Abstract
Platelet hyperactivity associated with hyperlipidemia contributes to development of a pro-thrombotic state. We previously showed that oxidized LDL (oxLDL) formed in the setting of hyperlipidemia and atherosclerosis initiated a CD36-mediated signaling cascade leading to platelet hyperactivity. We now show that the guanine nucleotide exchange factors Vav1 and Vav3 were tyrosine phosphorylated in platelets exposed to oxLDL. Pharmacologic inhibition of src family kinases abolished Vav1 phosphorylation by oxLDL in vitro. Coimmunoprecipitations revealed the tyrosine phosphorylated form of src kinase Fyn was associated with Vav1 in platelets exposed to oxLDL. Using a platelet aggregation assay, we demonstrated that Vav1 deficiency, Fyn deficiency, or Vav1/Vav3 deficiency protected mice from diet-induced platelet hyperactivity. Furthermore, flow cytometric analysis revealed that Vav1/Vav3 deficiency significantly inhibited oxLDL-mediated integrin αIIbβIII activation of platelets costimulated with ADP. Finally, we showed with an in vivo carotid artery thrombosis model that genetic deletion of Vav1 and Vav3 together may prevent the development of occlusive thrombi in mice fed a high-fat diet. These findings implicate Vav proteins in oxLDL-mediated platelet activation and suggest that Vav family member(s) may act as critical modulators linking a prothrombotic state and hyperlipidemia.
Introduction
Hyperlipidemia is recognized as a major risk factor for atherosclerosis and its complications including acute coronary syndromes. Hyperlipidemic individuals typically have elevated plasma level of low-density lipoprotein (LDL) as well as decreased plasma level of high-density lipoprotein (HDL). Hyperlipidemia is also associated with oxidant stress, resulting in the generation of oxidized LDL (oxLDL).1 OxLDL contains many classes of atherogenic oxidized lipids, including specific phosphatidylcholine (PC) species that are high affinity ligands for the type B scavenger receptor CD36 and that we have termed oxPCCD36.2
OxPCCD36 are present in atherosclerotic lesions as well as in the plasma of hyperlipidemic individuals.3,4 OxLDL particles carrying OxPCCD36 bind to macrophage CD36 and transmit intracellular signals that lead to inhibition of migration and promotion of cholesterol accumulation and foam cell formation.3,5 OxLDL and oxPCCD36 also bind to platelets via CD36 leading to platelet activation.4,6,7 This process may contribute mechanistically to the well known clinical association between hyperlipidemia, oxidant stress, enhanced platelet reactivity, and the prothrombotic state.4
The mechanism by which CD36-oxLDL interactions promotes platelet reactivity is not completely understood. We previously showed that on oxLDL binding, platelet CD36 recruits the src family kinases Fyn and Lyn into a multiprotein complex and that src kinase mediated activation of MAP kinases, specifically JNK, was required for oxLDL-mediated platelet activation.8 Although these findings provide valuable insights into the mechanism underlying the initiation of platelet CD36 signaling triggered by oxLDL, the intracellular signaling molecules responsible for transducing prothrombotic signals remain to be elucidated. Candidate molecules that could potentially link the platelet CD36 signaling complex to downstream events may include the proto-oncogene Vav family members. Vav proteins have been shown to be substrates for tyrosine kinases including src family members Syk, Fyn, and Lyn.9-11 They are large multi-domain proteins composed of a calponin-homology domain, an acidic region, Dbl and plekstrin homology domains, a zinc finger domain, and 2 SH3 domains flanking a single SH2 region.12 The SH2 region binds phosphotyrosine residues, mediating the interaction of Vav with tyrosine kinases.12 There are 3 Vav family members: Vav1, Vav2, and Vav3 in the mammalian genome. Vav2 and Vav3 proteins are widely expressed while Vav1 is specifically expressed in hematopoietic cells.12
Vav proteins have been studied extensively for their enzymatic activity as guanine nucleotide exchange factors (GEF) for Rho/Rac family proteins, although it has been suggested that Vav molecules may also function as adaptor proteins.12,13 The individual Vav proteins have specificity toward different Rho family G proteins. The GEF activity of Vav proteins is tightly regulated by tyrosine phosphorylation and the functional role of the Vav family coevolved with tyrosine kinase pathways. In the nonphosphorylated state, Vav proteins are in an auto-inhibited state and cannot interact with their small G-protein substrates. In contrast, on phosphorylation, Vav proteins adapt an “open” configuration capable of interacting with their substrates.
Among the 3 Vav family members, Vav1 has been implicated in CD36 signaling in monocytes and microglial cells exposed to β amyloid.9 In this setting, Vav1 undergoes an increase in tyrosine phosphorylation after the assembly of cell-surface receptor complex including CD36 and integrin-associated protein/CD47.9 In platelet biology, the role of Vav family members has not been extensively studied although Vav1 and Vav3 have been implicated in platelet activation in vitro by collagen.14,15 In studies outlined here, we have demonstrated that a novel signaling cascade involving Fyn and Vav family member(s) was induced by oxLDL and was responsible for enhanced platelet reactivity associated with high-fat feeding. These data indicate that Vav proteins play an essential role in the association between a prothrombotic state and hyperlipidemia and may provide new insights in targeting pathologic platelet activation in the setting of hyperlipidemia.
Methods
Materials
Rabbit Abs to Fyn, Vav1, and Vav3 were from Santa Cruz Biotechnology Inc. Anti-phosphotyrosine Ab (4G10) was from Upstate Biotechnology Inc. Mouse Ab to Fyn was from BD Biosciences. The broad-spectrum src kinase inhibitor AG1879 was purchased from Calbiochem. All other chemicals were obtained from Sigma-Aldrich.
LDL was isolated from fresh plasma as previously described and stored under nitrogen until use.16 LDL protein concentration was determined by the Lowry assay. All LDL concentrations were given in terms of their protein content. Native LDL (nLDL) was oxidized with CuSO4 for 24 hours at 37°C and the extent of oxidation was determined by measuring malondialdehyde (MDA) formation as thiobarbituric acid–reacting substances (TBARS). The amount of MDA in the starting nLDL material was between 0.6 and 0.8 nmol/mg LDL proteins. Only oxLDL with final MDA values between 2 and 10 nmol/mg were used in these studies to avoid any potential disruption of the LDL particles by excessive oxidation.
Human platelet preparation
Whole blood was collected from healthy human volunteers into 0.109M sodium citrate (1:9 dilution) in accordance with the Cleveland Clinic Institutional Review Board (IRB). These donors were not taking aspirin, nonsteroidal anti-inflammatory drugs or any other medication. Platelet-rich plasma (PRP) was obtained by centrifugation at 100g for 12 minutes at room temperature. Washed platelets were prepared by centrifuging PRP at 600g for 10 minutes in the presence of 100nM prostaglandin E1 (PGE1). The platelet pellet was washed twice with Pipes/saline/glucose (5mM Pipes, 145mM NaCl, 4mM KCl, 50μM Na2HPO4, 1mM MgCl2- 6H2O, and 5.5mM glucose) in the presence of 100nM PGE1 and then resuspended in modifed Tyrode buffer (137mM NaCl, 2.7mM KCl, 12mM NaHCO3, 0.4mM NaH2PO4, 5mM HEPES, 0.1% glucose, and 0.35% bovine serum albumin, pH 7.2). Platelet concentration was adjusted to 2 × 108 platelets/mL, unless otherwise indicated. CaCl2 (final concentration of 2mM) and MgCl2 (final concentration of 1mM) were added immediately before platelet stimulation.
Immunoprecipitation and immunoblotting
Stimulated platelets were lysed in 2mM Tris (pH 7.5), 150mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, and 1 μg/mL leupeptin, and protein concentrations were measured with a Bio-Rad Protein Assay Kit based on the method of Bradford. For immunoprecipitation, precleared lysates containing 500 μg of proteins were incubated with 3 μg of Abs to Vav1, Vav3, or Fyn immobilized on protein A agarose beads overnight at 4°C. Beads were washed, boiled in 2× SDS-PAGE loading buffer, and the bound material was analyzed by immunoblotting following standard protocols. After chemiluminescence detection of phosphorylated proteins, blots were stripped and reprobed with Abs to total relevant proteins for normalization.
Experiment animals
Vav1−/− mice created by Tybulewicz, were generously provided by Juan Rivera (National Institutes of Health–National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIH-NIAMS]).17 These mice were backcrossed 6 times to C57Bl/6. Vav1−/−;vav3−/− mice were generously provided by Wojciech Swat (Washington University) and were as described.18 Fyn−/− mice were purchased from The Jackson Laboratory (1× backcrossed) and also backcrossed 6 times to C57Bl/6. After the last backcross, heterozygotes were mated to create background matched wild-type (WT), vav1−/−, vav1−/−;vav3−/−, and fyn−/− mice for experiments in the present studies. Mice were maintained as homozygotes by sibling mating.
Murine platelet preparation, aggregometry, and flow cytometry
To isolate platelets, mice were anesthetized with ketamine (90 mg/kg) and xylazine (15 mg/kg). Blood (600 μL) was obtained from the inferior vena cava (IVC) into syringes containing 100 μL of 3.8% sodium citrate and then 500 μL of Ca2+- and Mg2+-free modified Tyrode buffer were added. Platelet-rich plasma (PRP) was separated by centrifugation at 100g for 10 minutes at 22°C. CaCl2 (final concentration of 1mM) was added immediately before platelet aggregation. Platelet-poor plasma (PPP) was obtained by centrifugation at 800g for 5 minutes at 22°C. Platelet aggregation in vitro in response to ADP was assessed at 37°C in an aggregometer type 500 VS (Chrono-log) with stirring at 1200 rpm. For flow cytometry, platelets were column purified using Sepharose 2B from Sigma-Aldrich, equilibrated in modified Tyrode buffer, and then incubated with native LDL 40 μg/mL, or oxLDL 40 μg/mL for 20 minutes before addition of Jon/A PE-conjugated Ab and ADP (1μM final concentration). After 10 minutes of incubation, platelets were fixed in 4% paraformaldehyde. Flow cytometry was performed on a FACScan (BD Biosciences) and data analyzed using FloJo software.
Murine carotid artery thrombosis
Mice were anesthetized with ketamine (90 mg/kg)/xylazine (15 mg/kg) and the right jugular veins and the left carotid artery exposed via a middle cervical incision. One hundred microliters of rhodamine 6G (0.5 mg/mL) were injected directly into the right jugular vein to label platelets. The left carotid artery was stripped of adventitia and a piece of black plastic was placed under the vessel to reduce the background fluorescence. A 1 × 2 mm piece of filter paper saturated with 7.5% FeCl3 solution was applied to the carotid artery for 1 minute. The filter paper was removed, and the vessel was rinsed with saline. Thrombus formation was observed in real-time under a Leica DMLFS fluorescent microscope with an attached Gibraltar Platform (EXFO) and a water immersion objective at ×10 magnification. Time to thombosis was determined through visual inspection using real-time video image capture with a QImaging Retigo Exi 12-bit mono digital camera and Streampix version 3.17.2 software (Norpix). The end points were set as either cessation of blood flow for > 30 seconds or no occlusion after 30 minutes, in which case the time was recorded as 30 minutes.
Cholesterol assay
Blood (600 μL) were obtained from the inferior vena cava (IVC) of mice into syringes containing 100 μL of 3.8% sodium citrate. Plasma was promptly separated from cells and stored at 4°C or frozen at −20°C. Assays were performed within 24 hours of specimen collection. Cholesterol esters were converted to cholesterol by reaction with cholesterol esterase. Total cholesterol was then measured enzymatically by a modification of the method of Allain et al.19
Mass spectrometric analysis of phospholipids
LC-ESI/MS/MS was used to quantify plasma oxidized phosphatidylcholine species.4 Briefly, 4 ng of internal standard, ditetradecyl phosphatidylcholine (DTPC) were added to 200 μL of plasma and phospholipids were extracted 3 times following the method of Bligh and Dyer. Pooled extracts were dried under nitrogen flow, resuspended in 120 μL of 85% (vol/vol) methanol and stored under argon at −80°C. Mass spectrometric analysis was performed within 24 hours of plasma collection. Forty microliters of the extract was then introduced onto a 2690 HPLC system (Waters) to separate phospholipids using a C18 column (2 × 150 mm, 5 μm of octadecyl silane; Phenomenex) under gradient conditions at flow rate of 0.2 mL/min. The HPLC effluents were then introduced onto a Micromass Quattro Ultima triple quadrupole mass spectrometer (Micromass) and analyzed by positive electrospray ionization in the multiple reaction monitoring (MRM) mode. Following collision-induced fragmentation with argon gas, phosphatidylcholine molecules generated a diagnostic product ion with mass-to-charge ratio (m/z) 184, corresponding to the protonated phosphocholine headgroup. The ion pairs for the MRM transitions monitored were each molecular ion [MH]+ of the phosphotidylcholines and the diagnostic product ion (m/z 184). Calibration curves were constructed with a fixed amount of DTPC.
Statistical analysis
Each experiment was repeated at least 3 times with different platelet donors or different mice. Values are expressed as mean ± SE. The statistical significance was evaluated using an unpaired t test and considered statistically significant when P < .05.
Results
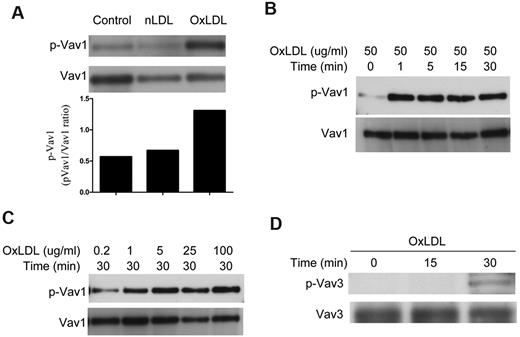
OxLDL induces phosphorylation of Vav1 and Vav3 in platelets
To assess the phosphorylation state of Vav1 during platelet activation by oxLDL, we incubated isolated human platelets with 50 μg/mL oxLDL or native LDL for 30 minutes. Cells were then lysed and Vav1 was immunoprecipitated with a specific Ab. After immunoprecipitation, we examined the phosphorylation state of Vav1 by immunoblot with 4G10 anti-phosphotyrosine Ab. We found that human platelets exposed to oxLDL (50 μg/mL) but not native LDL, had a ∼ 2- to 3-fold increase in phosphorylation of Vav1 (Figure 1A). No detectable band was observable when normal rabbit IgG was used to immunoprecipitate (data not shown). Evaluation of Vav1 phosphorylation revealed an increase in tyrosine phosphorylation within 1 minute of oxLDL addition that was sustained for at least 30 minutes (Figure 1B). Vav1 phosphorylation in platelets exposed to oxLDL was dose-dependent; concentrations as low as 1 μg/mL were effective at inducing phosphorylation (Figure 1C). The rapid kinetics of Vav1 phosphorylation suggests that Vav1 phosphorylation may be one of the earliest signaling events in platelets exposed to oxLDL.
OxLDL induces phosphorylation of Vav1 and Vav3 in platelets. Washed human platelets (2 × 108/mL) containing 2mM CaCl2 and 1mM MgCl2 were incubated with 50 μg/mL native LDL or oxLDL for 30 minutes (A) or 50 μg/mL oxLDL over varying time points (B,D) or various concentrations of oxLDL for 30 minutes (C) and then lysed. Vav1 (A-C) or Vav3 (D) was precipitated by specific Abs and precipitates were analyzed by immunoblot with 4G10 anti-phosphotyrosine Ab. The membranes were then stripped and reprobed with Abs to the total relevant proteins to normalize the protein loaded. Results are representative of at least 3 independent experiments from different donors. nLDL indicates native LDL; and oxLDL, oxidized LDL.
OxLDL induces phosphorylation of Vav1 and Vav3 in platelets. Washed human platelets (2 × 108/mL) containing 2mM CaCl2 and 1mM MgCl2 were incubated with 50 μg/mL native LDL or oxLDL for 30 minutes (A) or 50 μg/mL oxLDL over varying time points (B,D) or various concentrations of oxLDL for 30 minutes (C) and then lysed. Vav1 (A-C) or Vav3 (D) was precipitated by specific Abs and precipitates were analyzed by immunoblot with 4G10 anti-phosphotyrosine Ab. The membranes were then stripped and reprobed with Abs to the total relevant proteins to normalize the protein loaded. Results are representative of at least 3 independent experiments from different donors. nLDL indicates native LDL; and oxLDL, oxidized LDL.
Platelets mainly express 2 members of the Vav family, Vav1 and Vav3.14 Therefore, we also evaluated the phosphorylation state of Vav3 during platelet activation by 50 μg/mL oxLDL by immunoprecipitation with a specific Vav3 Ab followed by immunoblot with 4G10 anti-phosphotyrosine Ab. Platelets exposed to oxLDL had a significant increase in phosphorylation of Vav3 (Figure 1D), but the kinetics were much slower than was seen for Vav1. These results suggest that Vav3 may also participate in platelet activation by oxLDL.
OxLDL-induced Vav1 phosphorylation is mediated by the src family kinase Fyn
Src family kinases have been shown to induce Vav1 phosphorylation in other cellular systems. To determine whether oxLDL-induced Vav1 phosphorylation was mediated by src kinases, we treated platelets with the broad spectrum src inhibitor AG1879 before addition of oxLDL and found that Vav1 phosphorylation was completely abolished (Figure 2A). This result suggests that src family kinases mediate the tyrosine phosphorylation of Vav1 in platelets exposed to oxLDL.
OxLDL-induced Vav1 phosphorylation is mediated by src kinase Fyn. (A) Human platelets were incubated with the broad-spectrum src inhibitor AG1879 before incubation with 50 μg/mL oxLDL. The platelet lysates were then analyzed as in Figure 1 for Vav1 phosphorylation. (B) Platelets were incubated without or with oxLDL and then lysed. Vav1 was precipitated by an Ab specific for Vav1. Precipitates were analyzed by immunoblot with 4G10 anti-phosphotyrosine Ab, Vav1 Ab, and Fyn Ab (mouse). (C) Platelets were incubated without or with oxLDL and then lysed. Fyn was precipitated with an anti-Fyn Ab (mouse). Precipitates were analyzed by immunoblot with Vav1 Ab and Fyn Ab (Rabbit).
OxLDL-induced Vav1 phosphorylation is mediated by src kinase Fyn. (A) Human platelets were incubated with the broad-spectrum src inhibitor AG1879 before incubation with 50 μg/mL oxLDL. The platelet lysates were then analyzed as in Figure 1 for Vav1 phosphorylation. (B) Platelets were incubated without or with oxLDL and then lysed. Vav1 was precipitated by an Ab specific for Vav1. Precipitates were analyzed by immunoblot with 4G10 anti-phosphotyrosine Ab, Vav1 Ab, and Fyn Ab (mouse). (C) Platelets were incubated without or with oxLDL and then lysed. Fyn was precipitated with an anti-Fyn Ab (mouse). Precipitates were analyzed by immunoblot with Vav1 Ab and Fyn Ab (Rabbit).
Both Fyn and Lyn have been implicated in platelet oxLDL-induced signaling events and interestingly both have also been shown to be involved in tyrosine phosphorylation of Vav1 in other cell types.9,10 To assess the role of these specific kinases in oxLDL-induced platelet Vav1 phosphorylation, we performed immunoprecipitation studies with anti-Vav1 Ab and then examined the state of coprecipitated Fyn and Lyn. We found that under control conditions Fyn coprecipitated with Vav1 (Figure 2B left lane) and that the coprecipitated Fyn was not tyrosine phosphorylated. After exposure to oxLDL, the total amount of coprecipitated Fyn did not increase but there was a significant increase in tyrosine phosphorylation (Figure 2B right lane). We also performed reverse coimmunoprecipitation studies with an Ab to Fyn and immunoblotted the precipitates with anti-Vav1 Ab. As before, there was an association between Fyn and Vav1 under basal condition with no increase after exposure to oxLDL (Figure 2C). We also found that Lyn was coprecipitated by anti-Vav1 Abs, but unlike the case of Fyn, there was little or no increase in its phosphorylation level after oxLDL exposure (data not shown). These results suggest that platelet Vav1 is associated with both Fyn and Lyn under basal conditions and that oxLDL exposure induces specific phosphorylation of Vav1-associated Fyn, implying that Fyn may be the immediate upstream src family kinase responsible for tyrosine phosphorylation of Vav1 in platelets.
High-fat diet–induced platelet hyperreactivity in mice is dependent on Fyn/Vav signaling axis
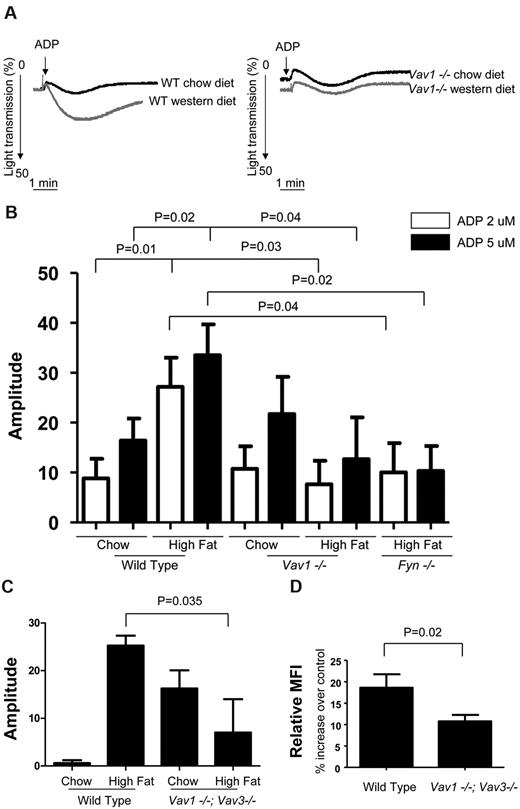
The observation that Vav1 and Vav3 phosphorylation in platelets can be induced by oxLDL raises the possibility that Vav family members may transduce the prothrombotic signal triggered by oxLDL in vivo. We previously demonstrated that platelets from hyperlipidemic apoe−/− mice and hyperlipidemic human subjects had an increase in their in vitro aggregation response that was mediated by CD36.4 To determine the role of Vav family members in this phenotype, we studied platelet aggregation in platelet-rich plasma obtained from vav1−/− mice fed either a high-fat “western” diet or normal chow. On normal chow there was no difference in aggregation responses to 2 or 5μM ADP of platelets from vav1−/− animals compared with wt mice (Figure 3A-B) or to a maximal dose of 20μM (not shown). On the high-fat diet, however, wild-type mice showed significant enhancement of platelet aggregation compared with that seen in platelets fed chow (Figure 3A left panel). Platelets from the vav1−/− animals fed the high-fat diet however showed no such enhancement of aggregation (right panel). Quantification of these data (Figure 3B) revealed a 2- to 3-fold increase in maximum amplitude of aggregation induced by 2 and 5μM ADP in platelets from the high-fat diet fed wt mice (P < .03), but no increase in those from the vav1−/− mice. Interestingly, platelets from vav1/vav3 double null mice on chow diet were more reactive than wt mice, but the double Vav1/Vav3-deficient mice were significantly protected from the hyperreactivity induced by the high-fat diet (Figure 3C). These studies show that the diet-induced platelet hyperreactivity previously reported in mice is not dependent on deletion of the apoe or ldlr genes, and that a CD36 signaling pathway involving Vav family members are involved. The protection from diet-induced platelet hyperreactivity seen in the vav1−/− mice was not because of failure of these mice to respond to the diet. The cholesterol level of WT and vav1−/− mice on the high-fat diet was increased to a similar extent in comparison to those on the normal chow diet (data not shown). In addition, both mouse strains showed identical weight gain on the diet and mass spectrometry analysis revealed a significant amount of oxPCCD36 present in the plasma of vav1−/− mice fed the high-fat diet (data not shown).
High-fat diet-induced platelet hyperreactivity in mice is dependent on Fyn/Vav1 signaling axis. (A) Platelets from WT and vav1−/− mice on chow or western diet for 2 weeks were stimulated with 2μM ADP. Aggregation was assessed turbidimetrically with a dual-channel aggregometer. Shown are representative curves. (B) Aggregation amplitude of platelets from WT or vav1−/− mice on chow or western diet or fyn−/− mice on western diet for 2 weeks incubated with 2 or 5μM ADP as indicated. (C) Platelets from WT and vav1−/−;Vav3−/− mice on chow or western diet for 2 weeks were treated with 1μM ADP. Aggregation was assessed turbidimetrically with a dual-channel aggregometer. Aggregation amplitudes were shown as indicated. (D) Platelets from WT and vav1−/−;Vav3−/− mice were column purified and incubated with 40 μg/mL native LDL or oxLDL. After 20 minutes, platelets were further incubated with or without 1μM ADP and with JonA Ab for 10 minutes and then fixed before subject to flow cytometric analysis. Percentage increases in MFI of stained platelets treated with ADP and oxLDL in comparison to that of platelets treated with ADP and native LDL were shown.
High-fat diet-induced platelet hyperreactivity in mice is dependent on Fyn/Vav1 signaling axis. (A) Platelets from WT and vav1−/− mice on chow or western diet for 2 weeks were stimulated with 2μM ADP. Aggregation was assessed turbidimetrically with a dual-channel aggregometer. Shown are representative curves. (B) Aggregation amplitude of platelets from WT or vav1−/− mice on chow or western diet or fyn−/− mice on western diet for 2 weeks incubated with 2 or 5μM ADP as indicated. (C) Platelets from WT and vav1−/−;Vav3−/− mice on chow or western diet for 2 weeks were treated with 1μM ADP. Aggregation was assessed turbidimetrically with a dual-channel aggregometer. Aggregation amplitudes were shown as indicated. (D) Platelets from WT and vav1−/−;Vav3−/− mice were column purified and incubated with 40 μg/mL native LDL or oxLDL. After 20 minutes, platelets were further incubated with or without 1μM ADP and with JonA Ab for 10 minutes and then fixed before subject to flow cytometric analysis. Percentage increases in MFI of stained platelets treated with ADP and oxLDL in comparison to that of platelets treated with ADP and native LDL were shown.
We also studied the aggregation of platelets from fyn−/− mice fed the high-fat diet. We found that the aggregation of platelets from these mice was impaired in comparison to the aggregation of those from wild-type mice fed high-fat diet (Figure 3B). These results suggest that Fyn/Vav signaling axis may link high-fat feeding and platelet hyperreactivity.
Platelet hyperreactivity induced by oxLDL-CD36 interaction is dependent on platelet Vav1/Vav3
To further demonstrate the function of platelet Vav1/Vav3, we analyzed by flow cytometry oxLDL-mediated αIIbβ3 integrin activation in platelets costimulated with low-dose ADP using an Ab specifically for the active form of the integrin. We found that platelets from Vav1/Vav3-deficient mice had a defect in oxLDL-mediate integrin activation in comparison to that of WT mice (Figure 3D). In contrast, there were no differences between Vav1/Vav3-deficient and WT platelets in response to ADP alone, native LDL alone, oxLDL alone or native LDL with ADP (data not shown). The data showed that Vav molecules are required for platelet CD36-initiated signaling that leads to platelet activation.
Vav1 and Vav3 may play redundant but critical roles in platelet hyperreactivity associated with hyperlipidemia
To further determine the role of Vav proteins in thrombus formation under hyperlipidemic condition in vivo, we induced carotid artery thrombi with 7.5% FeCl3 in wild-type and vav1−/− mice on high-fat diet and then determined vessel occlusion time with intravital microscopy. We found all wild-type mice and 5 of the 6 vav1−/− mice formed occlusive thrombi within 30 minutes and no significant difference in the mean vessel occlusion time between wild-type and vav1−/− mice (Figure 4A). Because platelet vav3 phosphorylation was induced by oxLDL and because previous studies showed that in platelets Vav3 may compensate for Vav1 deficiency, and Vav1 and Vav3 together play critical roles in platelet activation induced by collagen in vitro,14 we tested the hypothesis that genetic deletion of vav1 and vav3 together may protect mice fed a high-fat diet from forming occlusive thrombi. In this model, carotid occlusion in wild-type mice fed high-fat diet occurred at a mean of 14.1 minute, while in vav1−/−;vav3−/− mice fed high-fat diet the mean was prolonged to 23.2 minutes (P = .03, Figure 4B). It is important to note that the mean occlusion time reported for the vav1−/−;vav3−/− mice is an underestimate because 5 of the 8 animals studied failed to form occlusive thrombi during the 30 minutes of observation while all 5 wild-type mice formed occlusive thrombi within 19 minutes. The results suggest that Vav3 and Vav1 may play redundant but critical roles in protecting hyperlipidemic mice from developing occlusive thrombi in vivo.
Vav1 and Vav3 may play redundant but critical roles in in vivo thrombus formation in mice fed high-fat diet. (A) Time to thrombotic occlusion of carotid arteries from vav1−/− mice and their age-matched wild-type mice fed high-fat diet for 2 weeks was measured after 1 minute topical application of 7.5% FeCl3. Platelets were labeled by direct injection of fluorescence dye Rhodamine 6G into the jugular vein. Thrombi formation in the carotid artery was assessed under intravital microscopy. (B) Time to thrombotic occlusion of carotid arteries from vav1−/−;vav3−/− and their age-matched wild-type mice fed high-fat diet for 4 weeks was measured after 1-minute topical application of 7.5% FeCl3.
Vav1 and Vav3 may play redundant but critical roles in in vivo thrombus formation in mice fed high-fat diet. (A) Time to thrombotic occlusion of carotid arteries from vav1−/− mice and their age-matched wild-type mice fed high-fat diet for 2 weeks was measured after 1 minute topical application of 7.5% FeCl3. Platelets were labeled by direct injection of fluorescence dye Rhodamine 6G into the jugular vein. Thrombi formation in the carotid artery was assessed under intravital microscopy. (B) Time to thrombotic occlusion of carotid arteries from vav1−/−;vav3−/− and their age-matched wild-type mice fed high-fat diet for 4 weeks was measured after 1-minute topical application of 7.5% FeCl3.
Discussion
Previous studies from our group and others have demonstrated that CD36 plays an important role in platelet biology by transducing activating signals in response to oxidized phospholipids or cell-derived microparticles.4,6,7,20 Through this mechanism pathologic ligands generated in the context of hyperlipidemia and/or inflammation and oxidant stress enhance platelet reactivity and contribute to a prothrombotic state. Using oxLDL as a model ligand, we found that the initial signaling events triggered by CD36 involve ligand-dependent recruitment and activation of specific src family kinases Fyn and Lyn.8 Subsequent activation of the MAP kinase JNK followed Fyn/Lyn activation and was required for platelet activation.8 The signaling events immediately downstream of Src family kinases have not been defined. The studies outlined here have identified the Rho/Rac GEF Vav family members as critical modulators transducing prothrombotic signals. The studies also provide insights into the mechanism by which the Src family kinase Fyn mediates tyrosine phosphorylation of Vav1 in platelets in response to oxLDL as we demonstrated by coimmunoprecipitation that the amount of Vav1-associated Fyn did not change with ligand exposure, but that Fyn underwent an increase in tyrosine phosphorylation.
We previously demonstrated that apoe−/− mice fed a high-fat western diet had significantly increased thrombotic responses in vivo in comparison to wt mice on high-fat diet as well as to apoe−/− mice fed normal chow.4 This was associated with increased platelet reactivity in vitro. The effects of high-fat diet on thrombosis and platelet reactivity in the absence of apoe or ldlr mutations have not been carefully examined. High-fat diet fed C57Bl/6 mice are known to develop a moderate increase in plasma cholesterol mainly in the non-HDL fraction and to develop small atherosclerotic lesions after 14 weeks.21 In the current study, we challenged C57Bl/6 mice with high-fat feeding for 2 weeks and compared the ADP-induced aggregation of platelets from these mice with those from mice on normal chow. We found a significant diet-induced increase in platelet aggregation compared with mice on chow. Interestingly, we demonstrated by platelet aggregation assay that genetic deletion of vav or fyn eliminated the diet-induced platelet hyperreactivity, suggesting that Fyn/Vav signaling axis transduces diet-induced prothrombotic signals in platelets. We also investigate for the first time the role of Vav family members in in vivo thrombosis. We found that genetic deletion of both vav1 and vav3 also protects mice fed high-fat diet from developing occlusive thrombi, confirming that Vav family members play a role in in vivo thrombosis associated with hyperlipidemia and the in vivo role of Vav1 may be compensated by Vav3.
The role of Vav family members in platelet biology has not been extensively studied, although it has been shown that the Vav1 and/or Vav3 tyrosine phosphorylation was induced by thrombin and collagen and that Vav proteins contributed to platelet activation by these agonists.14,15,22 Our study extends the knowledge about the functional role of Vav family members in platelets by showing that tyrosine phosphorylation of Vav1 and Vav3 were induced by oxLDL and that Vav family member(s) may play an essential role in transducing a pro-thrombotic signal in response to high-fat diet. The precise role of Vav proteins in platelet biology remains to be determined. The impairment of thrombotic responses in mice deficient in Vav family member(s) that are fed high-fat diet could be explained by a role in inside-out or outside-in signaling by the integrin αIIbβ3. Although Vav family members are best known for their GEF activity toward Rac/Rho, they also functions as adaptor proteins. It thus remains to be determined whether GEF or adaptor activity or both are involved in transducing CD36 prothrombotic signals.
OxLDL is a complex particle and can contain a variety of biologically active lipids other than CD36 ligands, including lysophosphatidylcholine (LPC), platelet-activating factor (PAF), lysophosphatidic acid (LPA), and 9- and 13-HODE.23 The present studies identified Vav proteins as critical modulators for pro-thrombotic signals. The studies also revealed the presence of oxPCCD36 in the plasma of vav1−/− mice on western diet. The data suggests that oxPCCD36 may be responsible for the tyrosine phosphorylation of Vav1 and Vav3 in platelets, however, it remains to be determined if oxPCCD36 induces the tyrosine phosphorylation of Vav1 and Vav3 in vitro. The role of these other lipids in this process also remains unknown. Others have shown that LPA in oxLDL can induce platelet shape change via a specific G protein–coupled LPA receptor.24 The signaling pathways triggered by LPA involve tyrosine phosphorylation of specific proteins including Syk and an increase of cytosolic Ca2+.25 It is also possible that LPA or PAF receptors may work in a synergic way with oxPCCD36 and contribute to prothrombotic signals.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health HL81011; Specialized Center for Clinically Oriented Research (SCCOR) in Thrombosis (R.L.S. and M.F.); and American Heart Association Predoctoral Fellowship (0715088B, K.C.).
National Institutes of Health
Authorship
Contribution: K.C. performed and designed experiments, analyzed results, and wrote the paper; W.L. and J.M. performed and designed experiments, analyzed results, and contributed to writing; S.O.R. performed and designed experiments, analyzed results, and contributed to writing; M.F. analyzed results and contributed to writing; and R.L.S. analyzed results, designed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.C. is Brigham and Women's Hospital, Boston, MA.
Correspondence: Roy L. Silverstein, MD, Department of Cell Biology, NC-10, Lerner Research Institute, Cleveland Clinic Foundation, 9500 Euclid Ave, OH 44195; e-mail: silverr2@ccf.org.