Abstract
Although plasmacytoid dendritic cells (pDCs) are involved in HIV-1 pathogenesis, the precise mechanism of interaction between pDCs and HIV-1 in vivo is not clear. The conflicting reports in HIV-1–infected patients highlight the importance of studying the interaction between HIV-1 and pDCs in relevant in vivo models. The rag2/γC double knockout (DKO) mouse supports reconstitution of a functional human immune system in central and peripheral lymphoid organs. We report here that functional pDCs were developed in the BM and peripheral lymphoid organs in humanized DKO (DKO-hu) mice. We show that pDCs from both BM and spleen were activated and productively infected during early HIV infection. The activation level of pDCs correlated with that of CD4+ T-cell activation and apoptosis. Although CD4+ T cells were preferentially depleted, pDCs were maintained but functionally impaired in the BM and spleen of HIV-infected DKO-hu mice. We conclude that HIV-1 can efficiently infect, activate, and impair pDCs in the BM and spleen, in correlation with CD4+ T-cell depletion. The humanized mouse will serve as a relevant model to investigate the development and function of pDCs and their role during HIV-1 pathogenesis in vivo.
Introduction
Plasmacytoid dendritic cells (pDCs) are innate immune effector cells that can mature to become APCs and play a key role in bridging innate and adaptive immunity. As innate immune effector cells, pDCs rapidly produce type 1 IFN on exposure to virus infection. After activation/maturation, pDCs will become functional APCs, expressing high levels of MHC and T-cell costimulatory molecules such as CD80 and CD86. Compared with conventional DCs (ie, cDCs or myeloid DCs [mDCs]), activated pDCs also express human inducible costimulator-ligand (ICOS-L) and indoleamine 2,3-dioxygenase, which may contribute to IL-10 expression or T-cell suppression. Therefore, in addition to rapidly producing IFNα, pDCs also function as APCs to up- or down-modulate adaptive immunity, establishing themselves as critical players in coordinating antiviral immunity1 (and reviewed in Colonna et al2 and Liu3 ).
Besides high levels of CD4 and other pDC-specific receptors, such as blood dendritic cell antigen (BDCA)2, BDCA4, and immunoglobulin-like transcripts (ILT)7, Toll-like receptor (TLR)7 and TLR9 are preferentially expressed in the endosome of pDC, endowing them as the major sensors of viral RNA and DNA, respectively.4,5 On exposure to viral RNA or DNA in the endosome, pDCs are rapidly activated to produce type 1 IFN, IL-6, and TNFα. It has been reported that viral RNA (or DNA) binds to TLR7 (or TLR9) to initiate a cascade of signaling events to activate the MyD88-IRAK-TRAF-IRF7 complex. Activated IRF7 migrates into the nucleus to induce expression of IFN genes. In addition, a distinct but overlapping signaling pathway also leads to activation of IL-6 and TNFα expression, mainly via the NFκB and MAPK pathways. In contrary, cross linking the BDCA2 or ILT7 receptors on pDCs appears to lead to the inhibition of IFN induction via protein tyrosine kinase– and immunoreceptor tyrosine-based activation motif–containing signaling molecules.6
The natural ligand for ILT7 has been recently characterized as BM stromal cell antigen 2 (BST2; CD317), an IFN-induced gene.7 Therefore, the activation of pDCs is modulated by several cell-surface and intracellular receptors to ensure their proper activation and function during host immune responses. HIV-1 Env gp120 interacts with CD4, CXCR4, and CCR5. CD4 binding is proposed to trigger endocytosis to expose the HIV genome to TLR in the endosome.8,9 Interestingly, the authors of one report suggest that gp120 may bind BDCA2 to affect TLR9-mediated activation of pDCs.10 In addition, BST2 (aka tetherin) plays an inhibitory role in HIV budding11 and is degraded or down-regulated by the HIV-encoded protein Vpu to promote HIV virion release from infected target cells. Down-regulation of BST2/tetherin in HIV-1–infected cells may lead to elevated pDC activation because BST2 binds ILT7 to inhibit pDC activation. Therefore, HIV infection can potentially affect pDC activation via multiple mechanisms.
Several lines of evidence have indicated that pDCs may be important in HIV infection and pathogenesis.2,9 First, pDCs express high levels of CD4, CCR5, and CXC4, and HIV can productively infect pDCs in vitro. Second, pDCs (but not mDCs) are efficiently activated by HIV in the absence of a productive infection.9 Third, HIV-positive patients are usually associated with lower levels of pDC activity. In fact, early study and discovery of human pDCs are determined by the finding that IFN-producing cells are reduced in AIDS patients.12 The pDCs, therefore, are likely critical modulators of HIV infection and immunopathogenesis.
Pathogenic HIV infections of humans and SIV infections of rhesus macaques are characterized by generalized immune activation and progressive CD4+ T-cell depletion.13 In contrast, natural SIV hosts such as sooty mangabeys show a lack of aberrant immune activation, no CD4+ T-cell depletion, and do not progress to having AIDS, despite high levels of SIV replication. Some early reports support the idea that HIV infection leads to decreased numbers and activity of pDC in the peripheral blood.14-17 In addition, the nonpathogenic SIV infection in its native host sooty mangabeys is associated with stable levels of pDC, whereas reduced pDC levels are reported in SIV-infected rhesus monkeys during late chronic stages of infection.18-21
However, it was recently reported that aberrantly activated pDCs are accumulated in the lymphoid organs during HIV infection. It is proposed that chronic activation of pDCs and IFN production may play a critical role in CD4+ T-cell depletion and AIDS progression.22 This hypothesis is supported by recent reports that although both pathogenic and nonpathogenic SIV infection induce pDC activation in acute-phase infection, only nonpathogenic infection is associated with down-regulation of pDC activation.23-27 The conflicting reports in patients highlight the importance of studying the interaction between HIV and pDCs in relevant models. A robust animal model is urgently needed to study the modulation by, and role of, pDCs in HIV infection.
The Rag2/γC double knockout (DKO) mouse lacks T and B lymphocytes and natural killer cells and serves as an optimal host for the engraftment of human cells/tissues.28 Remarkably, long-term human T-cell development occurs efficiently in the mouse thymus, and normal human T, B, natural killer, and dendritic cells (both mDC and pDC) are readily detected in the peripheral lymphoid tissues such as spleen, lymph nodes, and peripheral blood. Human T cells developed in the DKO-hu mouse are tolerant to both human and mouse antigens, indicating efficient negative selection by both murine and human APCs. Importantly, de novo human B- and T-cell responses are elicited in the DKO-hu mouse by standard immunization (human TT-specific IgG induction) or infection with the human tumor virus EBV (expansion of EBV-specific CD8 T cells).28 These EBV-reactive T cells respond to EBV antigens in a human MHC-dependent fashion.29,30 We and others have shown that HIV infection is efficiently established and persistently detectable in DKO-hu mice with CXCR4, CCR5, or dual-tropic isolates. In addition, human CD4+ T cells are gradually depleted during HIV infection.31-33
We report here that functional pDCs were developed in all lymphoid organs in DKO-hu mice. We show that pDCs from both spleen and BM were productively infected and activated by acute HIV infection. Although CD4+ T cells were preferentially depleted, pDCs were maintained in lymphoid organs. However, pDCs were functionally impaired in HIV-infected DKO-hu mice. Therefore, HIV infection rapidly activated and impaired pDC functions in lymphoid organs, including the BM. The DKO-hu mouse will serve as a relevant model to investigate the development and function of pDCs and their roles during HIV pathogenesis in vivo.
Methods
Construction of DKO-hu mice
DKO-hu mice were constructed as previously reported.32,33 In brief, human CD34+ cells were isolated from 17- to 20-week-old fetal liver tissues. The cell suspension released from the liver was filtered through a 70-μm cell strainer (BD Falcon) centrifuged at 150g for 5 minutes to remove hepatocytes. The mononuclear cells were purified through a Ficoll gradient (GE Healthcare Bio-science AB). Cells were labeled with the CD34 MicroBead Kit from Miltenyi Biotec, then CD34+ cells were positive selected with autoMACS by following the manufacturer's instructions (Miltenyi Biotec). A total of 1-5 × 105 CD34+ HSPC cells were injected into the liver of each DKO mouse at 1-3 days of age, and the mouse has been previously irradiated at 400 rad. Mice who received a transplant were bled through tail vein at 3-4 months after transplantation to check human cell reconstitution by flow cytometry. All animal experiences were reviewed and approved by the University of North Carolina-Chapel Hill Institutional Animal Care and Use Committee.
HIV infection in DKO-hu mice
We used an HIV molecular clone with a highly pathogenic dual tropic envelope, R3A in NL4-3 backbone,34 for infection. R3A-HSA was constructed by replacing the vpr gene with mouse heat stable antigen (HAS; CD24) as reported.35 HIV-1 viral stocks were produced in 293T cells and expanded in PHA-activated PBMCs and titered on Hela-CD4-LTR-gal cells (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID). DKO-hu mice with stable human leukocyte reconstitution were infected with HIV at 4000 infectious units/mouse by intravenous injection. DKO-hu mice that were infected with mock supernatant were included as control groups. HIV replication (genome copy/mL in the plasma) was measured by the Roche Amplicor Monitor v.1.5 qRT-PCR assay (Roche Diagnostics Corporation) or by p24 intracellular staining as previously described.32,33
Flow cytometry
At termination, all lymphoid organs, including thymus, BM, spleen, and lymph nodes, were harvested.32,33 Total lymphocytes were isolated from mouse lymphoid organs, red blood cells were lysed with ACK buffer, and the remaining cells were stained and fixed with 1% (wt/vol) formaldehyde before analysis. Dead cells were excluded by violet fluorescence dead cell dye (VLD; cat. no. L34955, Invitrogen). Total cell number was quantified by Guava Easycytes with Guava Express software (Guava). pDC (CD4+CD123+) numbers were calculated by total cell number from Guava cell counts and percentage of total cells from flow cytometry analysis.
For p24, caspase 3, and IFNα staining, cells were stained with surface antibodies first, then permeabilized with cytofix/cytoperm buffer (BD Bioscience), followed by intracellular staining. Human leukocytes (CD45+) were analyzed for CD3, CD4, CD8, CD45RO, CD45RA, HLA-DR, CCR5, and CXCR4 by CyAn FACS machine (Dako). FITC-conjugated anti–human CCR5(2D7), CXCR4(12G5), CD45RA(HI100), and APC-conjugated CD86 were purchased from BD Biosciences; PE/Cy7-anti–human CD3(HIT3a), PE/Cy5-conjugated anti–human CD4(RPA-T4), APC/Cy7-conjugated anti–human CD45, Pacific blue–conjugated anti–mouse CD45(30-F11), PE-conjugated anti-CD80 (2D10), and FITC-conjugated HLA-DR(L243) were purchased from Biolegend; and PE/Texas red–conjugated anti–human CD8 (3B5) antibody and live/dead fixable violet dead cell dye (VLD) were purchased from Invitrogen/Caltag. PE-conjugated anti–human CD303 (BDCA2) was purchased from Miltenyi Biotec. FITC-conjugated anti-HIV p24 (FH190-1-1) was purchased from Beckman Coulter. The cells were analyzed on a Cyan ADP (Dako).
pDC purification
Total BM cells were stained with biotin-labeled antibody mix to human CD3, CD19, mouse CD45, and mouse Ter119. Lineage-positive cells were depleted by streptavidin-labeled magnetic beads with AutoMACS (Miltenyi Biotec). The negative cells were stained with CD4 and CD123. CD4+CD123+ cells were sorted by FACS (> 95% purity).
In vitro stimulation of pDCs
Total BM cells (2 × 105 in 100 μL of culture medium) or purified pDCs (1 × 104 in 100 μL of culture medium) were stimulated with CpG2216 (2 μg/mL; InvivoGen), influenza virus A/PR8/34 (2 μg/mL; Charles River), or UV-inactivated HSV (1 × 107/mL, kindly provided by Dr Steven Bachenheimer at University of North Carolina).
Human cytokine luminex assay
Cytokines in the mouse plasma or culture supernatant were quantified with Human Cytokine 25-Plex kit (Invitrogen/Biosource). Samples were collected and stored at −70°C until assay. Triton X-100 were added (1%, vol/vol) before the assay to inactivate HIV. The assays were performed at the Clinical Proteomics Laboratory at University of North Carolina at Chapel Hill.
Statistical analysis
The significance of all comparisons was calculated by use of a Student 2-tailed t test assuming unequal variance between mock and HIV-infected groups, and results were considered significant when P < .05. Correlations between parameters were assessed by the use of the Spearman rank correlation test; P < .05 was considered to be statistically significant.
Results
Development of functional human pDCs in central and peripheral lymphoid organs of DKO-hu mice
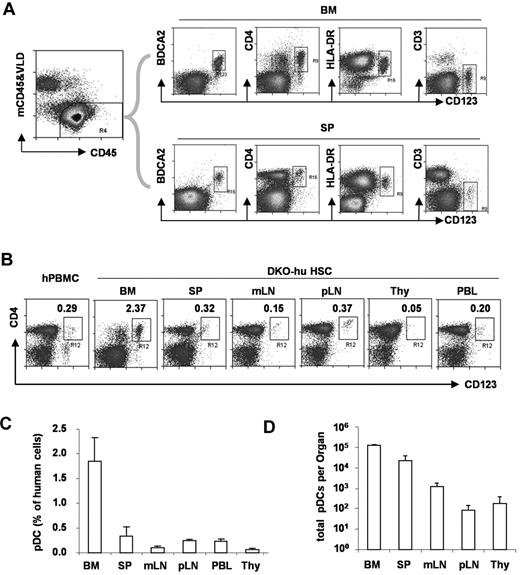
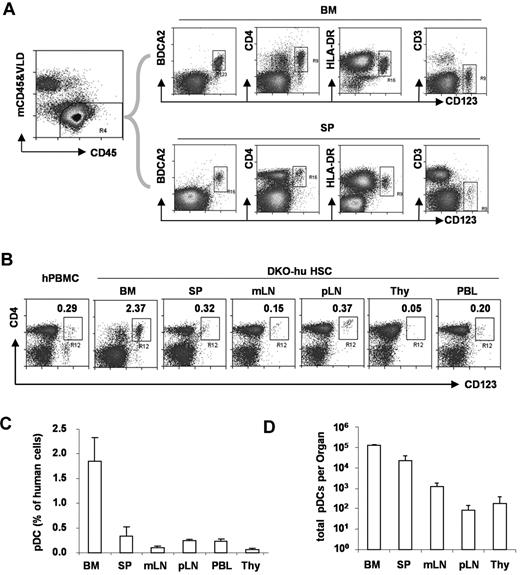
To define human pDCs from various lymphoid organs, we analyzed human CD45+ and murine CD45− cells in blood or lymphoid tissues from DKO-hu mice. Human pDCs (hCD45+CD3−CD19−CD11c−CD4+CD123+BDCA2+) were developed in the BM and other periphery lymphoid organs (Figure 1). Compared with human PBMCs, a similar low frequency of human pDCs was detected in the blood, spleen, or lymph nodes of humanized mice. A relatively greater fraction of human pDCs was detected in the BM, whereas only low numbers of pDCs were detected in the thymus of DKO-hu mice.
Development of pDC in lymphoid organs in DKO-hu mice. (A) Total live human cells (VLD-mCD45−hCD45+) from BM or spleen (SP) were analyzed for CD123 expression relative to BDCA2, CD4, HLA-DR, and CD3 expression. (B) pDCs in different lymphoid organs from the DKO-hu mice were analyzed. The number in each plot represents the percentage of CD4+CD123+ pDCs of total human CD45+ cells. Human PBMCs were used as a control. (C) Summarized data show average percentage of pDCs (CD3−CD4+CD123+ of total human CD45+ cells) in different lymphoid organs from DKO-hu mice at 12 weeks after transplantation (n = 7). (D) Total numbers of pDCs in each lymphoid organ are calculated as described in “Flow cytometry.” Error bars indicate SD (n = 7).
Development of pDC in lymphoid organs in DKO-hu mice. (A) Total live human cells (VLD-mCD45−hCD45+) from BM or spleen (SP) were analyzed for CD123 expression relative to BDCA2, CD4, HLA-DR, and CD3 expression. (B) pDCs in different lymphoid organs from the DKO-hu mice were analyzed. The number in each plot represents the percentage of CD4+CD123+ pDCs of total human CD45+ cells. Human PBMCs were used as a control. (C) Summarized data show average percentage of pDCs (CD3−CD4+CD123+ of total human CD45+ cells) in different lymphoid organs from DKO-hu mice at 12 weeks after transplantation (n = 7). (D) Total numbers of pDCs in each lymphoid organ are calculated as described in “Flow cytometry.” Error bars indicate SD (n = 7).
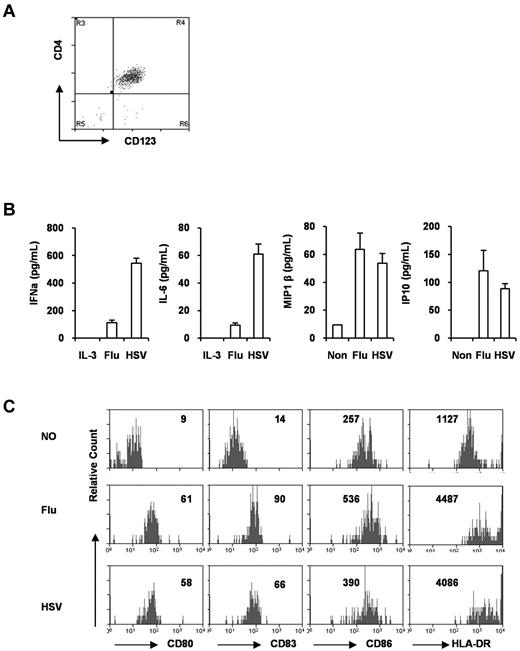
When pDCs from the BM were purified by FACS (Figure 2A), they responded to stimulation with influenza virus (TLR7) and HSV (TLR9) and rapidly produced IFNα, IL-6, and chemokines such as IP10, MIP1β, and IP10 (Figure 2B). In response to TLR7 or TLR9 stimulation, the pDCs also matured to up-regulate CD80, CD83, CD86, HLA-DR, ICOS-L, and other APC receptors (Figure 2C and data not shown). Therefore, pDCs developed in humanized mice were functional in response to TLR7 or TLR9 ligands.
pDCs from DKO-hu mice are functional. (A) pDCs (CD45+CD3−CD4+CD123+) were purified as described in “pDC purification,” and the purity was monitored by CD4 and CD123 staining (> 95% pure). (B) Purified pDCs (10 000) were cultured in the presence of influenza virus or HSV for 16 ours. Supernatants were collected and IFNα, IL-6, MIP1β, and IP10 were measured with the Human Cytokine Luminex kit. Error bars are SDs from triplicate samples. (C) Purified pDCs were stimulated with influenza virus or HSV for 48 hours, and then stained with anti–human CD80, CD83, CD86, and HLA-DR monoclonal antibodies. The number in each plot is the mean fluorescence intensity of total pDCs after culture. Flu indicates influenza virus.
pDCs from DKO-hu mice are functional. (A) pDCs (CD45+CD3−CD4+CD123+) were purified as described in “pDC purification,” and the purity was monitored by CD4 and CD123 staining (> 95% pure). (B) Purified pDCs (10 000) were cultured in the presence of influenza virus or HSV for 16 ours. Supernatants were collected and IFNα, IL-6, MIP1β, and IP10 were measured with the Human Cytokine Luminex kit. Error bars are SDs from triplicate samples. (C) Purified pDCs were stimulated with influenza virus or HSV for 48 hours, and then stained with anti–human CD80, CD83, CD86, and HLA-DR monoclonal antibodies. The number in each plot is the mean fluorescence intensity of total pDCs after culture. Flu indicates influenza virus.
pDCs in DKO-hu mice express both CCR5 and CXCR and are efficiently infected by HIV in vivo
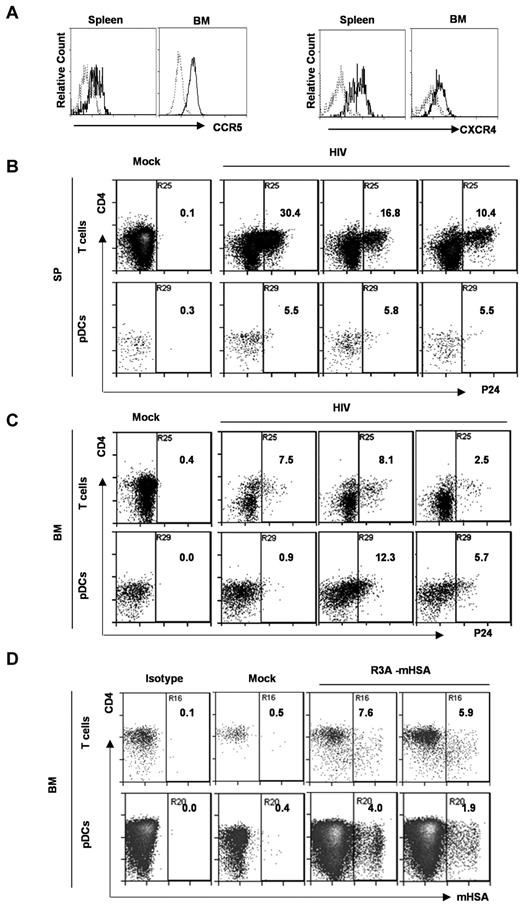
We showed that human pDCs (Lin−CD4+CD123+) in DKO-hu mice expressed both CCR5 and CXCR4 coreceptors (Figure 3A). We investigated whether pDCs could be directly infected by HIV-1 in the DKO-hu model in various lymphoid organs in vivo. We first measured HIV infection of pDCs by HIV p24 intracellular staining at 1-2 weeks after infection. pDCs were efficiently infected both in spleen and BM. Relative to the infection of human CD4+ T cells, similar levels of HIV infection of pDCs were detected in the BM of some infected mice (Figure 3B-C). Because the HIV-1 virion-associated p24 may be endocytosed by pDCs, the p24 detected in pDCs did not definitively prove that pDCs were productively infected by HIV-1. We thus further confirmed the productive infection with recombinant HIV-1–expressing mouse heat stable antigen in the vpr gene (HIV-R3A-mouse heat stable antigen; Figure 3D). Our data clearly show that HIV-1 efficiently infected pDCs in the BM and spleen during early HIV-1 infection in vivo.
pDCs from DKO-hu mice express both HIV coreceptors, and are productively infected by HIV-1 in vivo. (A) Total BM cells or splenocytes were analyzed by FACS. CD3−CD4+CD123+ pDCs were further analyzed for CCR5 and CXCR4 expression. Dotted gray lines represent IgG isotype control. (B,C) At 8 days after HIV infection (R3A virus), splenocytes (B) and BM cells (C) were stained with surface markers, followed by HIV p24 intracellular staining. The numbers in the plots represent p24+ percentage of CD3+ T cells and CD3−BDCA2+CD123+ pDCs. Samples from 1 mock and 3 HIV-infected DKO-hu mice are shown. (D) BM cells from HIV-R3A-HAS–infected DKO-hu mice were analyzed by flow cytometry. The percentage of HSA (mouse CD24) expression on CD3+CD8− T cells and CD3−CD123+ pDCs from 1 mock and 2 HIV infected DKO-hu mice are shown.
pDCs from DKO-hu mice express both HIV coreceptors, and are productively infected by HIV-1 in vivo. (A) Total BM cells or splenocytes were analyzed by FACS. CD3−CD4+CD123+ pDCs were further analyzed for CCR5 and CXCR4 expression. Dotted gray lines represent IgG isotype control. (B,C) At 8 days after HIV infection (R3A virus), splenocytes (B) and BM cells (C) were stained with surface markers, followed by HIV p24 intracellular staining. The numbers in the plots represent p24+ percentage of CD3+ T cells and CD3−BDCA2+CD123+ pDCs. Samples from 1 mock and 3 HIV-infected DKO-hu mice are shown. (D) BM cells from HIV-R3A-HAS–infected DKO-hu mice were analyzed by flow cytometry. The percentage of HSA (mouse CD24) expression on CD3+CD8− T cells and CD3−CD123+ pDCs from 1 mock and 2 HIV infected DKO-hu mice are shown.
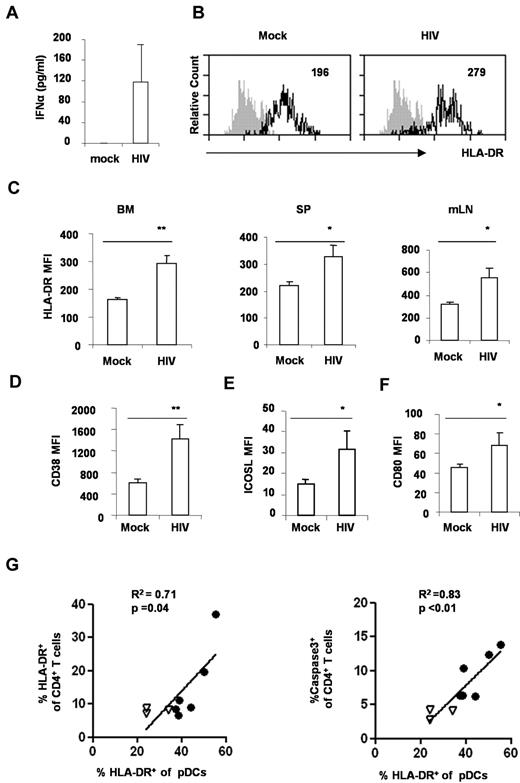
HIV-1 infection leads to rapid activation of pDCs in vivo
Activation of pDCs is proposed to contribute to the progression of HIV disease.22 We thus measured the production of IFNα in the blood from DKO-hu mice infected with mock or HIV. Significant induction of IFNα was detected in the blood of HIV-infected mice (Figure 4A). In addition, we analyzed the activation markers on pDC from mock and HIV-infected mice. In HIV-infected DKO-hu mice, pDCs were induced to express high levels of HLA-DR, CD38, CD80, and ICOS-L (Figure 4B-F). Interestingly, the relative activation of pDCs correlated well with CD4+ T-cell activation (Figure 4G), apoptosis, and depletion (Figure 4H). Thus, human pDCs were rapidly activated in lymphoid organs after HIV-1 infection in DKO-hu mice, and the activated pDCs may contribute to activation and depletion of CD4+ T cells.
HIV-1 infection induces pDC activation in DKO-hu mice. (A) Elevated IFNα in HIV-1–infected DKO-hu mice. Plasma from mock or HIV-1–infected mice at 1 week after infection was analyzed for IFNα with the Human Cytokine Luminex kit. IFNα in the mock-infected plasma was lower than the detection limit (< 13 pg/mL). SD is shown as error bar (n = 7 mice). (B) HIV-1 infection activated pDCs in the BM in vivo. BDCA2+CD123+ pDCs from BM of mock or HIV-infected mice were analyzed for HLA-DR expression by FACS. The number indicates the mean fluorescence intensity (MFI) of total pDCs. Shaded plots are IgG isotype controls. (C) Summarized data show relative expression of HLA-DR on pDCs from BM, spleen (SP), or mesenteric lymph node (mLN) cells. Error bars indicate SDs. pDCs from mock or HIV-infected mice were analyzed for CD38 (D), ICOS-L (E), or CD80 (F) expression. The number is MFI of total pDCs. Shown is summarized data from 3 mock and 4 HIV-infected DKO-hu mice at 2 weeks after HIV infection. P values between mock and HIV-infected groups were calculated by nonparametric Student t test. *P < .05, **P < .01, (G) pDC activation is correlated with CD4+ T-cell activation and apoptosis. BM cells from 3 mock (▿) and 6 HIV-infected (●) mice were analyzed. Cells were stained with surface markers, followed by caspase3 intracellular staining. CD123+BDCA2+ pDCs and CD3+CD4+ T cells from BM were analyzed for caspase3 or HLA-DR expression. Correlations were analyzed with the Spearman nonparametric test; squared correlation coefficients (R2), and P values were shown.
HIV-1 infection induces pDC activation in DKO-hu mice. (A) Elevated IFNα in HIV-1–infected DKO-hu mice. Plasma from mock or HIV-1–infected mice at 1 week after infection was analyzed for IFNα with the Human Cytokine Luminex kit. IFNα in the mock-infected plasma was lower than the detection limit (< 13 pg/mL). SD is shown as error bar (n = 7 mice). (B) HIV-1 infection activated pDCs in the BM in vivo. BDCA2+CD123+ pDCs from BM of mock or HIV-infected mice were analyzed for HLA-DR expression by FACS. The number indicates the mean fluorescence intensity (MFI) of total pDCs. Shaded plots are IgG isotype controls. (C) Summarized data show relative expression of HLA-DR on pDCs from BM, spleen (SP), or mesenteric lymph node (mLN) cells. Error bars indicate SDs. pDCs from mock or HIV-infected mice were analyzed for CD38 (D), ICOS-L (E), or CD80 (F) expression. The number is MFI of total pDCs. Shown is summarized data from 3 mock and 4 HIV-infected DKO-hu mice at 2 weeks after HIV infection. P values between mock and HIV-infected groups were calculated by nonparametric Student t test. *P < .05, **P < .01, (G) pDC activation is correlated with CD4+ T-cell activation and apoptosis. BM cells from 3 mock (▿) and 6 HIV-infected (●) mice were analyzed. Cells were stained with surface markers, followed by caspase3 intracellular staining. CD123+BDCA2+ pDCs and CD3+CD4+ T cells from BM were analyzed for caspase3 or HLA-DR expression. Correlations were analyzed with the Spearman nonparametric test; squared correlation coefficients (R2), and P values were shown.
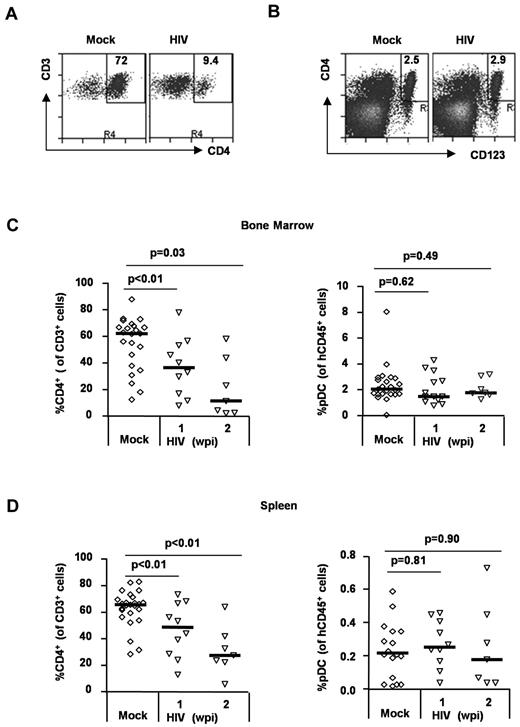
HIV-1 infection preferentially depletes human CD4+ T cells but not pDCs in vivo
To analyze relative depletion of human CD4+ T cells and pDCs by HIV-1 infection in vivo, we measured the relative frequency and number of human T cells and pDCs in each lymphoid organ. Human CD4+ T cells were efficiently depleted by HIV infection (in relative frequency and total cell number) in the BM and spleen. In contrast, the frequency of human pDCs was maintained in the BM (Figure 5A,C) and in the spleen (Figure 5D). The total pDC cell number was also maintained in both BM and spleen (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, HIV infection preferentially depleted human CD4+ T cells but not human CD4+ pDCs, even though they were also productively infected during HIV infection.
Differential depletion of CD4+ T cells and pDCs in HIV-infected DKO-hu mice. (A) Human CD4+ T cells were depleted by HIV-1 infection. Total BM cells from HIV-infected DKO-hu mice were analyzed by FACS. The numbers in each plot represent the percentage of CD4+ T cells of total CD3+ T cells. (B) Human pDCs were not depleted. Total human CD45+ cells were analyzed for CD4+CD123+ pDC. The number is the percent pDCs of total human CD45+ cells. (C) Summarized data of human CD4+ T cells and pDCs in the BM. (D) Summarized data of CD4+ T cells and pDCs in the spleen. Student t test was used to calculate P values.
Differential depletion of CD4+ T cells and pDCs in HIV-infected DKO-hu mice. (A) Human CD4+ T cells were depleted by HIV-1 infection. Total BM cells from HIV-infected DKO-hu mice were analyzed by FACS. The numbers in each plot represent the percentage of CD4+ T cells of total CD3+ T cells. (B) Human pDCs were not depleted. Total human CD45+ cells were analyzed for CD4+CD123+ pDC. The number is the percent pDCs of total human CD45+ cells. (C) Summarized data of human CD4+ T cells and pDCs in the BM. (D) Summarized data of CD4+ T cells and pDCs in the spleen. Student t test was used to calculate P values.
pDC function is impaired by HIV-1 infection in the BM and spleen in vivo
Although pDC levels were not reduced in HIV-1–infected DKO-hu mice, the level of IFNα was significantly diminished at 2 wpi in comparison with 1 wpi (Figure 6A-B). We postulated that the pDC function in HIV-1–infected lymphoid organs may be impaired as reported in HIV-1–infected patients. To analyze the pDC function, we measured the activation of BM pDCs isolated from DKO-hu mice infected with mock or HIV in response to TLR7 or TLR9 ligands. We demonstrated that pDCs from HIV-infected mice were impaired in response to either TLR9 (HSV; Figure 6C-D) or TLR7 (influenza virus; Figure 6E-F) stimulation. We concluded that pDCs in the BM of HIV-1–infected DKO-hu mice were functionally impaired to produce IFN in response to TLR7 and TLR9 stimulation.
pDCs from HIV infected DKO-hu mice are functionally impaired. (A) Relative HIV-1 replication level in the blood at 1 and 2 weeks after infection was shown. (B) IFNα in the plasma of mock or HIV-infected DKO-hu mice was measured by the Human Cytokine Luminex kit. Error bars are SD (n = 4). *P < .05. (C-D) BM cells from mock or HIV-infected DKO-hu mice were stimulated with HSV for 16 hours. The expression of intracellular IFNα in pDCs were measured by FACS (C). (D) Summarized data from 4 mock and 4 HIV-infected DKO-hu mice are shown. (E-F) BM cells were also stimulated with the influenza virus and analyzed for the expression of IFNα in pDCs. (F) Summarized data from 4 mock and 4 HIV infected DKO-hu mice are shown. Error bars represent SD. *P < .05.
pDCs from HIV infected DKO-hu mice are functionally impaired. (A) Relative HIV-1 replication level in the blood at 1 and 2 weeks after infection was shown. (B) IFNα in the plasma of mock or HIV-infected DKO-hu mice was measured by the Human Cytokine Luminex kit. Error bars are SD (n = 4). *P < .05. (C-D) BM cells from mock or HIV-infected DKO-hu mice were stimulated with HSV for 16 hours. The expression of intracellular IFNα in pDCs were measured by FACS (C). (D) Summarized data from 4 mock and 4 HIV-infected DKO-hu mice are shown. (E-F) BM cells were also stimulated with the influenza virus and analyzed for the expression of IFNα in pDCs. (F) Summarized data from 4 mock and 4 HIV infected DKO-hu mice are shown. Error bars represent SD. *P < .05.
Discussion
On the basis of findings from HIV-infected humans and SIV-infected monkeys, pDC activation has been implicated in playing a critical role in CD4+ T-cell depletion and AIDS pathogenesis.19-21 However, the function of human pDC is poorly understood because of its paucity in human peripheral blood and the difficulty of studying pDC in human or monkey lymphoid organs. Here we report that functional pDCs were developed in all lymphoid organs of DKO-hu mice. In addition, HIV-1–infected pDCs efficiently and productively in lymphoid organs in vivo. Interestingly, HIV-1 infection preferentially depleted human CD4+ T cells but only functionally impaired pDCs in the BM and spleen. HIV-1 infection rapidly activated pDCs in both BM and spleen, and relative activation of pDC correlated with CD4+ T-cell activation and depletion. Our data suggest that the activated but functionally impaired pDCs may contribute to the depletion of CD4+ T cells in HIV-1–infected DKO-hu mice, which will serve as an important model to study development and function of human pDCs in vivo.
Consistent with their expression of CD4 and HIV coreceptors, human pDCs support productive HIV-1 infection in vitro.15,36-38 HIV-1 antigen or proviral DNA have been detected in pDCs isolated from HIV-1–infected patients.36,39,40 However, it is not clear whether, and how efficiently, HIV-1 can productively infect pDCs in various lymphoid organs in vivo. Here we report that pDCs were efficiently infected both in the BM and spleen during the early phase of HIV infection. In addition to our direct detection of HIV-1 gag p24 in pDCs with the use of FACS, the HIV-1 virus encoding the murine HSA reporter clearly demonstrated the productive infection of pDCs in vivo because HSA expression in target cells depended on productive HIV-1 infection.
We also showed that, although both efficiently infected, human pDCs in lymphoid organs were not significantly depleted. This finding is consistent with the recent finding that activated pDCs are accumulated in lymph nodes of SIV-infected monkeys41 and HIV-infected patients.42-44 However, it has been reported that HIV-1 infection induces apoptosis of pDCs through fusion-dependent mechanisms in vitro.45 The fate of the HIV-infected pDCs in vivo and their contribution to HIV-1 reservoir will be further investigated in the DKO-hu model.
It has been documented that pDC activity in the blood is impaired or reduced in HIV-infected patients.17,46,47 We observed that pDCs from the BM of HIV-infected DKO-hu mice also were functionally impaired. However, it has been reported recently that blood pDCs during acute phase of SIV infection in rhesus macaques are functionally normal to TLR7 stimulation.41 In addition, a recent study also reports that pDCs from the blood of acute-phase HIV-1 patients are hyperresponsive on TLR7 ligand stimulation, including HIV virions.48 In an intriguing recent report, pDCs isolated from women show enhanced activation than pDCs isolated from men, which correlates with the preferential AIDS disease progression in HIV-infected women.49 . The discrepancy may be because of the different response of pDCs in the blood and in lymphoid organs, including the BM. Because of the limited human cells in the DKO-hu mouse blood, it is not possible to compare the function of human pDCs in the blood and lymphoid organs of HIV-1–infected DKO-hu mice.
In summary, the role of pDCs in HIV infection and AIDS progression is likely critical but poorly defined. HIV-1 infection–induced aberrant pDC activation may have a deleterious effect on immune system and contribute to disease progression 22,50 The humanized mouse model with a functional human immune system will serve as a valuable model to study development and function of human pDCs in central and peripheral lymphoid organs in vivo. To “genetically” define the role of pDCs in HIV infection and pathogenesis in vivo, a pDC-specific antibody will be useful to deplete pDCs in humanized mice during HIV-1 infection as we have defined the role of Treg cells in HIV-1 infection in the model.32 Findings regarding HIV-1 interaction with pDCs in vivo and the role of pDCs in HIV disease progression will shed light on the development of a novel therapeutic intervention that targets pDC functions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Yongjun Liu for discussions and Dedeke Brouwer, Selena Barbour, and Anthony Curtis for technical support.
This work was supported in part by grants from the National Natural Science Foundation of China (30872365 to L.Z.), from Ministry of Science and Technology Grants 2006CB910901 and KSCX2-YW-R-150 (to L.Z.); from the Ministry of Health (2009ZX10604 to L.G. and 2008ZX10002-011 to L.S.); and from the National Institutes of Health (R01-AI080432 and R01-AI077454 to L.S.). We would also like to thank the UNC CFAR, DLAM, and FACS Cores.
National Institutes of Health
Authorship
Contribution: L.Z., Q.J., and L.S. designed the project, analyzed data, and wrote the paper; and L.Z., Q.J., G.L., J.J., and G.I.K. performed experiments and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Liguo Zhang, Key Laboratory of Infection and Immunity, Institute of Biophysics, Chinese Academy of Sciences, 15 Da Tun Rd, Chaoyang District, 100101 Beijing, China; e-mail: liguozhang@ibp.ac.cn; and Lishan Su, Key Laboratory of Infection and Immunity, Institute of Biophysics, Chinese Academy of Sciences, 15 Da Tun Rd, Chaoyang District, 100101 Beijing, China; e-mail: lsu@med.unc.edu.
References
Author notes
L.Z. and Q.J. contributed equaled to this work.