threshold cycle values. Multiplexing the target and control probes in the reaction mixture facilitated the best determination of relative expression and provided a positive signal (GADPH) in the event a particular target gene was not expressed. This provided an internal positive control, confirming that intact RNA was isolated from the sorted cells. Because the total amount of RNA that could be isolated from the sorted cells was very low, it was not feasible to directly measure the quality of the RNA. Nevertheless, the sorted cells did give a strong signal for the housekeeping gene GADPH, and the curvilinear analysis on the ABI 7500 prism did not show any problems in the PCR.
For spectratype and TREC analyses, the number of cells from the sorted population was determined compared with RNaseP expression. In addition, the purity of the sorted population was > 98%. Furthermore, Foxp3 gene expression analysis (by quantitative PCR) of the Foxp3− and Foxp3+ populations (isolated flow cytometrically) showed that Foxp3 was only expressed in the positive population.
Statistical analyses
Statistical analyses were performed with the SigmaStat program, Version 3.01A (Systat Software Inc). Data for between-group analyses were first subjected to tests of normality. If they passed, they underwent unpaired t tests or factorial analysis of variance as applicable. If normality tests failed, Mann-Whitney rank sum tests or Kruskal-Wallis tests were done as applicable. For all comparisons, P < .05 was considered statistically significant.
Results
Patient demographics
As depicted in Table 1, the 20 patients (14 men, 6 women) ranged in years of age from 22 to 70 with a median age of 52.5 years and a mean age of 51.0 years. All patients had newly diagnosed AML and were undergoing induction TST. Eleven patients had AML de novo, 7 patients had AML developing from myelodysplastic syndrome, and 2 patients had treatment-related AML (1 chronic lymphocytic leukemia, 1 breast cancer). Eleven patients received AcDVP16,18,19 and 9 patients received FLAM.20
All patients had follow-up data for a minimum of 2 years or until death. Age at induction TST had no relationship with survival at 2 years (Mann-Whitney; medians: alive 56 years, deceased 47 years; P = .571). Two-year overall survival was 50% (10 of the 20 patients). Fifteen of 20 patients (75%) achieved complete remission (CR) with a median 2-year disease-free survival of 17.9 months (range, 2-24 months at last follow-up). Seven of 15 patients in CR relapsed (range, 2-17 months) of whom 2 died of persistent disease, 3 died of complications of treatment for relapsed disease, and 2 survived to > 24 months. Of the 5 patients failing to achieve CR, 4 died of persistent disease and 1 died of staphylococcal sepsis during induction TST.
Leukocyte count recovery
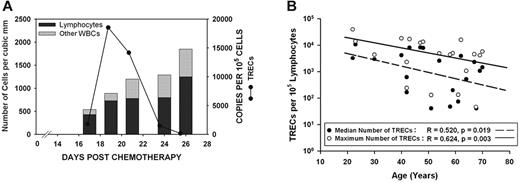
WBC count nadir occurred on median day 14.5 and mean day 13.9 (range, 8-25 days). Early leukocyte recovery started on median day 18 and mean day 19.7 (range, 12-29 days). The start day of leukocyte recovery was not significantly associated with age (linear regression: r = 0.257, P = .273) or specific chemotherapy regimen (Mann-Whitney; medians: AcDVP16, 18: FLAM, 24; P = .177). Of the recovering leukocytes, most were lymphocytes. On the first day of defined leukocyte recovery, lymphocytes constituted a median of 97% and mean of 90.0% of each person's total WBC count (range, 50%-100%) (Figure 1A).
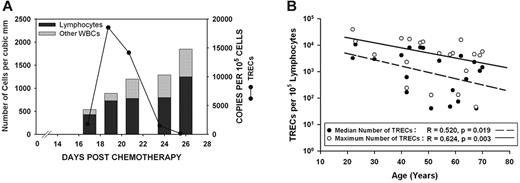
Although TREC expression drops off significantly with age, most patients exhibit an initial burst of thymopoietic activity that contributes to early lymphocyte recovery. (A) Total WBC count, percentage of lymphocytes, and expression of TRECs were assessed serially on all patients during the early leukocyte recovery period after timed sequential chemotherapy. The results from 1 patient who received AcDVP16 are shown. These results were representative of nearly all patients, regardless of chemotherapeutic regimen received. The majority of recovering leukocytes were lymphocytes. Although all patients had detectable TREC expression, 16 of 20 patients had a spike in TREC levels coinciding with initial lymphocyte recovery, suggesting that there is an initial burst of thymopoiesis that occurs during early lymphocyte recovery. (B) Age at start of chemotherapy had a significant negative predictive effect on both the median TREC expression (linear regression: r = 0.520, P = .019) and the maximum TREC expression (r = 0.624, P = .003) for a given patient. Median TREC expression was elevated in T lymphocytes derived from patients after chemotherapy compared with healthy controls. Healthy, age-matched persons had negligible TREC expression with a median of 47 copies/105 lymphocytes compared with a median of 1873 TRECs/100 000 cells for patients (P = .015).
Although TREC expression drops off significantly with age, most patients exhibit an initial burst of thymopoietic activity that contributes to early lymphocyte recovery. (A) Total WBC count, percentage of lymphocytes, and expression of TRECs were assessed serially on all patients during the early leukocyte recovery period after timed sequential chemotherapy. The results from 1 patient who received AcDVP16 are shown. These results were representative of nearly all patients, regardless of chemotherapeutic regimen received. The majority of recovering leukocytes were lymphocytes. Although all patients had detectable TREC expression, 16 of 20 patients had a spike in TREC levels coinciding with initial lymphocyte recovery, suggesting that there is an initial burst of thymopoiesis that occurs during early lymphocyte recovery. (B) Age at start of chemotherapy had a significant negative predictive effect on both the median TREC expression (linear regression: r = 0.520, P = .019) and the maximum TREC expression (r = 0.624, P = .003) for a given patient. Median TREC expression was elevated in T lymphocytes derived from patients after chemotherapy compared with healthy controls. Healthy, age-matched persons had negligible TREC expression with a median of 47 copies/105 lymphocytes compared with a median of 1873 TRECs/100 000 cells for patients (P = .015).
Despite an early burst of thymopoietic activity, most recovering lymphocytes are peripherally derived
The TREC is an excision product of T-cell receptor gene rearrangements that occurs in maturing thymocytes.25 Therefore, the presence of TRECs in cells is a marker for recent thymic emigration. We assessed for TREC expression in unsorted T lymphocytes isolated serially every 2 days after the start of early lymphocyte recovery for 4-5 blood draws from each of all 20 patients.
All patients had detectable levels of TRECs. In 16 of 20 patients, TREC expression rapidly rose to high levels before declining, suggesting that there is an initial burst of thymopoiesis that occurs during early PBL recovery (Figure 1). Age at the start of induction TST had a significant negative correlation with both the median TREC expression and the maximum TREC expression for a given patient (linear regression; median TREC, r = 0.520 and P = .019; maximum TREC, r = 0.624 and P = .003; Figure 1B).
Both median TREC expression and maximum TREC expression were significantly elevated in T lymphocytes derived from patients after TST compared with healthy controls. The daily median for all patients was 1873 TRECs per 100 000 cells (range, 0-10 720 cells), indicating that on average ≥ 1.9% of early recovering PBLs were of thymic origin compared with 0.047% for controls (Mann-Whitney rank sum test; P = .015). All healthy controls had negligible levels of TRECs detected with a median of 47 TRECs per 100 000 cells. In terms of the maximum TREC expression on any day, the median for all patients was 4708 TRECs per 100 000 cells (range, 47-39 304 cells), indicating that during the burst of thymopoiesis, ≥ 4.7% of cells were derived from the thymus.
Recovering lymphocytes are predominantly CD45RO+ and CD27+, corroborating peripheral derivation from antigen stimulation
Flow cytometry for expression of CD45RO and CD45RA was performed on unsorted T lymphocytes derived from 5 patients during early PBL recovery after TST. Most of the unsorted PBLs (median, 83.1%; range, 64.8%-92.5%) expressed CD45RO+. A small minority of unsorted PBLs (median, 12.5%; range, 3.3%-16.1%) expressed CD45RA+. These data substantiated the implication of the TREC data described earlier, namely that unsorted T lymphocytes during early PBL recovery were chiefly peripherally derived.
CD27 expression is highly up-regulated in response to TCR/CD3 activation as opposed to down-regulated in response to direct activation of protein kinase C by phorbol esters.26,27 With the use of flow cytometry, 77% of CD3+CD4+ T lymphocytes were found to have significant expression of CD27+, suggesting that the peripheral derivation of these cells may have been antigen stimulated.
Immunophenotypic profile of recovering PBLs: an expanded population of CD3+CD4+CD25+Foxp3+ T cells
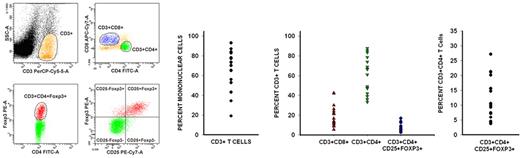
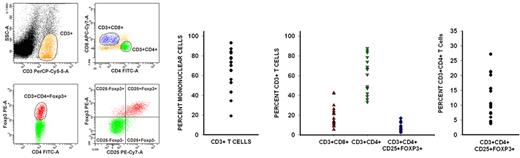
CD3+ cells constituted a median of 73.4% (range, 19%-93%) of the peripheral blood mononuclear cells during early PBL recovery. Of these CD3+ cells, CD8+ cells constituted a median of 16.7% (range, 9%-42%), whereas CD4+ cells constituted a median of 66.4% (range, 33%-88%). The median ratio of CD3+CD4+ cells to CD3+CD8+ cells for these 17 patients was 2.7:1 (range, 1.1:1 to 9.3:1) (Figure 2).
Recovering lymphocytes are mostly CD3+CD4+, and there is a much larger than expected population of CD3+CD4+CD25+Foxp3+ T cells. Flow cytometric analysis of peripheral blood lymphocytes isolated from patients during the early phase of lymphocyte recovery after induction chemotherapy showed that there is significant recovery of CD3+CD4+ and CD3+CD8+ cells. Data from 1 representative patient are shown on the left. CD3+ cells constituted a median of 73.4% (range, 19%-93%) of the recovering mononuclear cells. Of CD3+ cells, CD8+ cells constituted a median of 16.7%, whereas CD4+ cells constituted a median of 66.4%. Surprisingly, there also was a rapid and significant recovery of regulatory T cells (median, 10.5% of CD4+ T cells), which were phenotypically identified by the coexpression of CD3, CD4, CD25, and Foxp3.
Recovering lymphocytes are mostly CD3+CD4+, and there is a much larger than expected population of CD3+CD4+CD25+Foxp3+ T cells. Flow cytometric analysis of peripheral blood lymphocytes isolated from patients during the early phase of lymphocyte recovery after induction chemotherapy showed that there is significant recovery of CD3+CD4+ and CD3+CD8+ cells. Data from 1 representative patient are shown on the left. CD3+ cells constituted a median of 73.4% (range, 19%-93%) of the recovering mononuclear cells. Of CD3+ cells, CD8+ cells constituted a median of 16.7%, whereas CD4+ cells constituted a median of 66.4%. Surprisingly, there also was a rapid and significant recovery of regulatory T cells (median, 10.5% of CD4+ T cells), which were phenotypically identified by the coexpression of CD3, CD4, CD25, and Foxp3.
Regulatory T cells normally constitute 1%-2% of circulating CD4+ T cells.28 Flow cytometric analysis showed a much larger than expected population of CD3+CD4+CD25+Foxp3+ regulatory T cells with a median of 10.5% of the CD3+CD4+ subpopulation expressing Foxp3 (range, 3.7%-27.2%) (Figure 2). These cells expressed transforming growth factor-β (relative transcripts 0.4/GAPDH) and IL-10 (relative transcripts 0.01/GAPDH) but did not express detectable levels of IFN-γ, all consistent with a regulatory T-cell phenotype.
Regulatory T cells present during early lymphocyte recovery are activated regulatory T cells
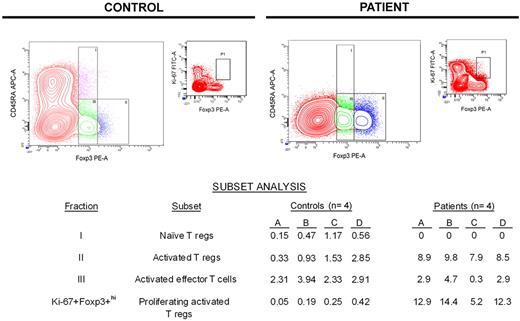
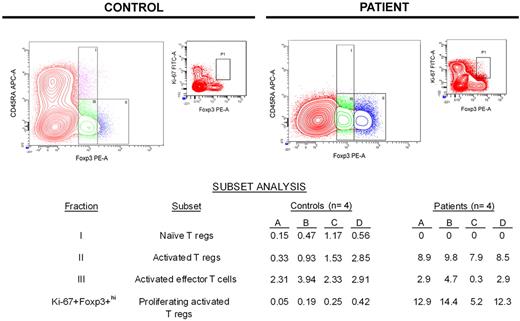
The specificity of Foxp3 for identifying regulatory T cells has been questioned because activated effector T cells without suppressive activity (not regulatory T cells) also may express Foxp3, although relative expression of Foxp3 in such cells is low.29-32 Miyara et al identified a strategy for distinguishing regulatory T cells from effector T cells without regulatory activity on the basis of relative CD45RA and Foxp3 expression.33 We used this strategy to partition T cells expressing Foxp3 into 3 distinct subsets: fraction I (CD45RA+Foxp3+lo) represents naive regulatory T cells, fraction II (CD45RA−Foxp3+hi) represents activated regulatory T cells, and fraction III (CD45RA−Foxp3+lo) represents activated effector T cells that do not have regulatory activity.
The data summarized in the table of Figure 3 show that patients had no naive regulatory T cells (fraction I) during lymphocyte recovery, in contrast to healthy controls who had a small but detectable naive regulatory T-cell population. Conversely, patients had a significantly larger population of activated regulatory T cells (fraction II) compared with controls (medians: controls, 1.4%; patients, 8.8%; P < .001). Meanwhile, the size of the activated effector T-cell population (fraction III) was similar between controls and patients (medians: controls, 2.9%; patients, 2.7%; P = .866). On the basis of the expression of Ki-67 within activated regulatory T cells (CD45RA−Foxp3+hi; fraction II), patients had a much higher percentage of proliferating activated regulatory T cells compared with controls (medians: controls, 0.2%; patients, 12.6%; P = .029).
Most Foxp3+ T cells during early lymphocyte recovery after induction chemotherapy are proliferating activated regulatory T cells. On the basis of a previous study by Miyara et al with the use of relative expression of Foxp3 and CD45RA,33 flow cytometric analysis was used to partition Foxp3+ T cells into 3 subsets. Data from a representative healthy control and patient are shown graphically, whereas the subset analyses for all controls and patients tested are shown in the table. The population of CD45RA−Foxp3+lo effector (nonregulatory) T cells in patients during early lymphocyte recovery after TST is similar to that seen in healthy controls (P = .866). Unlike healthy controls, patients do not have an appreciable population of naive regulatory T cells. Conversely, patients have a significantly expanded population of activated regulatory T cells (P < .001), which are proliferating much more rapidly than activated regulatory T cells found in controls (P = .029). APC indicates allophycocyanin.
Most Foxp3+ T cells during early lymphocyte recovery after induction chemotherapy are proliferating activated regulatory T cells. On the basis of a previous study by Miyara et al with the use of relative expression of Foxp3 and CD45RA,33 flow cytometric analysis was used to partition Foxp3+ T cells into 3 subsets. Data from a representative healthy control and patient are shown graphically, whereas the subset analyses for all controls and patients tested are shown in the table. The population of CD45RA−Foxp3+lo effector (nonregulatory) T cells in patients during early lymphocyte recovery after TST is similar to that seen in healthy controls (P = .866). Unlike healthy controls, patients do not have an appreciable population of naive regulatory T cells. Conversely, patients have a significantly expanded population of activated regulatory T cells (P < .001), which are proliferating much more rapidly than activated regulatory T cells found in controls (P = .029). APC indicates allophycocyanin.
CD3+CD4+CD25+Foxp3+ T cells also express CD27 and glucocorticoid-induced tumor necrosis factor receptor, further supporting an activated regulatory T-cell phenotype
CD27 expression is a marker of regulatory activity in CD3+CD4+CD25+ T cells.34 Flow cytometry was used to sort CD3+CD4+CD25+Foxp3+ T cells on the basis of their expression of CD27. In recovering lymphocytes after TST, 91.6% of CD3+CD4+CD25+Foxp3+ T cells also expressed CD27.
Glucocorticoid-induced tumor necrosis factor receptor (GITR) is found on regulatory T cells and is up-regulated in response to recent activation.35 cDNA was generated from the mRNA message in CD4+CD25+Foxp3+hi T cells and amplified by PCR. CD4+CD25+Foxp3+hi T cells expressed GITR (relative transcripts 1.32/GAPDH). Taken together, these additional studies further support an activated regulatory T-cell phenotype for CD4+CD25+Foxp3+ T cells during early lymphocyte recovery after TST.
CD4+CD25+ T cells exert suppressive action on cytokine release in a MLR
To address the question of whether CD4+CD25+ T cells identified phenotypically were regulatory T cells with functional activity, CD4+CD25+ cells were assessed for their ability to inhibit cytokine production in a MLR. The limited number of cells precluded assessment of proliferation. In this assay, the unfractionated lymphocytes were markedly unresponsive to in vitro stimulation in MLR. Only 1 of 3 patients weakly responded. Even for the responding patient, expression of > 75% of inflammatory cytokine (IL-2, IFN-γ, TNF-α, and MIP1-α; data not shown) was suppressed with the addition of CD4+CD25+ T cells.
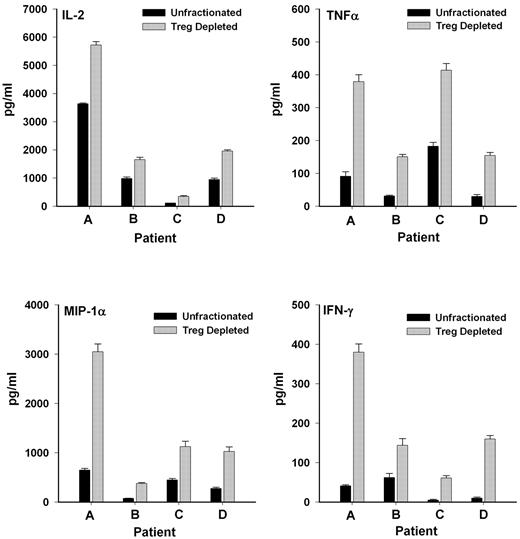
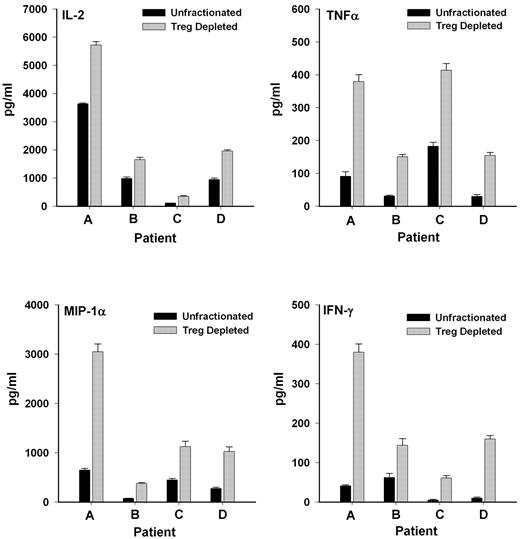
Because the cells were largely unresponsive presumably because of the suppressive actions of regulatory T cells already contained in the unfractionated lymphocytes, another approach was used to assess whether CD25+ T cells functionally were regulatory T cells. The response of unfractionated cells was compared with the response of cells after depletion of CD25+ T cells (leaving CD4+CD25− helper T cells intact to respond). As shown in Figure 4, depletion of the CD25+ population significantly (P < .01) enhanced the ability of the remaining lymphocytes to produce inflammatory cytokines after allogeneic cell stimulation in a MLR. These results also suggested that the poor response of the patient cells to in vitro stimulation in the first set of experiments was, in fact, due to the presence of CD4+CD25+ T cells, which, as regulatory T cells, were exerting suppressive effects.
CD4+CD25+ T cells exert suppressive effects on cytokine production in a MLR. Release of inflammatory cytokines was significantly elevated (P < .01) in cultures depleted of CD25+ T cells compared with unfractionated T lymphocyte cell cultures when exposed to irradiated allogeneic lymphocytes in a MLR.
CD4+CD25+ T cells exert suppressive effects on cytokine production in a MLR. Release of inflammatory cytokines was significantly elevated (P < .01) in cultures depleted of CD25+ T cells compared with unfractionated T lymphocyte cell cultures when exposed to irradiated allogeneic lymphocytes in a MLR.
CD3+CD4+CD25+Foxp3+ regulatory T cells are peripherally derived
TREC expression was minimally elevated in CD3+CD4+CD25+Foxp3+ T cells during early PBL recovery. The median TREC expression for CD3+CD4+CD25+Foxp3+ T cells was 922 TRECs per 100 000 cells, approximately one-half that observed in the unsorted T-lymphocyte population. These data suggested that nearly all CD3+CD4+CD25+Foxp3+ T cells (∼ 99%) were peripherally derived.
Furthermore, CD3+CD4+CD25+Foxp3+ T cells expressed high levels of CD45RO (median, 82.0%; range, 77.8%-88.8%) and low levels of CD45RA (median, 8.7%; range, 4.8%-13.4%). These CD45RO/CD45RA expression levels were similar to the relative expression levels seen in unsorted T lymphocytes and further substantiated the implication of the TREC data that CD3+CD4+CD25+Foxp3+ T cells during PBL recovery were predominantly peripherally derived. This contrasted with CD3+CD4+CD25+Foxp3+ T cells from controls, wherein expression of CD45RO was only 60%-65% and expression of CD45RA was 35%-40%.
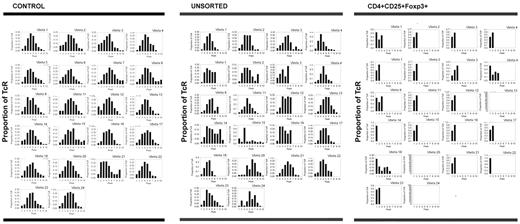
CD3+CD4+CD25+Foxp3+ regulatory T cells show oligoclonal skewing
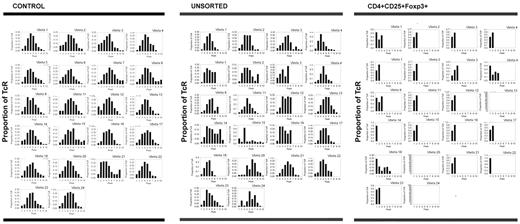
TCR spectratyping analysis was used to assess the diversity of recovering T lymphocytes. The Vβ CDR3 chain type, distribution, and deviations from a normal Gaussian distribution were quantified in our patient samples. Deviations (≥ 3 standard deviations from the normal) were labeled as “driven peaks.” For comparison, PBLs derived from healthy controls also were spectratyped. Analysis of control persons showed that there was a Gaussian distribution of CDR3 domains for the majority (> 97%) of Vβ genes. Of the 2 controls, 1 control had 175 peaks with only 5 being driven peaks (2.9%), whereas the other control had 164 peaks with only 3 being driven peaks (1.8%) (Table 2). For healthy controls, the diversity of the TCR repertoire of regulatory T cells is similar to the diversity of unsorted T lymphocytes.36
Comparatively, unsorted T lymphocytes from 3 patients showed a median of 157 peaks (range, 144-172 peaks) with a median of 32 driven peaks (range, 28-40 driven peaks; median 20.4% of peaks were driven peaks). Thus, the total number of peaks was similar to controls, but the percentage of driven peaks was higher. Moreover, regulatory T cells from 7 patients (n = 10 because 3 patients were assayed on 2 separate days) displayed a median of 29 total peaks (range, 1-100 total peaks) with a median of 21 driven peaks (range, 1-42 driven peaks; median 80.6% of peaks were driven peaks), indicating that recovering regulatory T cells were oligoclonal (Table 2; Figure 5).
Regulatory T cells recovering after chemotherapy show clear oligoclonal skewing, suggesting that their proliferation is antigen-driven. TCR spectratyping analysis was used to assess the diversity of the recovering T lymphocytes, specifically the types and lengths of TCR expression. Unsorted T lymphocytes derived from healthy controls expressed a full range of Vβ chains with approximately normal Gaussian distributions of the lengths of the Vβ chains for each Vβ (< 3% driven peaks). Comparatively, unsorted T lymphocytes from a representative patient on day 21 after AcDVP16 showed a full complement of Vβ chains, but the lengths of these chains varied somewhat from a normal distribution (27.8% driven peaks). Conversely, regulatory T cells from that same patient on that same day expressed a markedly constricted range of TCR Vβ chains with striking oligoclonal skewing (75.6% driven peaks). These data suggest that the proliferation of regulatory T lymphocytes after timed sequential chemotherapy is antigen-driven.
Regulatory T cells recovering after chemotherapy show clear oligoclonal skewing, suggesting that their proliferation is antigen-driven. TCR spectratyping analysis was used to assess the diversity of the recovering T lymphocytes, specifically the types and lengths of TCR expression. Unsorted T lymphocytes derived from healthy controls expressed a full range of Vβ chains with approximately normal Gaussian distributions of the lengths of the Vβ chains for each Vβ (< 3% driven peaks). Comparatively, unsorted T lymphocytes from a representative patient on day 21 after AcDVP16 showed a full complement of Vβ chains, but the lengths of these chains varied somewhat from a normal distribution (27.8% driven peaks). Conversely, regulatory T cells from that same patient on that same day expressed a markedly constricted range of TCR Vβ chains with striking oligoclonal skewing (75.6% driven peaks). These data suggest that the proliferation of regulatory T lymphocytes after timed sequential chemotherapy is antigen-driven.
Thus, TCRs in healthy controls (n = 2) showed a full range of Vβ chains with approximately Gaussian distributions of the length of the chains for each Vβ. Although unsorted T lymphocytes from patients (n = 3) after TST showed a full complement of Vβ chains, the lengths of these chains varied somewhat from a normal distribution. Conversely, CD3+CD4+CD25+Foxp3+ regulatory T lymphocytes isolated from patients (n = 10) after TST during early PBL recovery displayed a markedly constricted range of TCR Vβ chains with striking oligoclonal skewing (Figure 5), suggesting that the proliferation of these regulatory T lymphocytes after TST was antigen-driven. This oligoclonality seemed to persist because the 2 patients (patients 5 and 8) who had spectratyping repeated at 3 months after induction TST still had marked restriction of their regulatory T-cell Vβ expression, whereas the unsorted T lymphocytes still showed relatively normal Vβ expression (Table 2).
Discussion
Our work is unique in immunophenotypically characterizing early lymphocyte recovery within the first month after induction TST for AML. We show that CD3+CD4+CD25+Foxp3+ T cells comprise an unexpectedly high percentage of recovering PBLs after TST in all patients with AML. These CD4+CD25+ T cells show suppressive activity and phenotypically are CD45RA−, Foxp3 highly positive, CD27+, and GITR+, confirming that they are activated regulatory T cells. These regulatory T cells are peripherally derived and show marked oligoclonal skewing, thus suggesting an antigen-driven mechanism for their expansion.
T lymphocyte recovery can be due to thymic-dependent T-cell development or peripheral expansion of circulating T lymphocytes that survive chemotherapy. While the thymus plays a role in PBL reconstitution,37,38 eventual recovery of CD4+ T cells is thought to be largely thymic independent.38-40 Although our data do show that most early recovering PBLs are peripherally derived, surprisingly, most patients, regardless of age, show a burst of appreciable thymopoiesis during early PBL recovery.
It is important to note that TRECs are not replicated during mitosis and thus become diluted with each successive iteration of cell division. Therefore, lower levels of TRECs in rapidly dividing cells can underestimate the amount of thymic regeneration.41 On the other hand, some terminally differentiated effector T cells can lose their CD45RO expression and gain CD45RA expression.42 Thus, the TREC data potentially underestimate thymic derivation, whereas the CD45RO/CD45RA data potentially underestimate peripheral derivation. This would explain the lower relative contribution of the thymus to PBL recovery with the TREC data compared with the CD45RO/CD45RA data.
Nevertheless, when taken together with these 2 caveats, the TREC and CD45RO/CD45RA data would set lower limits on the minimum thymic derivation and the minimum peripheral derivation, respectively. Thus, for unsorted T cells, the peripheral derivation of cells would be a minimum of 83% and a maximum of 98%. Similarly, for CD4+CD25+Foxp3+ T cells, the peripheral derivation would be a minimum of 82% and a maximum of 99%. Overall, these data together confirm the peripheral derivation of the vast majority of both unsorted CD4+ and sorted CD4+CD25+Foxp3+ T cells during initial lymphocyte recovery after TST.
Peripheral expansion of T cells can occur either through an antigen-driven mechanism or through homeostatic proliferation of naive T cells. The latter mechanism has been shown to occur in murine subjects that were irradiated and subjected to adoptive transfers of T lymphocytes.43,44 Although this homeostatic mechanism has been shown to occur in these murine models that received a transplant, there are no data on whether similar mechanisms occur in human subjects undergoing autologous reconstitution after induction chemotherapy for AML. Although we cannot absolutely rule out homeostatic proliferation rather than antigen stimulation as the cause of peripheral expansion in our patients, the available evidence would argue against it. Homeostatic proliferation has been shown to involve a few rounds of proliferation by a broad base of cells, whereas in our study the oligoclonal skewing would suggest extensive proliferation of a few clones. Furthermore, relatively high expression of CD25, CD27, and GITR as seen in our patients is consistent with antigen-driven expansion.44
Because an expanded oligoclonal population of CD4+CD25+Foxp3+ T cells during early lymphocyte recovery after TST is the central finding of this work, it was important to identify these cells as regulatory T cells functionally as well as phenotypically. Our suppression assay shows that CD25+-depleted lymphocyte populations have much higher inflammatory cytokine production than unfractionated populations containing CD4+CD25+ T cells, confirming regulatory activity of these CD4+CD25+ T cells.
Depletion of the CD25+ population alone was specifically performed as CD4+CD25− T cells were needed to coordinate the inflammatory response to the allogeneic lymphocytes in culture. Although the ideal experiment would have involved depleting these populations of CD4+CD25+Foxp3+ T cells, the permeabilization process necessary to identify Foxp3+ expression would have affected the functionality of these cells and limited assessment of their suppressive action. Even so, on the basis of our flow cytometric data, CD25 and Foxp3 expression sorted together such that there were negligible numbers of CD25+Foxp3− T cells.
Although the presence of an expanded population of regulatory T cells in newly diagnosed AML before treatment has been previously described,45,46 there are limited data in patients with AML after treatment. A study45 (concurrent to our own) that focused primarily on regulatory T-cell expansion before treatment also noted that in the 7 patients achieving complete remission, the regulatory T-cell percentage was still elevated at the time of remission and actually was increased compared with pretreatment levels.
While we confirm this expansion of regulatory T cells after chemotherapy, our study is distinct both in examining initial lymphocyte recovery in the first month after induction chemotherapy and in analyzing the regulatory T-cell population in all patients irrespective of whether they achieved CR. Furthermore, our work is the first to show peripheral oligoclonal expansion of these regulatory T cells during initial lymphocyte recovery after induction chemotherapy. Although we would have liked to compare the populations of these regulatory T cells before and after TST, the lack of sufficient quantities of pretreatment cells precluded such comparisons. Nevertheless, the mere presence of this regulatory T-cell population has independent clinical prognostic implications, given that larger regulatory T-cell populations before treatment in AML correlates with decreased achievement of remission.45
The discovery of an oligoclonally expanded population of regulatory T cells present during early PBL recovery after TST has potentially important implications for immune-based treatment. Any vaccination or other immunomodulatory technique introduces an antigen into the system with the hopes of priming an antileukemic response. Antigen-driven expansion of the regulatory T-cell population likely plays a critical role in the net response to any immunomodulatory stimulation during the early PBL recovery period. Presumably, the nature of the regulatory T-cell response to antigen stimulation depends on the type and timing of antigen stimulation.
The most surprising aspect of this study is that the same basic immunologic phenomenon (peripheral oligoclonal expansion of a regulatory T-cell population during initial lymphocyte recovery after TST) is conserved and shared across this heterogeneous patient population. This discovery points to a certain stereotyped immunologic response to TST in patients with AML that is independent of the AML etiology, cytogenetics, or molecular characteristics for the particular patient. A better understanding of this immunologic response is essential to designing therapies that will effectively manipulate immunologic states after chemotherapy toward a survival advantage for patients with AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by grants from the National Institutes of Health (CA06973, CA70095, CA15396, and M01-RR0052).
National Institutes of Health
Authorship
Contribution: C.G.K. analyzed the data, wrote the manuscript, and helped make the figures; A.D.H. designed the research, performed experiments, analyzed data, made the figures, and edited the manuscript; C.D.G. and H.L. contributed to the study design and edited the manuscript; C.T. and L.L. performed experiments; F.K. performed the flow cytometric studies; C.M. and J.B. enrolled patients and collected and collated all of the samples; B.D.S. contributed to the study design; and J.E.K. designed the research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allan D. Hess, Division of Immunology, The Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, 417 N Caroline St, Bond St Bldg, Rm 302, Baltimore, MD, 21231; e-mail: adhess@jhmi.edu.