Abstract
Because cancer at its origin must acquire permanent genomic mutations, it is by definition a disease of DNA repair. Yet for cancer cells to replicate their DNA and divide, which is the fundamental phenotype of cancer, multiple DNA repair pathways are required. This produces a paradox for the cancer cell, where its origin is at the same time its weakness. To overcome this difficulty, a cancer cell often becomes addicted to DNA repair pathways other than the one that led to its initial mutability. The best example of this is in breast or ovarian cancers with mutated BRCA1 or 2, essential components of a repair pathway for repairing DNA double-strand breaks. Because replicating DNA requires repair of DNA double-strand breaks, these cancers have become reliant on another DNA repair component, PARP1, for replication fork progression. The inhibition of PARP1 in these cells results in catastrophic double-strand breaks during replication, and ultimately cell death. The exploitation of the addiction of cancer cells to a DNA repair pathway is based on synthetic lethality and has wide applicability to the treatment of many types of malignancies, including those of hematologic origin. There is a large number of novel compounds in clinical trials that use this mechanism for their antineoplastic activity, making synthetic lethality one of the most important new concepts in recent drug development.
Cancer as a disease of DNA repair
The concept of synthetic lethality is based on the reliance of cancer cells on DNA repair to maintain cell division. DNA repair promotes faithful transmission of genomes in dividing cells by reversing extrinsic or intrinsic DNA damage, and is required for cell survival during replication. Carefully regulated repair pathways cooperate and sometimes compete in response to different types of DNA damage, and these pathways are not just important for cancer cell survival, they are essential for the origins of all malignancy. The concept that cancer is a result of defective DNA repair is demonstrated by the finding that inherited mutations in DNA repair genes result in malignancies.1,2 Examples of inherited DNA repair defects predisposing to cancer include mutations of ataxia telangiectasia mutated (ATM) gene3 in several types of malignancies including acute leukemia,4 mutations in NBS1 in Nijmegen breakage syndrome in acute leukemia,4 and BRCA15 and BRCA26 mutations in breast, ovarian, and peritoneal malignancies. In addition, mutations in the nucleotide excision repair genes in xeroderma pigmentosum result in skin cancer,7 defects in the Fanconi anemia genes can cause acute leukemia,2 and mutations in the mismatch repair proteins8 occur in colorectal and other cancers. As whole genome sequence analysis is applied to more tumor types, 2 further principles have become clear. First, many tumor types, not just the inherited deficiencies listed here, have mutations in genes involved in DNA repair. Second, and more importantly, every tumor genome sequenced thus far has multiple DNA mutations,9 implying that genomic instability can be both the cause and the effect of neoplastic transformation.10
Thus, because cancer has at its origin DNA mutation, it is a disease of DNA repair. Yet for cancer cells to replicate their DNA and proliferate, the fundamental phenotype of cancer, multiple DNA repair pathways are required.11 This produces an essential difficulty for the cancer cell—its origin is at the same time its weakness. To overcome this difficulty, cancer cells often become addicted to DNA repair pathways other than the one that led to its initial mutability, because that originating pathway is often defective. This addiction can be exploited therapeutically; by inhibiting the DNA repair pathway to which the cancer cell has become addicted, it is possible to prevent repair of the intrinsic damage that occurs during DNA replication, or enhance DNA damage from chemotherapy. This therapeutic exploitation of the overreliance of the cancer cell on a specific DNA repair pathway is based on the concept of synthetic lethality.
Mechanisms of DNA repair
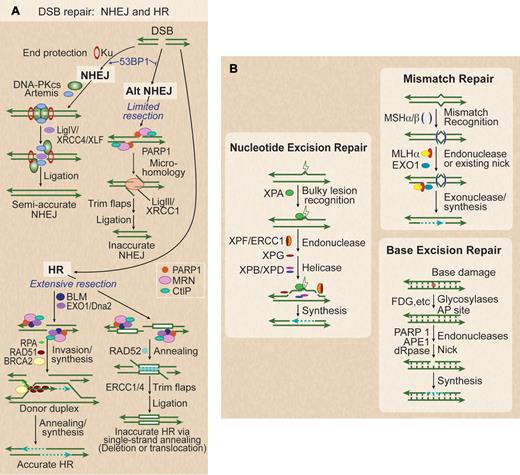
To understand the concept of synthetic lethality, one must first understand how cells repair DNA, especially during replication.11 DNA damage is reversed by 5 major DNA repair pathways (Figure 1). Double-strand breaks (DSBs) are the most dangerous of all types of DNA damage; when unrepaired, DSBs can be lethal and trigger apoptosis. There are 2 major pathways for DSB repair, nonhomologous end-joining (NHEJ) and homologous recombination (HR), each of which has 2 subpathways (Figure 1A). NHEJ repair is the result of the direct ligation of the free ends of the DNA DSB, whereas HR uses strand invasion of the homologous chromatid to synthesize DNA across the DSB using this homologous chromatid sequence as a template. Thus, HR can take place only after DNA replication, in S/G2/M cell-cycle phases, whereas NHEJ is preferred in G0/G1. Because HR uses a homologous template to repair a DSB, it is quite accurate, whereas NHEJ may require end trimming that makes it less accurate.11-14
Five DNA repair pathways. (A) DSBs are repaired by 2 NHEJ and 2 HR subpathways. Classic NHEJ initiates with broken ends bound by Ku, which protects ends, leading to accurate or semi-accurate repair. Mutations in classic NHEJ factors shunt DSBs toward alternative NHEJ, which involves limited resection by MRN/CtIP and annealing via microhomology, yielding inaccurate repair. 53BP1 also serves to increase NHEJ accuracy by blocking MRN/CtIP resection. PARP1 promotes more extensive end-resection by EXO1 and BLM to reveal ssDNA and promote HR. RPA binds to ssDNA, BRCA2 mediates replacement of RPA with RAD51, the RAD51 nucleoprotein filament invades a homologous donor sequence (typically the sister chromatid in S/G2 phase), and repair synthesis extends the invading 3′ end, which then anneals with resected end to provide accurate repair. If long homologous repeats flank the DSB (white boxes), extensive resection can reveal complementary ssDNA that is annealed in a reaction promoted by RAD52, leading to deletion of one repeat and DNA between repeats, or translocations if DSBs occur on different chromosomes. (B) Base damage, often from oxidation, triggers base excision repair (BER). This results in a short repaired single-strand segment, also called a patch. Bulky nucleotide lesions, such as thymidine dimmers from ultraviolet light, are repaired by nucleotide excision repair (NER). These involve a long repaired single-strand patch. Mismatch repair (MMR), used to replace nucleotides mistakenly placed opposite a nonpaired template nucleotide during DNA synthesis, involves long excision of single strands and resynthesis repair patches initiated from existing or induced nicks.
Five DNA repair pathways. (A) DSBs are repaired by 2 NHEJ and 2 HR subpathways. Classic NHEJ initiates with broken ends bound by Ku, which protects ends, leading to accurate or semi-accurate repair. Mutations in classic NHEJ factors shunt DSBs toward alternative NHEJ, which involves limited resection by MRN/CtIP and annealing via microhomology, yielding inaccurate repair. 53BP1 also serves to increase NHEJ accuracy by blocking MRN/CtIP resection. PARP1 promotes more extensive end-resection by EXO1 and BLM to reveal ssDNA and promote HR. RPA binds to ssDNA, BRCA2 mediates replacement of RPA with RAD51, the RAD51 nucleoprotein filament invades a homologous donor sequence (typically the sister chromatid in S/G2 phase), and repair synthesis extends the invading 3′ end, which then anneals with resected end to provide accurate repair. If long homologous repeats flank the DSB (white boxes), extensive resection can reveal complementary ssDNA that is annealed in a reaction promoted by RAD52, leading to deletion of one repeat and DNA between repeats, or translocations if DSBs occur on different chromosomes. (B) Base damage, often from oxidation, triggers base excision repair (BER). This results in a short repaired single-strand segment, also called a patch. Bulky nucleotide lesions, such as thymidine dimmers from ultraviolet light, are repaired by nucleotide excision repair (NER). These involve a long repaired single-strand patch. Mismatch repair (MMR), used to replace nucleotides mistakenly placed opposite a nonpaired template nucleotide during DNA synthesis, involves long excision of single strands and resynthesis repair patches initiated from existing or induced nicks.
In NHEJ the choice between classic and alternate pathways is regulated by 53BP1,12 which promotes classic NHEJ, and PARP1, which promotes alternative NHEJ.13 Alternative NHEJ involves more resection of the free DNA ends to find microhomologies than classic NHEJ, which directly ligates the free ends. Alternative NHEJ is therefore less accurate and can result in large deletions or translocations.13,14 Classic NHEJ is initiated by KU70/86 end binding, which protects broken ends,15 and recruits the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs).16 Artemis and Metnase are nucleases that process free ends for more efficient ligation during NHEJ.17-19 These factors promote ligation of broken ends involving little or no microhomology.14 Phosphorylation of DNA-PKcs, either trans-autophosphorylation or by ATM, promotes its dissociation from DNA ends,20 and enhances access by the Ligase IV/XRCC4/XLF protein complex, which completes the ligation reaction.21 Defects in classic NHEJ proteins channel DSBs toward alternative NHEJ (Alt-NHEJ), a robust but less accurate pathway that is regulated by PARP113 binding to the free DNA ends instead of the Ku complex. This pathway requires single-strand end-resection by the MRE11/RAD50/NBS1 (MRN) complex,22 and the CtIP tumor-suppressor.23 This resection exposes microhomologies that promote pairing of broken ends, which are then ligated by Ligase III/XRCC1.24 Alt-NHEJ is suppressed by 53BP1, which restricts end-processing by MRN/CtIP25
HR also includes accurate and inaccurate subpathways, both of which require extensive single-strand end-resection to allow for invasion by the remaining strand of the homologous template. End resection occurs in 2 phases, with limited resection initiated by MRN/CtIP and extensive resection mediated by the BLM helicase and the EXO1 and DNA2 exonucleases. In the accurate HR pathway, homologous DNA sequences are used as templates to copy genetic information for repair. As mentioned, homologous sequences are typically sister chromatids in S/G2 phase cells, but may be homologous chromosomes or linked or unlinked repeated sequences. After resection, accurate HR involves binding of RPA to single strands, and BRCA2-mediated replacement of RPA with RAD51 to form a RAD51 nucleoprotein filament, which searches for and invades the homologous template.26 The invading strand is extended by synthesis of new DNA. The newly synthesized strand can then anneal with the other resected end, and additional synthesis and ligation completes high-fidelity repair. Inaccurate HR is termed single-strand annealing (SSA), in which extensive resection exposes complementary sequences in linked direct repeats, which anneal in a reaction promoted by RAD52.27 SSA is always inaccurate because it deletes one of the repeats and the intervening sequence, or it results in translocations when 2 DSBs occur within or near repeats on different chromosomes.28 Inducing and then preventing repair of DSBs in cancer cells is the goal of synthetic lethal approaches.
Three other repair pathways operate on nucleotide lesions occurring on single strands (Figure 1B). These are termed base excision repair (BER),29 nucleotide excision repair (NER),7 and mismatch repair (MMR).30 In all cases, the complementary strand serves as the repair template. BER repairs base damage and is initiated by several glycosylases that produce apurinic or apyrimidinic (AP) lesions that recruit PARP1, followed by strand nicking by APE1 and deoxyribophosphodiesterase, repair synthesis, and ligation. NER repairs bulky lesions by excising ∼ 30 nt oligonucleotides containing the lesion by a multistep process that involves lesion recognition, helicase, and nuclease activities, followed by synthesis/ligation. MMR is also an excision-based repair system involving mismatch recognition, excision directed from induced or existing nicks, and synthesis/ligation. DNA damage is also dealt with by “lesion bypass” pathways. Translesion synthesis (TLS) polymerases are able to use damaged DNA templates to extend replicating strands, but with lower fidelity, hence TLS tends to be mutagenic.31 During replication, HR can also provide another mechanism for nucleotide lesion bypass.32 Defects in each of these repair pathways can lead to malignant transformation, as described earlier, and each can also be subverted to assist the cancer cell in replication of its genome. Thus, each can also be targets of synthetic lethality. Similar to HR, these nucleotide repair pathways are most active in S phase, as would be predicted, given their role in maintaining DNA replication.33 Not only do cell-cycle phases regulate which DNA repair pathway is used, but these pathways also regulate progression through the cell cycle.33 The best example of this is the S-phase arrest, mediated by Chk1 and 2 through p53, that occurs when DSBs are present.33
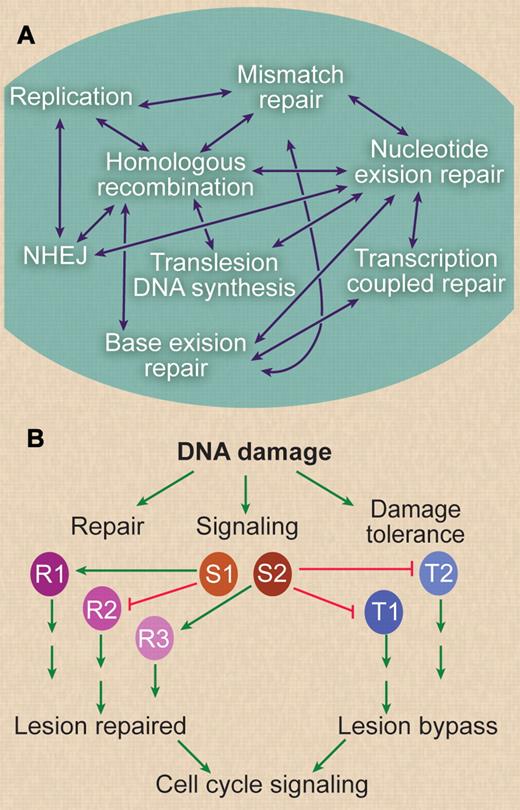
Although these DNA repair and damage tolerance pathways have been largely studied in isolation, it is becoming increasingly clear that there is significant functional overlap among pathways (Figures 2–3). This overlap can involve sharing of repair proteins among pathways (ie, PARP1 is involved in alt-NHEJ, HR, and BER), because specific lesions may be processed by multiple pathways (eg, DSB repair by NHEJ or HR), or because lesion processing produces intermediates that can be shunted to different pathways (eg, RAD51-dependent HR and SSA). The requirement of one component for the function of multiple repair path-ways plays a significant role in the synthetic lethal phenotypes discussed in the following paragraph.
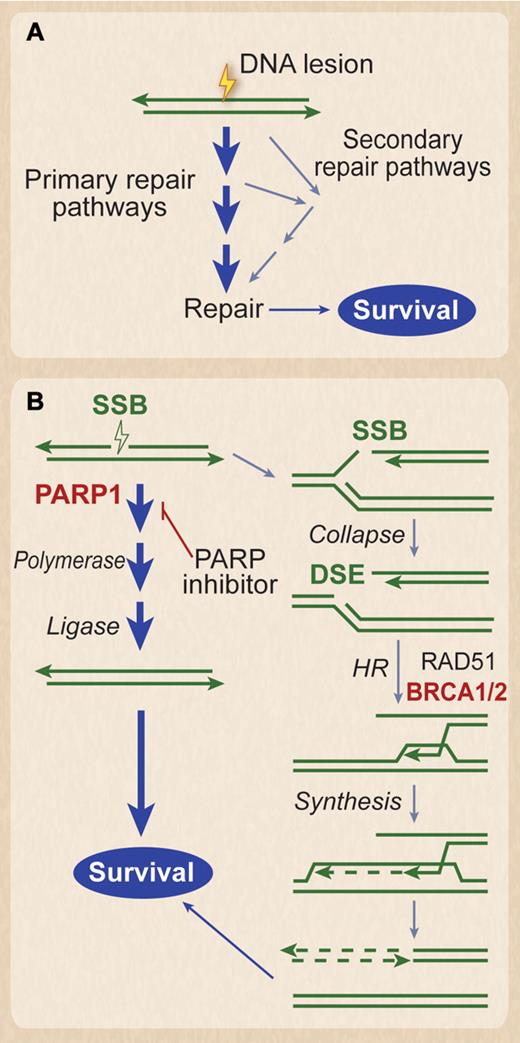
Synthetic lethality. (A) DNA lesions are often processed by multiple pathways that promote survival. Lesions can be lethal if all routes to repair are blocked. This is the basis for synthetic lethality. (B) Synthetic lethality in BRCA1/2-defective tumors. Naturally occurring single-strand breaks (SSBs) are primarily repaired by PARP1-dependent pathway before a replication fork hits. However, if they are not repaired in time before the replication fork hits, the SSB turns into a DSB. The DSB at the fork is repaired by HR, which requires BRCA1 and 2, denoted in red. Thus, BRCA1 and 2 are needed to restart the collapsed replication forks caused by the DSB. In BRCA1- or BRCA2-defective tumors, these SSBs must be repaired by the PARP1 pathway before the replication fork hits, as there is no mechanism of repairing the resultant DSB. Thus, PARP inhibitors result in synthetic lethality of the BRCA1- or BRCA2-defective cancer cell, because the SSB cannot be repaired in front of the replication fork, and the resultant DSB caused by the progression of the fork through the SSB also cannot be repaired. Note that normal tissue, which does not have the bi-allelic defects in BRCA1 or 2, would not be affected.
Synthetic lethality. (A) DNA lesions are often processed by multiple pathways that promote survival. Lesions can be lethal if all routes to repair are blocked. This is the basis for synthetic lethality. (B) Synthetic lethality in BRCA1/2-defective tumors. Naturally occurring single-strand breaks (SSBs) are primarily repaired by PARP1-dependent pathway before a replication fork hits. However, if they are not repaired in time before the replication fork hits, the SSB turns into a DSB. The DSB at the fork is repaired by HR, which requires BRCA1 and 2, denoted in red. Thus, BRCA1 and 2 are needed to restart the collapsed replication forks caused by the DSB. In BRCA1- or BRCA2-defective tumors, these SSBs must be repaired by the PARP1 pathway before the replication fork hits, as there is no mechanism of repairing the resultant DSB. Thus, PARP inhibitors result in synthetic lethality of the BRCA1- or BRCA2-defective cancer cell, because the SSB cannot be repaired in front of the replication fork, and the resultant DSB caused by the progression of the fork through the SSB also cannot be repaired. Note that normal tissue, which does not have the bi-allelic defects in BRCA1 or 2, would not be affected.
Relationships between DNA repair pathways. (A) DNA repair and DNA damage tolerance pathways are highly networked. Each double-headed arrow indicates a known functional interaction between pathways and/or cases where one or more proteins function in 2 pathways. For example, mismatches arise in strand exchange intermediates during HR and are acted on by the mismatch repair machinery, and interstrand crosslink repair involves proteins from several pathways, including NER, HR, and translesion DNA synthesis. Also, most DNA repair occurs in chromatin, and all repair and signaling pathways are also regulated by chromatin modification pathways. (B) Understanding DNA repair networks can lead to new cancer therapies based on synthetic lethal interactions, but these networks also are a mechanism by which the cancer cell can escape synthetic lethality. In this diagram, a hypothetical DNA repair and signaling network is illustrated. DNA damage can be processed by DNA repair pathways (denoted R1, R2, R3) or is bypassed during replication by damage tolerance pathways (T1, T2). DNA damage also activates multiple arrest signaling pathways for activation of repair and inhibition of cell-cycle progression, indicated by S1 and S2. Each repair or tolerance pathway leads to either repair or bypass of lesions and promotes survival. Signaling pathways may activate (arrows) or inhibit (blocked lines) specific DNA repair or mutation tolerance pathways. These pathways ultimately regulate cell-cycle progression. Blocking any of these pathways, not just repair (via mutation at the origination of the cancer, or with chemotherapy), provides numerous opportunities to exploit synthetic lethal interactions. However, highly interconnected networks also present challenges, because blocking 2 pathways may simply shunt damage to a third pathway. It is also possible that synthetic lethality may be suppressed through accumulation of additional mutations, that is, by activating positive regulatory pathways, or by inactivating negative regulatory pathways. Thus, one worry with this approach is that cancer cells will ultimately become resistant to these synthetic lethal drugs by adapting alternative repair or lesion bypass pathways.
Relationships between DNA repair pathways. (A) DNA repair and DNA damage tolerance pathways are highly networked. Each double-headed arrow indicates a known functional interaction between pathways and/or cases where one or more proteins function in 2 pathways. For example, mismatches arise in strand exchange intermediates during HR and are acted on by the mismatch repair machinery, and interstrand crosslink repair involves proteins from several pathways, including NER, HR, and translesion DNA synthesis. Also, most DNA repair occurs in chromatin, and all repair and signaling pathways are also regulated by chromatin modification pathways. (B) Understanding DNA repair networks can lead to new cancer therapies based on synthetic lethal interactions, but these networks also are a mechanism by which the cancer cell can escape synthetic lethality. In this diagram, a hypothetical DNA repair and signaling network is illustrated. DNA damage can be processed by DNA repair pathways (denoted R1, R2, R3) or is bypassed during replication by damage tolerance pathways (T1, T2). DNA damage also activates multiple arrest signaling pathways for activation of repair and inhibition of cell-cycle progression, indicated by S1 and S2. Each repair or tolerance pathway leads to either repair or bypass of lesions and promotes survival. Signaling pathways may activate (arrows) or inhibit (blocked lines) specific DNA repair or mutation tolerance pathways. These pathways ultimately regulate cell-cycle progression. Blocking any of these pathways, not just repair (via mutation at the origination of the cancer, or with chemotherapy), provides numerous opportunities to exploit synthetic lethal interactions. However, highly interconnected networks also present challenges, because blocking 2 pathways may simply shunt damage to a third pathway. It is also possible that synthetic lethality may be suppressed through accumulation of additional mutations, that is, by activating positive regulatory pathways, or by inactivating negative regulatory pathways. Thus, one worry with this approach is that cancer cells will ultimately become resistant to these synthetic lethal drugs by adapting alternative repair or lesion bypass pathways.
The evolution of the concept of synthetic lethality
The concept of synthetic lethality comes from genetic studies in drosophila,34,35 where the loss of one gene function is tolerated by overreliance on another gene in a redundant pathway. Synthetic lethality occurs when the gene from the redundant pathway is also deleted, or its product inhibited. Because the loss of tumor suppressor genes is prevalent in human malignancies, Hartwell and colleagues proposed that blocking the function of that other gene product, required to compensate for the tumor suppressor loss, could be utilized to kill cancer cells, similar to synthetic lethality in drosophila.36 Because in most situations both copies of tumor suppressor genes are deleted in tumors, while one normal copy is retained in the healthy tissue, it was hypothesized that synthetic lethality would be selective to the tumor, and spare normal cells.37,38
Hartwell et al screened a panel of 76 Food and Drug Administration–approved cancer drugs against a panel of yeast strains mutant for DNA repair and cell-cycle checkpoints genes, searching for drugs that were specifically lethal to yeast with gene defects analogous to human tumor suppressor gene mutations.39 They identified a number of strains with deleted genes that are particularly sensitive to certain cancer drugs. For example, yeast mutant for the DNA Post-Replication Repair (PRR) pathway components (Rad18 and Rad6) were far more sensitive to the DNA cross-linking drug cisplatin compared with other strains. HR DNA repair mutants were also sensitive to cisplatin, but not as much as the PRR mutants. In another, much more extensive screen for compounds that are toxic to yeast cells deficient in DNA DSB repair,40 126 of 85 000 compounds were selectively toxic to yeast deficient in DSB repair, and most of these compounds were inhibitors of topoisomerase I and II.
Although the terminology of synthetic lethality is traditionally associated with inhibition of a function that the cell relies on to compensate for another gene loss, there are examples where a gain of function could be synthetically lethal with another gain of function. For example, activating RAS mutations generate a state of dependency on the STK3341 and TKB142 kinases. Inhibiting each of these kinases is synthetically lethal in cells with RAS mutations. This is usually referred to in yeast as “synthetic dosage lethality.“43 Thus, synthetic lethality can be discussed in the setting of interactions of multiple cellular pathways, but we will limit this review to inhibiting DNA repair pathways.
Synthetic lethality in cancers with defective homologous recombination pathways
PARP1 adds ADP-ribose chains on target proteins and contributes to multiple DNA repair pathways, including BER and DSB repair.44 Two groups postulated that BRCA1- and BRCA2-deficient cell lines or tumors, which are defective in HR repair of DSBs, will be susceptible to PARP1 inhibition, and both groups demonstrated that this was indeed the case in vitro and in vivo.45,46 DSBs generated at replication forks are largely repaired by HR,47 and HR requires BRCA1 and 2 (Figures 1–2). Because PARP1 is required for BER, as described, its inhibition will result in the accumulation of SSBs in the genome, occurring after partial BER occurs, but cannot be completed. When a replication fork progresses to this SSB, that SSB will be converted into a DSB. These investigators postulated that the enhanced toxicity was because of accumulation of DSBs at replication forks, and these increased DSBs are not repaired appropriately in the BRCA1- or BRCA2-deficient background.
DSBs are the most toxic of all DNA lesions and can lead to apoptosis if unrepaired. One group reported 57- and 133-fold enhanced sensitivity to PARP1 inhibition of murine embryonic stem cells lacking BRCA1 and BRCA2 compared with the wild type, respectively.46 Nonembryonic cells such as BRCA2-deficient Chinese hamster ovary cells showed even a greater sensitivity to PARP1 inhibition, nearly 1000-fold, compared with a BRCA2-complemented derivative.46 PARP1 is not only important in BER, it is also required in restarting stalled replication forks after their repair, and it is hyperactive in BRCA1- or BRCA2-deficient cells.48 Since these reports were published, multiple clinical trials have been initiated or completed using a collection of PARP inhibitors in breast and ovarian cancers deficient in BRCA1 or BRCA237,38,49 (Table 1). These trials have demonstrated clear single-agent activity of PARP1 inhibition, demonstrating that this concept had immense clinical significance.
BRCA1 and 2 mutations are prevalent in familial breast and ovarian cancers, but they can also be seen in sporadic cases of these tumors. This led to the concept of identifying the phenotype of “BRCA-ness” in nonfamilial breast and ovarian cancer cases.50 This phenotype is observed in a subset of patients with ER-negative, PR-negative, and Her-2–negative (triple negative) breast cancer. A recent trial with combination chemotherapy in patients with triple negative breast cancer (which behave like BRCA1/2 mutants) demonstrated a superior outcome with the combination with PARP inhibition, compared with chemotherapy alone.51 Of note, other defects in other HR DSB repair components, such as RAD51 or the MRN complex, also sensitize cancer cells to inhibition by PARP1.52
A recent small interfering siRNA screen of DNA repair genes53 for synthetic lethality after PARP1 inhibition identified multiple other HR repair genes that mediated cytotoxicity besides BRCA1 and 2. Genes involved in involved in NER (DDB1 and XAB2), and the tumor suppressor gene PTEN, a negative regulator of the PI3K pathway, were also found to be synthetically lethal with a PARP inhibitor.53,54 Thus, PARP1 inhibition may also cause synthetic lethality in cancers with these genes deleted. Decreasing Tankyrase 1 level, which is another PARP family member involved largely in telomere maintenance, was found to be synthetically lethal with BRCA1 deficiency.55 Tankyrase I inhibitors have been identified, and Tankyrase I inhibition suppresses the WNT pathway in oncogenesis.56
Synthetic lethality between MMR components and DNA polymerases
Germline mutations in the MMR genes MLH1 or MSH2 predispose to hereditary nonpolyposis colorectal cancer (HNPCC). Classic HNPCC cases account for 5% of colorectal cancer cases.8,30 MSH2 and MLH1 are proposed to act as classic tumor suppressor genes, where tumor cells lose all MSH2 or MLH1 function, whereas normal cells retain at least one functional allele. Although these genes have essential functions in MMR, they have been implicated in other DNA repair pathways, such as HR. An MMR-deficient phenotype is also seen in sporadic colorectal cancer (because of MMR gene promoter hypermethylation). These cases tend to have a better prognosis, but they do not benefit from adjuvant fluorouracil,57 but respond better to cross-linking agents such as oxaliplatin.
A yeast synthetic lethal screen identified an interaction between deletion of these MMR components and DNA polymerases.58 Martin and coworkers further extended this observation to human cells by demonstrating that MSH2 deficiency is synthetically lethal with inhibition of the DNA polymerase POLB, and MLH1 deficiency is synthetically lethal with DNA polymerase POLG inhibition.59 Both POLB and POLG inhibition led to the accumulation of 8-oxoG oxidative DNA lesions. In the MSH2/POLB case, 8-oxoG accumulation was in the nuclear DNA, whereas synthetic lethality of MLH1/POLG led to a rise in mitochondrial DNA 8-oxoG levels. In both cases the lethality was rescued by silencing the adenine glycosylase MUTYH, suggesting that it was caused by the formation of DNA SSBs on 8-oxoG accumulation. Thus, inhibitors of these DNA polymerases may have a role in the treatment of HNPCC. This same group of investigators identified a known DNA polymerase inhibitor that might be useful clinically in these malignancies, reporting that methotrexate is particularly toxic to MSH2-deficient cells, because of induction of oxidative DNA damage.60 The role of methotrexate in HNPCC has not yet been fully tested clinically, but is an intriguing possibility.
Synthetic lethality dosage interactions
Whereas classic synthetic lethality involves suppressing one gene's function in the setting of another gene's deficiency, there are interactions among DNA repair components that can lead to a lethal phenotype without necessarily following this pattern. One example is the recently described interaction between P53 and ATM.61 In this model, inhibiting ATM in the context of wild-type P53 leads to dramatic sensitivity to DNA-damaging cancer drugs such as cisplatin and Topoisomerase II inhibitors. However, decreasing ATM levels, or its target Chk2, in the context of P53 deletion results in a survival advantage for cancer cells after exposure to the same DNA-damage agents.
Thus, ATM inhibition is synthetically lethal with DNA damage, but only in the setting of a functional P53 protein. These same investigators found that ATM-deficient (or ATM-inhibited) cancer cells are also addicted to DNA-PKcs for survival after DNA damage.61 DNA-PKcs inhibitors are already in clinical development, and these might be especially useful in tumors deficient in ATM, such as CLL (Table 162 ). There is a similar inverse interaction described between P53 and ATR. P53 loss potently sensitizes cells to inhibition of ATR63 by caffeine given with small doses of DNA-damaging drugs. This raises the question of whether caffeine can be used as a clinical enhancer of chemotherapy in cancers with P53 deficiency.
In a genome-wide siRNA screen for genes that enhance cisplatin sensitivity in the background of P53 deficiency, multiple hits were identified, including BRCA1 and 2, whose inhibition enhanced cisplatin cytotoxicity up to 7-fold more in P53-deficient cells than in matched P53 wild-type cells.64 Thus, tumor cells having disruptions in the BRCA1/2 network and P53 together are more sensitive to cisplatin than cells with either disruption alone. When P53-deficient cells were exposed to ionizing radiation, there was selective up-regulation of polo-like kinase 1 (PLK1) compared with the wild-type P53 cells.65 P53-deficient cancer cells were highly sensitive to PLK1 inhibitors, both in vivo and in vitro.65 Thus, PLK1 is an important future synthetic lethality target in the many tumors that are deficient in P53 function, and inhibitors of this kinase have proceeded past phase 1 clinical trial testing.65
Targeting other DNA repair proteins for cancer therapy
After malignant transformation, the survival of a cancer cell in the face of radiation or chemotherapy often relies on its ability to enhance DNA repair. Thus, DNA repair is a fundamentally important target to enhance the effectiveness of cancer therapy. One example is targeting the Chk1 protein to enhance chemotherapy. Chk1 is serine-threonine kinase that is activated because of phosphorylation by ATR, and to a lesser extent by ATM47,66 on DNA damage. Chk1 is especially important for the stability of stalled replication forks, which can be caused by many chemotherapeutic agents, such as cytarabine.47 UNC-01 is the first Chk1 inhibitor to be investigated in humans and blocks the G2/M cell-cycle checkpoint.67 UNC-01 is not specific for Chk1, but also inhibits other kinases, such as protein kinase C, which may explain its diverse toxicity profile (such as hyperglycemia).68 However, UCN-01 synergizes with multiple DNA-damage agents in killing cancer cells,69,70 demonstrating the usefulness of Chk1 as a target to enhance cancer therapy. Several other, more specific Chk1 inhibitors are in development, and some have finished phase 1 trials with minimal toxicity, but only mild activity.71 These have not yet been tested in conjunction with other known chemotherapeutic agents. Apart from classic chemotherapy, other types of lethal interactions with Chk1 inhibitors have also been reported, including synergistic cytotoxicity in combination with an Erk inhibitor in leukemia,72 and a Src inhibitor in myeloma cells.73 In addition, a recent study found that a novel Chk2 inhibitor (CCT241533) was synthetically lethal with PARP1 inhibitors in several human tumor cell lines.74 Thus, both Chk1 and 2 are potentially useful targets for preventing repair of endogenously derived DSBs in cancer cells and may imply that this mechanism of synthetic lethality may be useful for therapy.
ATM is another attractive target for inhibition to enhance cancer therapy. ATM inhibitors are still in preclinical development75 but are expected to be significant radio-sensitizers given the important role of ATM in DSB repair. An ATR inhibitor has been recently described as well76 and is in preclinical development. However, given the essential and pleotropic role of ATR in normal cells, one would worry about the potential toxicity of these agents to normal tissue.
As mentioned in “Mechanisms of DNA repair,” DNA-PKcs is an essential kinase for NHEJ of DSBs in human cells, and DNA-PKcs inhibitors are also undergoing preclinical evaluation. One of these has shown the ability to potentiate radiation and chemotherapy in cancer cells with minimal toxicity to normal tissue.77 In addition, an inhibitor of the MRN complex, which is involved in both HR and NHEJ DSB repair, was identified in a genetic-chemical screen.78 This too might have activity in enhancing DNA-damaging chemotherapy or radiation. Finally, DNA ligases mediate the bonding of the 2 DNA ends in different modes of DNA repair. Inhibitors of these ligases have been described, and they appear to potentiate the toxic effect of alkylating agents selectively in cancer cell lines.79
BER repairs DNA adducts caused by alkylating agents, and APE1 is the rate-limiting enzyme in this pathway. Thus, APE1 is an attractive target for synthetic lethal interactions.80 Several APE1 inhibitors have been described that could be used in combination with drugs like temozolomide.81 One particular compound (TRC102) is in a phase 1 trial. O6-methylguanine-DNA methyltransferase (MGMT) repairs the DNA adducts induced by monomethylating and chloroethylating chemotherapy drugs, respectively. Multiple inhibitors of MGMT have been introduced, and some have been studied in clinical trials in combination with alkylating drugs.82 Although these inhibitors had no major toxicities as single agents, they exacerbated the toxicities of alkylator chemotherapy and did not improve clinical outcome.83 In addition, strategies to enhance resistance in the hematopoietic system have been entertained to mitigate the bone marrow toxicity seen with this drug combination. Table 1 includes a list of some DNA repair protein inhibitors that are being tested in clinical trials.62
Synthetic lethality in the treatment of hematologic malignancies
Proteasome inhibition was reported to sensitize cancer cells to different DNA-damaging agents, including radiation. In their search of potential mechanisms of this phenomenon, one group discovered a delay in the localization of multiple DNA repair proteins (ATM, 53BP1, NBS1, BRCA1, FANCD2, and RAD51) to sites of DNA damage with the proteasome inhibitor bortezomib,83 while other foci kinetics (γH2AX, MDC1, and RPA) did not change. Bortezomib was also found to be a strong inhibitor of FancD2 mono-ubiquitination in response to DNA cross-linking.83 This would decrease repair of damage caused by many alkylating agents and platinum derivatives. Another group reported bortezomib to be synthetically lethal with the loss of DNA repair protein Fanconi group E (FANCE), or the loss of the histone variant H2Ax in a shRNA screen,84 whereas there was no increased toxicity with the loss of the DNA repair proteins Rad51, Rad21, and RPA3. Thus, bortezomib may have a role in potentiating the efficacy of DNA-damaging antineoplastic drugs in tumors where it has little single-agent activity. These findings also explain the synergy between bortezomib and the alkylating agents melphalan and cyclophosphamide in the treatment of myeloma.85
Chronic lymphocytic leukemia (CLL) is another hematologic malignancy in which synthetic lethality will be important. There is a subset of CLL with poor prognosis that has mutations in the ATM gene.86 These CLL cells would have defects in HR, and they have been found to be amenable to therapy with PARP1 inhibitors.86 Thus, the PARP1 inhibitors currently in trials in breast and ovarian cancer could also find a role for the treatment of CLL, perhaps in combination with an alkylating agent. In addition, the original finding by Hartwell et al that topoisomerase I and II inhibitors were synthetically lethal in yeast with defects in DSB repair might have applicability in CLL.36 Perhaps agents like camptothecin or etoposide might be useful in this subclass of aggressive CLL.
Finally, there is also a report that AML is sensitive to PARP1 inhibition because of its intrinsic genomic instability.87 PARP1 inhibition has been found to increase the cytotoxicity of alkylating agents in AML.88 Although alkylating agents are rarely useful in AML outside of preparative regimens for stem cell transplantation, it is possible that when given in conjunction with a PARP1 inhibitor they would have more effectiveness. This latter approach, in which PARP1 inhibition is combined with a DNA-damaging agent that would induce DSBs during replication, is one of the most promising.
Limitations of synthetic lethality in cancer drug development
Despite its strengths, there are multiple limitations to the synthetic lethality approach to drug development. First, inhibitors may increase side effects of other cancer drugs on normal tissue, as noted with temozolomide. Inhibition of a DNA repair pathway required in a proliferating normal tissue such as marrow or mucosa could lead to an increase in DNA damage in the normal tissue. One other possible long-term adverse result from this mechanism is that inhibiting DNA repair in normal tissues may increase the risk of secondary malignancies from this type of therapy.89
Second, many cancers do not depend on a single DNA repair pathway to overcome a defect in another pathway. Third, the tumor specificity of many DNA repair components is not well defined. Thus, the targets to develop inhibitors against are not understood for many cancer types. But this problem may be overcome soon, as more and more genome-wide sequencing efforts in various tumors are reported. Fourth, many DNA repair proteins not only share domains among themselves, they also share similar structures with essential cellular proteins with functions external to DNA repair. Thus, drugs developed using synthetic lethality to inhibit one DNA repair component could have off-target effects, whereby another important protein sharing a domain with the targeted repair component is also inhibited. An example of this is DNA PKcs, which shares kinase domain homology with SMG-1, ATM, ATR, and m-TOR, each of which has distinct and important functions.17,90
A fifth concern about synthetic lethality is that because DNA repair pathways can overlap, resistance can develop to a synthetically lethal drug. A given cancer can subvert an alternative pathway to fulfill the function of the inhibited pathway (Figure 3). Or, given the genomic instability of malignancies, resistance could develop by mutation in the targeted repair pathway itself. The best example of this is the mutation leading to re-expression of a functional BRCA2 in BRCA2-negative breast cancers treated with PARP1 inhibitors.91 In this situation, breast cancer cells, originally with a mutation in BRCA2, deleted a portion of this mutated sequence under the selective pressure of PARP1 inhibitor therapy, resulting in a smaller but functional BRCA2 protein. This allowed repair of DSBs that occur during replication in the presence of PARP1 inhibition, and rescued the ability of these breast cancer cells to proliferate. In a similar manner, it was found that deleting the NHEJ protein 53BP1 negated the embryonic lethal phenotype and genomic instability of BRCA1 deficiency, largely through restoration of functional HR DNA repair.92,93 53BP1 levels were found to be decreased in a subset of triple-negative breast cancer patients, which correlated with worse outcome, probably because of resistance to chemotherapy.93
Future applications of synthetic lethality in cancer treatment
The future applications of synthetic lethality depend on responding to the limitations described herein; otherwise, the applications of synthetic lethality will be short-lived and limited to a few tumors. Most cancers are not like the BRCA1- and 2-deficient tumors, with well-defined defects in DNA repair. However, because cancer can be thought of as a disease of genomic instability, each cancer most likely does have a specific defect in DNA repair that could have led to its transformation to malignancy. Different tumor types will perhaps have different DNA repair pathways that were defective for transformation, and each tumor type would need these to be defined at a molecular level. This would better define which pathways to target using the synthetic lethality approach in various tumor types, broadening the applicability of this approach in drug development. In a similar manner, once such pathways are defined for all tumor types, they can be used as biomarkers of predicted response to these agents.94 In this manner, synthetic lethality can have far wider application than originally thought.
One obvious area of future application of PARP1 inhibitors mentioned here is to apply them to other tumor types besides those with BRCA1 or 2 deficiencies. Other tumors may share the biochemical phenotype without sharing the genetic phenotype. For example, a 25-gene expression assay can be used to define BRCA1-like breast cancers, and this bioassay could be used to predict which breast cancers with genetically normal BRCA1 and 2 potentially could respond to PARP1 inhibition.95 Recently, efforts have mounted to define a biomarker for HR that could be exploited to predict sensitivity to PARP inhibitors in vivo. DNA damage–induced RAD51 nuclear foci have stood out as a potential biomarker.96 These foci decrease when there is a functional defect in the HR process. Thus, one could assay RAD51 foci before treatment, and this would define whether a PARP1 inhibitor could be effective in tumors without known BRCA1 or 2 defects.
Synthetic lethality drugs carry with them, by their very mechanism, the seeds of resistance against them. Because some component of DNA repair is inhibited, an increase in the mutation rate is a possible result. Such an increase in mutation rates could result in mutations in the target of the inhibitor. Thus, drugs developed using synthetic lethality could have a short lifespan of effectiveness. Resistance could develop rapidly, as noted for BRCA2.91 The best use of drugs developed using synthetic lethality may yet be in the future, when they may be deployed in combination with other known chemotherapeutic agents, especially those that damage DNA on their own. Such clinical trials have been initiated only to a limited extent. Because this might be where the most benefit of synthetic lethality agents lie, clinical trials testing this hypothesis are eagerly awaited. If a synthetic lethal agent can be combined with conventionally chemotherapy, it is possible that it can fundamentally alter the way cancer is treated. Therefore, this concept could represent one of the most important advances in the management of malignancy in the modern era, where molecular findings have defined targets of therapy.
Authorship
Contribution: M.S. and C.A. wrote the manuscript, J.A.N. created the figures and edited the manuscript, and R.H. wrote and edited the manuscript.
Conflict-of-interest disclosure: R.H. is a Scientific Advisor for Northlake Biosciences LLC. The remaining authors declare no competing financial interests.
Correspondence: Jac A. Nickoloff, Department of Environmental and Radiological Health Sciences, 1618 Campus Delivery, Colorado State University, Ft Collins, CO; e-mail: j.nickoloff@colostate.edu; or Robert Hromas, Division of Hematology-Oncology, Department of Medicine, University of Florida, 1600 SW Archer Rd, Gainesville, FL 32610; e-mail: robert.hromas@medicine.ufl.edu.