Abstract
Bone morphogenetic protein (BMP) signaling regulates embryonic hematopoiesis via receptor-mediated activation of downstream SMAD proteins, including SMAD1. In previous work, we showed that Smad1 expression is sufficient to enhance commitment of mesoderm to hemangioblast fate. We also found indirect evidence to support a subsequent repressive function for Smad1 in hematopoiesis. To test this hypothesis directly, we developed a novel system allowing temporal control of Smad1 levels by conditional knockdown in embryonic stem cell derivatives. Depletion of Smad1 in embryoid body cultures before hemangioblast commitment limits hematopoietic potential because of a block in mesoderm development. Conversely, when Smad1 is depleted in FlK1+ mesoderm, at a stage after hemangioblast commitment, the pool of hematopoietic progenitors is expanded. This involves enhanced expression levels for genes specific to hematopoiesis, including Gata1, Runx1 and Eklf, rather than factors required for earlier specification of the hemangioblast. The phenotype correlates with increased nuclear SMAD2 activity, indicating molecular cross-regulation between the BMP and TGF-β signaling pathways. Consistent with this mechanism, hematopoiesis was enhanced when Smad2 was directly expressed during this same developmental window. Therefore, this study reveals a temporally defined function for Smad1 in restricting the expansion of early hematopoietic progenitors.
Introduction
Mammalian hematopoiesis begins on the yolk sac, generating a transient population of primitive erythrocytes, as well as a subset of definitive myeloid lineages.1 Considerable progress has been made identifying the relevant signaling pathways that regulate early hematopoiesis.2,3 The bone morphogenetic protein (BMP) members of the TGF-β superfamily are particularly important in patterning the character of early embryonic tissue to generate ventral mesoderm,4,5 which serves as the origin of the developing hemangioblast, a cell type that is bipotent for the hematopoietic and vascular lineages.6 Forced expression of BMP ligands or signaling components expands hematopoiesis,7 while genetic deletion of Bmp4 or its cognate receptor results in developmental arrest during gastrulation, with attendant failure to generate the mesodermal precursors from the epiblast that are required for hematopoietic development.4,8
Key downstream effectors of TGF-β and BMP signaling are members of the SMAD family of transcription factors, that are activated by type I receptors through C-terminal serine/threonine phosphorylation, after ligand binding and subsequent trans-activation of type I/type II receptor heterodimers.9 There are 5 receptor-activated SMADs, or R-SMADS: SMAD2 and SMAD3 mediate the TGF-β/Activin/Nodal pathway, while SMADs 1, 5, and 8 respond to BMP signaling. Smad1 and Smad5 have roles in hematopoiesis, while Smad8 is apparently not expressed in hematopoietic cells.10 Activated R-SMADs compete for access to a single coactivator or coSMAD, SMAD4, that when bound allows translocation of R-SMADs to the nucleus, where they bind and activate transcription of target genes within the context of multimeric complexes.11 The R-SMADs share a high degree of similarity in amino acid sequence, with SMAD1 and SMAD5 sharing approximately 92% identity in mouse; additionally, all of the relevant functional domains in R-SMADs are conserved, with DNA binding mediated via the N-terminal MH1 domain.
Regardless of the high degree of sequence similarity between SMAD1 and SMAD5, several studies revealed striking differences in their biology. Notably, while deletion of either gene results in early embryonic lethality, the underlying phenotypes are distinct. Smad5-null mice die around embryonic day 10 (E10) with gross defects in yolk sac vasculogenesis, and impairments in the developing gut and cardiac compartments.12,13 Smad1-null embryos, on the other hand, die at day E9.5 because of a failure in chorio-allantoic fusion.14 In addition, Smad1 is required for development of extra-embryonic tissues and in regulation of visceral endoderm proliferation.15 In zebrafish, smad5 and smad1 are sequentially expressed during different stages of embryonic development, although they both function to impart ventralized character to mesoderm.16 Morpholino-based knockdown of smad1 or smad5 results in distinct and even opposite hematopoietic defects in zebrafish embryos, with smad1 able to rescue the smad5 morphant phenotype, but not vice versa.17
Embryonic hematopoiesis can be recapitulated faithfully in vitro using the embryonic stem cell/embryoid body (ES/EB) system, with the timing of progenitor commitment occurring similar to the mouse embryo.18 The early lethality caused by deletion of individual R-SMADs presents a challenge to understand their roles during specific stages of embryonic hematopoiesis. Given evidence for their importance, and the regulatory potential for individual SMAD factors, we exploited the advantage of manipulating SMAD expression in the ES/EB system. We showed previously that Smad1 expression is enriched in the BRY+/FLK+ subpopulation of blast colony-forming cells (BL-CFCs) that arises around day 3.5 of EB differentiation, and that has been identified as the in vitro equivalent of the hemangioblast.6,19 Furthermore, we used a conditional transgene expression system to show that a timed pulse of SMAD1 early in EB differentiation is sufficient to increase hematopoietic progenitors through expansion of the BL-CFC subset. Surprisingly, increased numbers of progenitors were not seen with continuous induction of Smad1 transgene expression. In other words, Smad1 expression needed to be transient to reveal the phenotype, suggesting that Smad1 has both an early positive role, and then a subsequent inhibitory role in the proliferation, survival, or differentiation of hematopoietic progenitors.19
To test this hypothesis directly, we created a novel transgenic system allowing conditional shRNA-mediated gene-specific knockdown to explore the contribution of Smad1 signaling to hematopoietic differentiation throughout embryoid body development. Our results demonstrate a repressive role for Smad1 in regulating hematopoietic progenitor numbers, as we had previously hypothesized. In addition, our results suggest a mechanism involving competition for limited coSMAD that reveals cross-talk of the BMP and TGF-β signaling pathways.
Methods
Generation of inducible Smad1 knockdown ES-cell lines
Three individual short interfering (22 nt) RNA target sites were identified in the mouse Smad1 MH1 domain (NM_008539) using the Cold Spring Harbor Laboratory shRNA design protocol (http://katahdin.mssm.edu/siRNA/RNAi.cgi?type = shRNA). Complete hairpin oligonucleotides were designed with 5′ and 3′ miR-30 flanking sequence sufficient to allow intracellular RNA-induced silencing complex (RISC) processing, and used to PCR-amplify target sites with 5′ Xho1 and 3′ EcoRI adapters as 130-bp fragments.20 Individual shRNA hairpins were combined in tandem using restriction site adapters to subclone cassettes into the plox-IRES-EGFP vector. The resulting 3-hairpin construct (PIE-miSmad1, 20 μg) was cotransfected by electroporation with 20 μg of pSALK-Cre into the parental ES-cell line AinV18 (8 × 106 cells). Multiple individual cell clones were isolated and expanded after selection with 300 μg/mL G-418, and then tested for site-specific integration by PCR analysis of cDNA. Loxin primers: F5′-CTAGATCTCGAAGGATCTGGAG, R5′-ATACTTTCTCGGCAGGAGCA. Smad1 shRNA 22-mer sequences: a (GGUGAAGAAACUGAAGAAGAAG), b (AGCCGAGUAACUGCGUCACCAU), and c (AAGGGACUACCUCAUGUCAUUU).
ES-cell growth and differentiation
ES cells were maintained on irradiated mouse embryonic fibroblast (MEF) feeder cells in DMEM supplemented with 20% heat-inactivated ES-qualified FCS (Hyclone), 50 IU of penicillin (Cellgro), 50 μg/mL streptomycin (Cellgro), Leukemia Inhibitory Factor (LIF; 2% conditioned medium), and 1.5 × 10−4M monothioglycerol (MTG; Sigma-Aldrich). After serial depletion of mouse embryonic fibroblast (MEF) feeders, ES cells were allowed to differentiate as EBs in ethylene oxide-treated Petri grade dishes or in Aggrewell 400 plates (StemCell Technologies). EBs were cultured in IMDM supplemented with 15% FCS, 5% protein-free hybridoma medium (PFHM-II; Gibco), 2 mM l-glutamine (Cellgro), 0.5mM ascorbic acid (Cellgro), 200 μg/mL transferrin (Roche), and 4.5 × 10−4M MTG. For EBs harvested between days 3 and 4.5 of development, ES cells were plated at a density of 4 × 104 cells/mL culture medium, and for EBs harvested between days 5 and 6, ES cells were plated at a density of 8 × 103 cells/mL. Continuous Smad1 knockdown via activation of integrated shRNA hairpins was induced by addition of 1 μg/mL doxycycline directly to either the ES-cell or EB-culture medium.
Colony assays
Differentiation of EB-derived cells into hematopoietic progenitor colonies was performed as described.19,21 Embryoid bodies contain subpopulations of cells that are asynchronously primed to undergo commitment to different early hematopoietic lineages. Therefore, primitive erythroid progenitors emerge from day 4 to day 7, with a peak around day 6 of differentiation. Similarly, there is an analogous distribution for in vitro definitive colony potential later in EB differentiation, overlapping with primitive erythroid potential. The time we probe, around day 6 is sufficient to interrogate both the primitive and initial definitive programs. Briefly, EBs were disaggregated by trypsinization, and single-cell suspensions were replated at a density of 1 × 105 cells/mL in IMDM containing 1% methylcellulose, 10% plasma-derived serum (PDS; Antech), 5% PFHM-II, 2mM l-glutamine, 0.25mM ascorbic acid, and 1.5 × 10−4 M MTG, supplemented with the following progenitor-specific cytokines: primitive erythroblasts (erythropoietin [EPO, 2 U/mL]); blast colonies (VEGF [5 ng/mL], SCF [5 ng/mL], IL-6 [10 ng/mL], 25% D4T endothelial cell-conditioned media); macrophages (IL-3 [1% conditioned media], M-CSF [5 ng/mL]); megakaryocytes (IL-3 [1% conditioned media], IL-11 [5 ng/mL], thrombopoietin [TPO, 5 ng/mL]); mixed colonies (SCF [5 ng/mL], IL-3 [1% conditioned media], G-CSF [30 ng/mL], GM-CSF [10 ng/mL], IL-11 [5 ng/mL], IL-6 [5 ng/mL], TPO [5 ng/mL], M-CSF [5 ng/mL); and definitive erythrocytes (EPO [2 U/mL], TPO [5 ng/mL], SCF [5 ng/mL]). Colonies were counted under a light microscope at 10× magnification 4 days (EryP), 7 days (MacP, MegaP, EryD), or 9 days (Mixed) after plating. IL-3 was derived from media conditioned by CHO cells transfected with an IL-3 expression vector. EPO was obtained from the Mount Sinai Medical Center hospital pharmacy. All other cytokines were purchased from R&D Systems.
Quantitative RT-PCR
Smad1 knockdown was induced on day 2 or day 4 of EB development by addition of 1 μg/mL doxycycline. Induced and control EBs were harvested at equivalent time points, and subjected to isolation of total RNA with TRIzol reagent (Invitrogen). One microgram of RNA was used in reverse transcription reactions using Superscript III First-Strand Synthesis kit (Invitrogen) to generate cDNA, which was analyzed directly by standard PCR, or diluted 1:40 in RNase-free H2O for quantitative PCR (qPCR) with Sybr green using the Roche 480 II LightCycler and the 2−ΔΔCT method.22 PCR primers and qPCR primers are listed in the supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Western blotting
Whole-cell extracts were collected from ES or EB cultures in complete lysis buffer (20mM Tris, 150mM NaCl, 50mM NaF, 1% NP-40 substitute, 1% aprotinin, 1mM PMSF, 1mM Na3VO4). For cell fractionation experiments, EBs were collected, washed with ice-cold PBS and prepared essentially as described.23 Coimmunoprecipitation was carried out using whole-cell lysates precleared with rabbit IgG (Jackson ImmunoResearch Laboratories) and protein A + G agarose beads (Santa Cruz Biotechnology), incubated overnight with anti-SMAD2 XP Ab (Cell Signaling) in a 1:50 dilution. Proteins were resolved by electrophoresis on pre-cast 10% NuPage Bis-Tris gels and transferred to nitrocellulose membranes using the iBlot system (both from Invitrogen). Membranes were blocked in 5% BSA in TBS + 0.5% Tween-20 and probed overnight with the following Abs: rabbit anti-SMAD1, anti-SMAD2/3, or anti-SMAD4 (Cell Signaling 9743, 3102, and 9515, respectively), mouse anti-SMAD5 (Invitrogen 39-5700), or mouse anti-γ-tubulin (Sigma-Aldrich). Loading of nuclear and cytoplasmic protein fractions was visualized by probing with Abs for histone deacetylase-1 (Cell Signaling 2062, rabbit Ab) and β-actin (Sigma-Aldrich, mouse mAb). Proteins were visualized with HRP-tagged secondary Abs (Bio-Rad or GE Healthcare) and West Pico chemiluminescence reagent (Pierce). Images were obtained and analyzed by densitometry to measure relative protein levels on a UVP Biospectrum 500 Imaging System.
Flow cytometry
Control untreated and doxycycline-induced EBs were collected between days 3 and 6 of growth in differentiation medium by low-speed centrifugation, washed in PBS, and disaggregated by trypsinization. Cells were resuspended in ice-cold FACS buffer (PBS + 1% FCS + 1mM EDTA), and incubated with Abs for the relevant cell-surface proteins in V-bottom plates on ice. Cells (2 × 105) were stained with the following fluorophore-conjugated Abs and reagents: anti-c-KIT-APC (eBioscience 17-1171-83), anti-DPP4-APC (R&D Systems FAB9541A), anti-epCAM-PE (eBioscience 12-5791-81), or anti-FLK1-PE (BD PharMingen 555308). Controls included unstained and single Ab-stained samples. Individual live cells were gated by forward and side scatter, and 10 000 events were recorded and analyzed per sample using an Accuri C6 flow cytometer and De Novo FCS Express version 4 software. For some experiments, disaggregated day 4 EBs were stained in complete EB medium + 2mM EDTA with a 1:100 dilution of anti-FLK1-PE, sorted using a FACSVantage SE (BD Biosciences), and recultured in a 1:1 ratio with cells derived from disaggregated day 2 AinV18 EBs with or without doxycycline, and subsequently analyzed by colony assays.
Results
Generation of a novel ES-cell line with the capacity for conditional depletion of Smad1
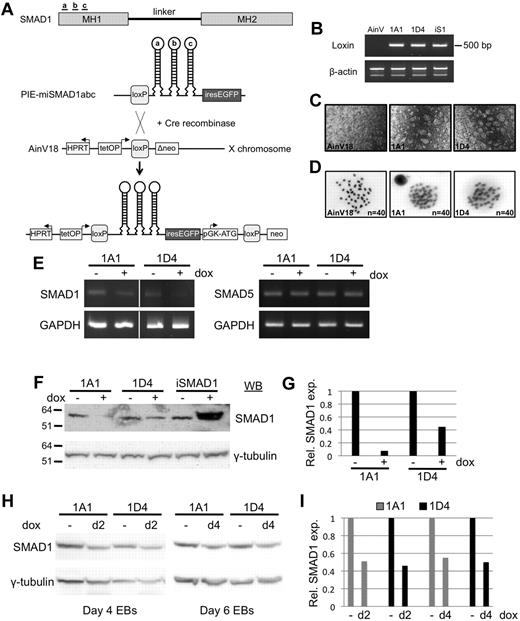
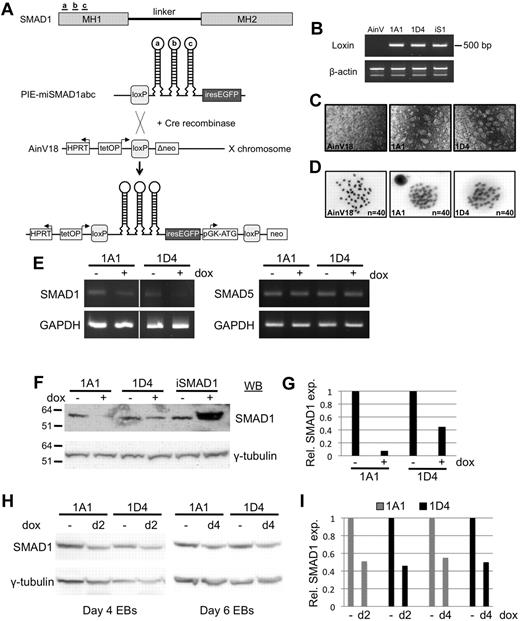
A system based on AinV murine ES cells has been described by Daley, Kyba, and colleagues for inducible control of transgene expression.19,24 The AinV parental cell line has the coding sequences for the reverse tet-transactivator (rtTA) pre-targeted at the constitutively active Rosa26 locus, the rtTA operator site inserted near the HPRT locus, and just downstream of the operator site is a loxP site and a defective neomycin phosphotransferase gene. We adapted this system for the conditional expression of tandem shRNA hairpin modules specific for Smad1. The modules contain flanking 5′ and 3′ sequence from the mouse miR-30 gene that promote processing of hairpins by the intrinsic cellular RNA interference apparatus to generate functional siRNA molecules.20 Three separate hairpins were designed to target the Smad1 MH1 domain and cloned in tandem into the plox-IRES-EGFP vector using restriction site adapters, as shown in Figure 1A. The complete 3-hairpin construct, miSmad1abc-IRES-EGFP, was coelectroporated with a vector expressing Cre recombinase into the parental AinV18 ES-cell line. Cre/loxP-mediated homologous recombination was selected using G418 by restoration of the neoR gene, and resulted in a single, site-specific insertion of the tet-inducible shRNA cassette into the X chromosome with IRES-driven EGFP as a reporter (Figure 1A).
Generation of inducible ES cells allowing temporal control of Smad1 depletion. (A) miSmad1abc-IRES-EGFP ES cells were generated by combining the transgenic technique reported by Kyba et al (2002)24 with the miR-30–based shRNA technique reported by Sun et al (2006).20 Three unique shRNA target sites were identified in the MH1 domain of Smad1, and hairpins were designed containing the miR-30 flanking sequence required for endogenous RISC processing. Cre/loxP-mediated recombination of a construct containing hairpins a, b, and c in tandem results in site-specific integration into a position downstream of the HPRT locus on the X chromosome, and confers neomycin resistance, allowing selection and isolation of transgenic cell clones amenable to tet-on induction of Smad1 knockdown, concomitant with GFP expression. (B) Genomic PCR analysis confirms single-copy site-specific integration of the shRNA-IRES-EGFP cassette. Two separate transgenic ES clonal cell lines are shown, 1A1 and 1D4. The parental AinV18 line serves as a negative control, and a transgenic iSmad1 clone that allows induced Smad1 expression16 provides a positive control. (C) GFP expression is seen in 1A1 and 1D4 ES cells 24 hours after induction of miSmad1abc hairpin expression, with parental AinV18 cells serving as negative control. (D) Analysis of metaphase karyotypes in the ES-cell lines, showing grossly normal chromosomal integrity in both clones, indistinguishable from parental AinV18 cells. (E) Representative RT-PCR analysis of Smad1 transcript levels in 2 clonal lines 30 hours after induction, showing a decrease in Smad1 transcript levels after addition of doxycycline, in contrast to unchanged levels of Smad5 transcripts, with Gapdh as a loading control. (F) Analysis of SMAD1 protein levels 30 hours after induction in 2 separate transgenic clonal lines by Western blotting. Overexpression of Smad1 using iSmad1 ES cells is used as a control to verify SMAD1 signal. (G) Densitometric analysis of SMAD1 protein levels normalized to the γ-tubulin loading control, demonstrating in a representative experiment the extensive knockdown (∼ 60%-90%) in both cell lines. (H) Representative Western blotting analysis of SMAD1 protein levels 48 hours after induction in embryoid bodies using 2 independent clonal cell lines (I) Densitometric quantification of Smad1 protein levels in a representative experiment, normalized to γ-tubulin controls, demonstrating approximately equivalent knockdown in 1A1 and 1D4 cell clones, with induction at either day 2 or day 4.
Generation of inducible ES cells allowing temporal control of Smad1 depletion. (A) miSmad1abc-IRES-EGFP ES cells were generated by combining the transgenic technique reported by Kyba et al (2002)24 with the miR-30–based shRNA technique reported by Sun et al (2006).20 Three unique shRNA target sites were identified in the MH1 domain of Smad1, and hairpins were designed containing the miR-30 flanking sequence required for endogenous RISC processing. Cre/loxP-mediated recombination of a construct containing hairpins a, b, and c in tandem results in site-specific integration into a position downstream of the HPRT locus on the X chromosome, and confers neomycin resistance, allowing selection and isolation of transgenic cell clones amenable to tet-on induction of Smad1 knockdown, concomitant with GFP expression. (B) Genomic PCR analysis confirms single-copy site-specific integration of the shRNA-IRES-EGFP cassette. Two separate transgenic ES clonal cell lines are shown, 1A1 and 1D4. The parental AinV18 line serves as a negative control, and a transgenic iSmad1 clone that allows induced Smad1 expression16 provides a positive control. (C) GFP expression is seen in 1A1 and 1D4 ES cells 24 hours after induction of miSmad1abc hairpin expression, with parental AinV18 cells serving as negative control. (D) Analysis of metaphase karyotypes in the ES-cell lines, showing grossly normal chromosomal integrity in both clones, indistinguishable from parental AinV18 cells. (E) Representative RT-PCR analysis of Smad1 transcript levels in 2 clonal lines 30 hours after induction, showing a decrease in Smad1 transcript levels after addition of doxycycline, in contrast to unchanged levels of Smad5 transcripts, with Gapdh as a loading control. (F) Analysis of SMAD1 protein levels 30 hours after induction in 2 separate transgenic clonal lines by Western blotting. Overexpression of Smad1 using iSmad1 ES cells is used as a control to verify SMAD1 signal. (G) Densitometric analysis of SMAD1 protein levels normalized to the γ-tubulin loading control, demonstrating in a representative experiment the extensive knockdown (∼ 60%-90%) in both cell lines. (H) Representative Western blotting analysis of SMAD1 protein levels 48 hours after induction in embryoid bodies using 2 independent clonal cell lines (I) Densitometric quantification of Smad1 protein levels in a representative experiment, normalized to γ-tubulin controls, demonstrating approximately equivalent knockdown in 1A1 and 1D4 cell clones, with induction at either day 2 or day 4.
We isolated multiple G418-resistant ES-cell clones (miSmad1ES), and examined them for site-specific integration of Smad1 shRNAs (Figure 1B) and doxycycline-induced GFP expression (Figure 1C). Next, we evaluated genome integrity using chromosome spreads to assess ploidy in the cell lines. In both miSmad1 lines tested, chromosome number did not deviate from the expected 40, and karyotypes were indistinguishable on a gross morphologic level compared with the AinV18 parental cells (Figure 1D). Data from 2 representative independent clones, 1A1 and 1D4, are shown in Figure 1 for purposes of ES-cell line validation. All following experiments were performed using both lines, which were found to be phenotypically similar, although only data for clone 1D4 is shown in subsequent figures. At 30 hours after addition of 1 μg/mL doxycycline to miSmad1ES-cell cultures, Smad1 RNA levels were clearly reduced according to semiquantitative RT-PCR analysis, while Smad5 transcript levels remained constant (Figure 1E). Likewise, 30 hours after shRNA induction, SMAD1 protein levels were decreased in both ES-cell clones, determined by Western blotting experiments (Figure 1F). As a control, the previous line we had generated for induced expression of Smad1 (iSmad1ES19 ) was included in this analysis, further validating that the depletion using the miSmad1 lines was because of shRNA expression rather than an indirect effect of doxycycline or some other component in the system. The graph in Figure 1G shows densitometric analysis of a representative experiment, with Smad1 expression normalized to the γ-tubulin loading control, documenting an approximately 90% depletion of Smad1 protein in clone 1A1 and 60% in clone 1D4. In multiple independent experiments miSmad1 lines were depleted of SMAD1 on induction within this typical range. Induction at day 2 or day 4 during EB differentiation resulted in approximately equivalent SMAD1 knockdown in cell clones 1A1 and 1D4 (Figure 1H and 1I). Therefore, while the lines do not eliminate SMAD1, induction of shRNAs reliably depleted SMAD1.
Inducible knockdown of Smad1 before or after the hemangioblast stage of EB development has opposite effects on primitive erythroid progenitors
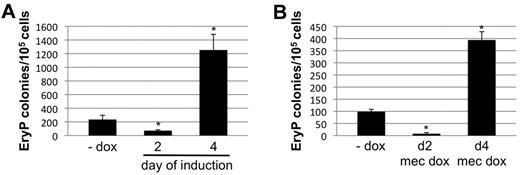
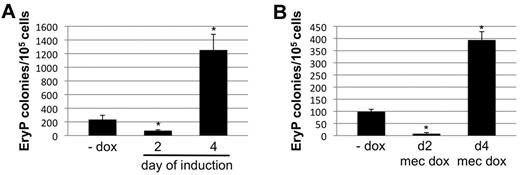
To study the contribution of Smad1 signaling to embryonic hematopoiesis, we used conditional depletion of Smad1 in the embryoid body system. Differentiating EBs recapitulate germ layer commitment in vitro, and disaggregation of EBs followed by reseeding in semisolid media with the relevant cytokines facilitates a quantitative assessment for progenitor numbers associated with primitive and early definitive hematopoiesis.25 We showed previously that forced expression of Smad1 during a limited developmental window around day 2, before hemangioblast formation, expands the progenitor population committed to hemato-vascular development.19 In contrast, when Smad1 knockdown was induced using the miSmad1 lines beginning at day 2 of EB differentiation, there was a major reduction in primitive erythroid (EryP) colony formation (Figure 2A). Conversely, when Smad1 was depleted beginning on EB day 4, subsequent to progenitor commitment, there was an approximately 5-fold increase in the number of EryP colonies compared with uninduced controls (Figure 2A). This biphasic control of primitive hematopoiesis by Smad1 is consistent with our previous published results,19 which suggested that Smad1 expression after the early limited pulse abrogates progenitor expansion. To rule out potential confounding effects of doxycycline on cellular differentiation, Smad1 knockdown was again induced on day 2 or 4 of EB differentiation, and doxycycline was also added directly to the erythroid-permissive methylcellulose differentiation medium. As shown in Figure 2B, the early suppression and late expansion of EryP potential was equivalent in terms of relative differences compared with uninduced controls, although the total number of EryP colonies was modestly reduced. These results demonstrate temporally separable and opposite functions for Smad1 in primitive erythropoiesis.
Early or late depletion of Smad1 has opposite effects on primitive erythroid potential. (A) The primitive EryP colony-forming potential was analyzed for EBs derived from a representative miSmad1 ES-cell clone. Smad1 knockdown was induced by 1 μg/mL doxycycline treatment starting either 2 or 4 days after initiation of growth in EB culture medium permissive for differentiation. The EBs were harvested at day 5.75 and recultured in 2 U/mL EPO in semisolid methylcellulose medium. Erythroid colonies were counted 4 days later, and the EryP potential of induced cells was compared with untreated cells. Results are shown as total mean colony number counted in each experimental condition, and each graph is a representative result from a pool of at least 4 independent experiments, each done in triplicate. (B) The EryP colony assays were repeated with the addition of doxycycline to the methylcellulose, to rule out potential confounding effects of doxycycline on cell differentiation.
Early or late depletion of Smad1 has opposite effects on primitive erythroid potential. (A) The primitive EryP colony-forming potential was analyzed for EBs derived from a representative miSmad1 ES-cell clone. Smad1 knockdown was induced by 1 μg/mL doxycycline treatment starting either 2 or 4 days after initiation of growth in EB culture medium permissive for differentiation. The EBs were harvested at day 5.75 and recultured in 2 U/mL EPO in semisolid methylcellulose medium. Erythroid colonies were counted 4 days later, and the EryP potential of induced cells was compared with untreated cells. Results are shown as total mean colony number counted in each experimental condition, and each graph is a representative result from a pool of at least 4 independent experiments, each done in triplicate. (B) The EryP colony assays were repeated with the addition of doxycycline to the methylcellulose, to rule out potential confounding effects of doxycycline on cell differentiation.
Early depletion of Smad1 impairs hematopoietic potential by affecting commitment of epiblast to derivatives
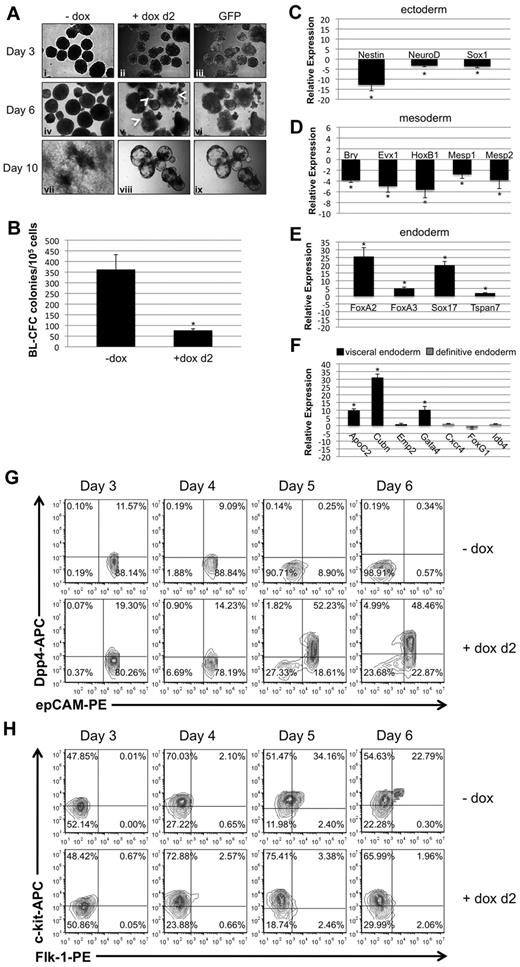
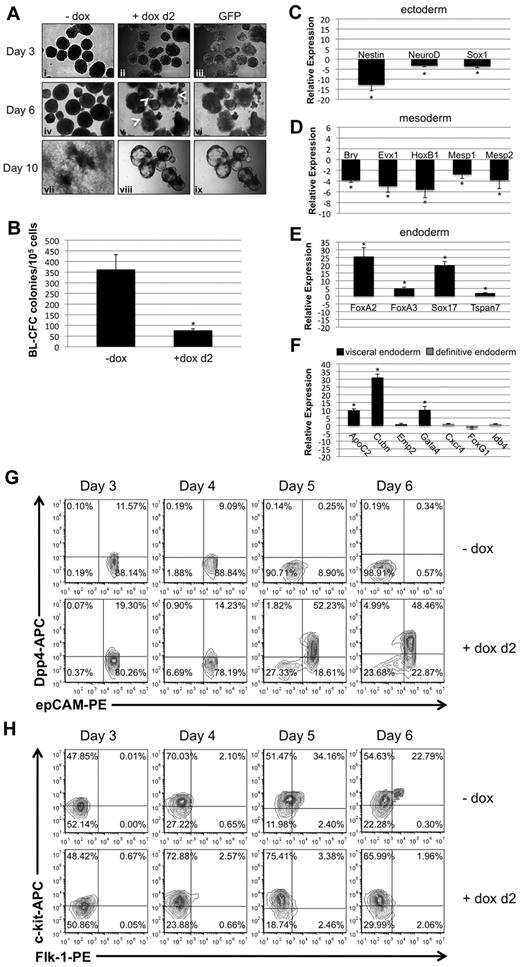
To investigate whether the phenotypes caused by early or late depletion of Smad1 during EB differentiation differ mechanistically, EB morphology and gene expression patterns were evaluated comparatively. Figure 3A shows a panel of miSmad1 EBs on successive days of differentiation, either uninduced or after induction on day 2. Day 2–induced EBs expressed GFP throughout EB differentiation, initially ubiquitously but less so at later time points. The uninduced EBs displayed a characteristic round morphology with darker regions toward the center, indicative of developing mesoderm and its derivatives. In contrast, day 2–induced EBs formed vesiculated protrusions by day 4, which continued to emerge at the EB periphery through day 6 (see arrowheads Figure 3Av), by which time the EBs had become entirely cystic. Unlike control EBs, when these induced EBs were replated onto gelatinized dishes at day 7, they failed to adhere and flatten on the plate surface by day 10, as indicated in Figure 3Aviii and ix. Also unlike controls, they failed to exhibit any beating morphologies indicative of cardiac derivatives (data not shown). Because depletion of Smad1 at day 2 of EB development ostensibly precedes commitment of epiblast to mesoderm and its derivative hemato-vascular progenitors,26 we analyzed the hemangioblast analog BL-CFC colony-forming potential of day 2–induced EBs. As shown in Figure 3B, there was an approximately 5-fold reduction in BL-CFC potential, roughly equivalent to that observed for EryP potential (see Figure 2A).
Early depletion of Smad1 results in disruption of epiblast derivatives and commitment to visceral endoderm. (A) Smad1 knockdown was induced on day 2 of EB differentiation, and EBs were analyzed for morphologic changes and GFP expression over the following several days. Induction was maintained by addition of doxycycline at day 5; day 7 EBs were transferred to gelatinized tissue-culture plates and examined both for their morphology and their ability to adhere to the plate surface. (B) Day 2–induced EBs were harvested at day 3.75 of differentiation and cultured in methylcellulose medium with cytokines permissive for differentiation into BL-CFC colonies. Colonies were identified and counted by their distinctive morphology 4 days later. For each sample, n = 3 and the graph is a representative example taken from a pool of at least 4 experiments. Error bars indicate SEM; *P < .01 compared with uninduced controls. Transcription of genes associated with ectoderm (C), mesoderm (D), endoderm (E), and, more selectively, visceral or definitive endoderm (F) were analyzed by qPCR in day 4.5 EBs that had been induced at day 2. Data were analyzed using the 2−ΔΔCT method19 and reported as fold change in mRNA transcript level, and were normalized to control cells that were not treated with doxycycline. Gapdh transcript levels were used as a reference control, and shown are representative results from an experiment that was repeated at least 3 times. Error bars indicate SEM; *P < .01 compared with uninduced controls. (G) Visceral endoderm commitment in day 2–induced EBs was corroborated by flow cytometric analysis after staining for epCAM (pan-endoderm) and DPP4 (visceral endoderm) -specific fluorophore-conjugated Abs using cells from dissociated EBs over a 4-day time course from day 3 to day 6 of EB cultures. (H) FLK1 and c-KIT staining was analyzed from day 3 to day 6 by flow cytometry to measure the potential population of hemato-vascular precursors in EBs induced at day 2, compared with control uninduced samples.
Early depletion of Smad1 results in disruption of epiblast derivatives and commitment to visceral endoderm. (A) Smad1 knockdown was induced on day 2 of EB differentiation, and EBs were analyzed for morphologic changes and GFP expression over the following several days. Induction was maintained by addition of doxycycline at day 5; day 7 EBs were transferred to gelatinized tissue-culture plates and examined both for their morphology and their ability to adhere to the plate surface. (B) Day 2–induced EBs were harvested at day 3.75 of differentiation and cultured in methylcellulose medium with cytokines permissive for differentiation into BL-CFC colonies. Colonies were identified and counted by their distinctive morphology 4 days later. For each sample, n = 3 and the graph is a representative example taken from a pool of at least 4 experiments. Error bars indicate SEM; *P < .01 compared with uninduced controls. Transcription of genes associated with ectoderm (C), mesoderm (D), endoderm (E), and, more selectively, visceral or definitive endoderm (F) were analyzed by qPCR in day 4.5 EBs that had been induced at day 2. Data were analyzed using the 2−ΔΔCT method19 and reported as fold change in mRNA transcript level, and were normalized to control cells that were not treated with doxycycline. Gapdh transcript levels were used as a reference control, and shown are representative results from an experiment that was repeated at least 3 times. Error bars indicate SEM; *P < .01 compared with uninduced controls. (G) Visceral endoderm commitment in day 2–induced EBs was corroborated by flow cytometric analysis after staining for epCAM (pan-endoderm) and DPP4 (visceral endoderm) -specific fluorophore-conjugated Abs using cells from dissociated EBs over a 4-day time course from day 3 to day 6 of EB cultures. (H) FLK1 and c-KIT staining was analyzed from day 3 to day 6 by flow cytometry to measure the potential population of hemato-vascular precursors in EBs induced at day 2, compared with control uninduced samples.
These observations prompted us to investigate possible effects on germ layer development in Smad1-depleted EBs. Therefore, the expression of representative molecular markers was evaluated by quantitative real-time PCR (qPCR). For this purpose, EBs were induced for Smad1 knockdown at day 2, and RNA was harvested at day 4.5 of EB differentiation. Compared with uninduced EBs, there was a statistically significant decrease in expression levels for ectoderm markers Nestin, NeuroD, and Sox1 (Figure 3C), and mesoderm markers including Brachyury, Evx1, HoxB1, Mesp1, and Mesp2 (Figure 3D). In contrast, there was a marked increase in the expression levels for markers of endoderm generally (Figure 3E), and visceral endoderm specifically (Figure 3F). The pan-endodermal markers were all transcriptionally up-regulated compared with untreated controls, including FoxA2 (≥ 20-fold), FoxA3 (≥ 5-fold), Sox17 (> 15-fold), and Tspan7 (> 3-fold). While many genes are coexpressed in both primitive and definitive endoderm, several markers have been shown to discriminate endoderm types27-29 and we found that expression levels were enhanced particularly for genes associated with visceral endoderm. Transcript levels for apolipoprotein C2 (ApoC2; > 8-fold), Cubilin (Cubn; > 25-fold), and Gata4 (7-fold) were all considerably increased, while transcripts associated with definitive endoderm development, including Cxcr4, Idb4, and FoxG1, were not changed significantly (Figure 3F). Flow cytometry was used to analyze quantitatively cells that express epithelial cell adhesion molecule (epCAM) and dipeptidyl peptidase IV (DPP4), pan-endoderm and visceral endoderm markers, respectively.27 As shown in Figure 3G, uninduced control EBs were depleted of epCAM+ cells by days 5-6 in culture. In contrast, Smad1-depleted EBs retained an epCAM+ population, a significant number (∼ 50%) of which were epCAM+/DPP4+ double-positive cells.
Flow cytometry was also used to compare hemato-vascular development in control or day 2-induced EBs, by quantifying the emergence of FLK1+/c-KIT+ double-positive progenitors. In uninduced miSmad1 control EBs, FLK1+ cells began to accumulate on day 4 of differentiation, and by day 5, there was a significant population of clearly defined FLK1+/c-KIT+ double-positive cells (Figure 3H). In contrast, EBs induced for Smad1 knockdown on day 2 exhibited severely diminished numbers of FLK1+ cells (∼ 6% on day 5, Figure 3H), and a striking absence of double-positive cells even by day 6 (∼ 2%). Overall, the data show that Smad1 is essential from day 2 of EB differentiation for the commitment of epiblast to all 3 embryonic germ layers, and that Smad1 depletion results in formation of predominantly visceral-like endoderm at their expense.
Depletion of Smad1 after progenitor commitment increases hematopoietic potential through up-regulation of specific regulatory factors, including Gata1
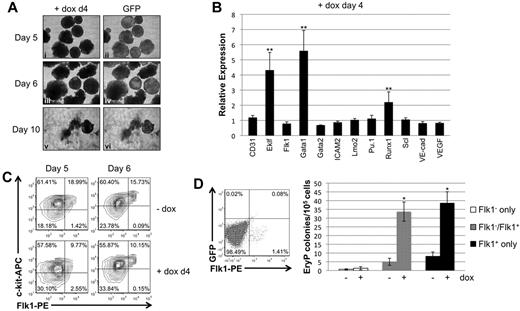
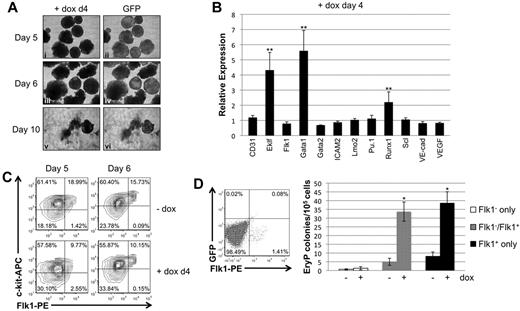
EB morphology and changes in gene expression were next evaluated after induction of Smad1 knockdown starting at day 4 of EB differentiation, at a time subsequent to specification of the hemato-vascular lineage. When induced on day 4 of differentiation, EBs were generated that on subsequent days were qualitatively similar to uninduced control EBs (Figure 4A). Moreover, day 4-induced EBs that were replated on gelatinized plates at day 7 generally adhered to the surface, although with some morphologic heterogeneity compared with control samples.
Late depletion of Smad1 enhances erythroid potential correlating with enhanced expression levels for Gata1. (A) EBs induced on day 4 of differentiation examined for morphologic changes and GFP expression until day 6. EBs were transferred on day 7 to gelatinized tissue-culture plates and analyzed for their ability to adhere to the plate surface. (B) Hemangioblast marker gene expression was analyzed by qPCR; RNA from day 4–induced EBs was harvested after 24 hours, on day 5 of differentiation. Data were collected and analyzed using Gapdh as a reference control as in Figure 3B-E. Each experimental condition was repeated a minimum of 3 times and a representative result is shown. Error bars denote the SEM; **P < .01 compared with uninduced controls. (C) Flow cytometric analysis of FLK1/c-KIT expression at days 5 or 6 of EB differentiation, after induction of Smad1 depletion at day 4. (D) EryP colony-forming analysis of FLK1+ mesoderm sorted by flow cytometry from day 3.75 EBs to examine cell autonomy of Smad1 functions. Defined populations were recultured as EBs for a further 2 days with or without day 4 doxycycline treatment, and disaggregated to generate primitive erythroid colonies under the relevant permissive conditions. Each experimental condition was repeated 3 times, and a representative result is shown. Error bars denote SEM; *P < .05 compared with uninduced controls.
Late depletion of Smad1 enhances erythroid potential correlating with enhanced expression levels for Gata1. (A) EBs induced on day 4 of differentiation examined for morphologic changes and GFP expression until day 6. EBs were transferred on day 7 to gelatinized tissue-culture plates and analyzed for their ability to adhere to the plate surface. (B) Hemangioblast marker gene expression was analyzed by qPCR; RNA from day 4–induced EBs was harvested after 24 hours, on day 5 of differentiation. Data were collected and analyzed using Gapdh as a reference control as in Figure 3B-E. Each experimental condition was repeated a minimum of 3 times and a representative result is shown. Error bars denote the SEM; **P < .01 compared with uninduced controls. (C) Flow cytometric analysis of FLK1/c-KIT expression at days 5 or 6 of EB differentiation, after induction of Smad1 depletion at day 4. (D) EryP colony-forming analysis of FLK1+ mesoderm sorted by flow cytometry from day 3.75 EBs to examine cell autonomy of Smad1 functions. Defined populations were recultured as EBs for a further 2 days with or without day 4 doxycycline treatment, and disaggregated to generate primitive erythroid colonies under the relevant permissive conditions. Each experimental condition was repeated 3 times, and a representative result is shown. Error bars denote SEM; *P < .05 compared with uninduced controls.
To investigate changes in the transcriptional program in response to late depletion of Smad1, the expression levels of transcripts associated with the hemangioblast or hematopoietic progenitors were evaluated by qPCR. When Smad1 knockdown was induced at day 4 there was already by day 5 more than a 5-fold increase in transcript levels for Gata1 (Figure 4B). In addition, transcript levels for the hematopoietic genes Eklf and Runx1 were significantly increased compared with uninduced controls. In contrast, transcript levels for hemangioblast-associated genes, including Flk1, Gata2, Lmo2, Scl, and Vegf, were unaffected or slightly decreased (Figure 4B). These results suggest that inhibitory effects of Smad1 on hematopoietic potential function at least in part through restricting levels of transcriptional activators including Gata1, and are consistent with an effect on committed hematopoietic progenitors rather than earlier mesoderm or hemangioblast cells. When depletion was induced at day 4, Flk1 expression levels persisted through days 5-6, as did the FLK1+/c-KIT+ double-positive population (Figure 4C). To probe whether Smad1 function is cell autonomous for hematopoietic progenitors, the initial wave of FLK1+ cells was isolated from day 4 EBs by cell sorting, and then recultured separately or after recombination with FLK− cells, under conditions of doxycycline-induced Smad1 depletion, and analyzed for erythroid progenitor potential. As shown in Figure 4D, induction of Smad1 knockdown specifically in this initial wave of FLK1+ hemato-vascular precursors is fully sufficient to expand erythroid progenitors, consistent with a cell-autonomous function for Smad1, or at least function within the FLK1+ progenitor population.
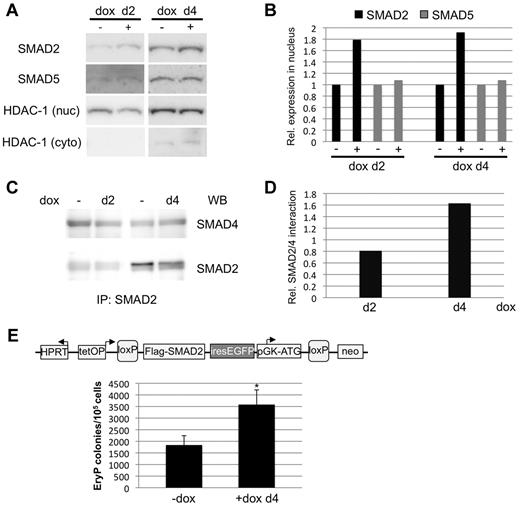
SMAD2 provides a mechanistic link between the BMP and TGF-β pathways in the context of Smad1 knockdown
BMP-responsive SMADs and TGF-β–responsive SMADs all function via the common co-SMAD, SMAD4. Therefore, it is possible for SMADs to compete for activity with each other, if SMAD4 levels are limiting. In the context of the EBs derived from miSmad1ES cells, depletion of Smad1 could result indirectly in enhanced activity of Smad5, or even of Smad2/3, the latter of which would imply a shift to a predominantly TGF-β–like activated pathway. To evaluate this possibility, the relative levels of nuclear SMAD2/3 (Abs do not distinguish) and SMAD5 were measured after depletion of Smad1 for 24 hpf. As shown in Figure 5A and B, levels of nuclear SMAD2 increased approximately 2-fold, while nuclear SMAD5 levels remained unchanged. Although nuclear SMAD2 increased on either day 2 or day 4 induction of Smad1 knockdown, SMAD2/SMAD4 complexes increased only with day 4 induction, which is the timing relevant to the observed hematopoietic increase (Figure 5C-D). To test directly if Smad2/3 activity can enhance hematopoietic fate, in the absence of Smad1 depletion, we generated a new ES-cell line, with Smad2 placed under doxycycline control (iSmad2ES cells, supplemental Figure 1). In EBs derived from these iSmad2ES cells, induction of SMAD2 expression on day 4 resulted in an approximately 2-fold expansion of EryP colonies (Figure 5E). These results are consistent with the hypothesis that depletion of Smad1 leads to enhanced SMAD2 activity, which impacts hematopoietic progenitor fate.
Smad1 knockdown correlates with activation of SMAD2, a TGF-β downstream effector. (A) miSmad1 EBs were left untreated or induced with 1 μg/mL doxycycline to deplete Smad1 on day 2 or day 4, and EBs were harvested and fractionated to generate nuclear extracts. Nuclear fractions were subjected to Western blotting to quantify SMAD2 and SMAD5 levels, and HDAC-1 was used as a loading control for total nuclear protein. (B) Densitometric analysis of relative nuclear SMAD2 and SMAD5 protein levels, with induced samples normalized to uninduced controls. (C) Whole-cell lysates were generated from day 2– or day 4–induced miSmad1 EBs and subjected to immunoprecipitation using a SMAD2-specific Ab. Immunoprecipitates were analyzed by Western blotting for SMAD2 and SMAD4. (D) Densitometric quantification of SMAD2/SMAD4 interaction, with day 2– and day 4–induced samples normalized to their respective uninduced controls. (E) An ES-cell line was engineered to allow doxycycline-induced expression of Smad2 (see supplemental Figure 1). Smad2 expression was induced on day 4 and on day 5.75 the EBs were analyzed for EryP colony-forming potential, as in Figure 2. For each sample, n = 3, and the graph is a representative example taken from a pool of at least 4 experiments. Error bars indicate SEM; *P < .01 compared with uninduced controls.
Smad1 knockdown correlates with activation of SMAD2, a TGF-β downstream effector. (A) miSmad1 EBs were left untreated or induced with 1 μg/mL doxycycline to deplete Smad1 on day 2 or day 4, and EBs were harvested and fractionated to generate nuclear extracts. Nuclear fractions were subjected to Western blotting to quantify SMAD2 and SMAD5 levels, and HDAC-1 was used as a loading control for total nuclear protein. (B) Densitometric analysis of relative nuclear SMAD2 and SMAD5 protein levels, with induced samples normalized to uninduced controls. (C) Whole-cell lysates were generated from day 2– or day 4–induced miSmad1 EBs and subjected to immunoprecipitation using a SMAD2-specific Ab. Immunoprecipitates were analyzed by Western blotting for SMAD2 and SMAD4. (D) Densitometric quantification of SMAD2/SMAD4 interaction, with day 2– and day 4–induced samples normalized to their respective uninduced controls. (E) An ES-cell line was engineered to allow doxycycline-induced expression of Smad2 (see supplemental Figure 1). Smad2 expression was induced on day 4 and on day 5.75 the EBs were analyzed for EryP colony-forming potential, as in Figure 2. For each sample, n = 3, and the graph is a representative example taken from a pool of at least 4 experiments. Error bars indicate SEM; *P < .01 compared with uninduced controls.
Knockdown of Smad1 after hematopoietic commitment expands progenitors for other lineages, including definitive erythroid and multipotent progenitors
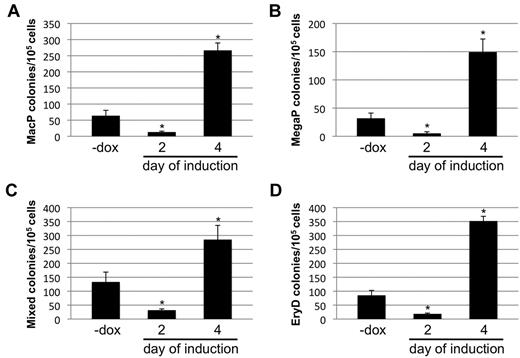
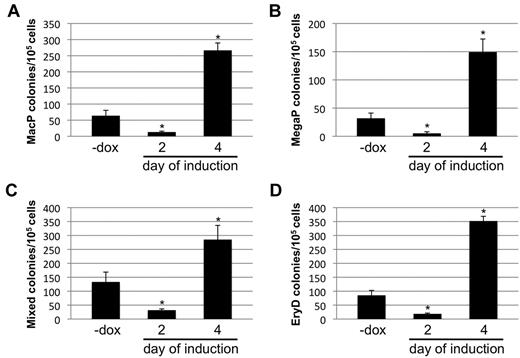
Finally, the ability of Smad1 levels to regulate the development of other hematopoietic progenitors was tested. The potential of day 2– and day 4–induced EBs to form colonies was quantitatively evaluated for progenitors to macrophages (MacP) and megakaryocytes (MegaP), as well as to definitive erythroid (EryD) and multilineage mixed colonies consisting of macrophages, megakaryocytes, erythroid cells, and granulocytes. Hematopoietic colonies were identified and scored using the morphologic criteria established by Kennedy and Keller.21 As expected, based on the effects on mesoderm development, induction of Smad1 knockdown on day 2 of EB development led to a consistent reduction in formation of all hematopoietic colonies compared with uninduced controls (Figure 6). Conversely, when EBs were induced at day 4, there was an increase in progenitor colony potential compared with uninduced controls (Figure 6A-B), regardless of the lineage. When Smad1 was depleted starting at day 4, the potential for MacP, MegaP, and EryD colony generation was enhanced approximately 5-fold, while the numbers of mixed lineage colonies showed a slightly more modest increase of approximately 3-fold (Figure 6).
The biphasic effects of Smad1 on EB-derived EryP potential extend to other hematopoietic progenitors. The miSmad1 EBs were left untreated or induced with doxycycline on day 2 or day 4, and day 5.75 EBs were harvested and recultured in semisolid methylcellulose media permissive for differentiation into (A) macrophage (MacP) colonies, (B) megakaryocyte (MegaP) colonies, (C) definitive mixed lineage colonies, or (D) EryD definitive erythroid colonies. After replating dissociated EBs in media containing the relevant permissive cytokines, MacP, MegaP, and EryD colonies were identified by their distinctive morphologies and counted on day 7; mixed lineage colonies were counted on day 9. For each sample, n = 3, and each graph is a representative example taken from a pool of at least 4 experiments. Error bars indicate SEM; *P < .01 compared with uninduced controls.
The biphasic effects of Smad1 on EB-derived EryP potential extend to other hematopoietic progenitors. The miSmad1 EBs were left untreated or induced with doxycycline on day 2 or day 4, and day 5.75 EBs were harvested and recultured in semisolid methylcellulose media permissive for differentiation into (A) macrophage (MacP) colonies, (B) megakaryocyte (MegaP) colonies, (C) definitive mixed lineage colonies, or (D) EryD definitive erythroid colonies. After replating dissociated EBs in media containing the relevant permissive cytokines, MacP, MegaP, and EryD colonies were identified by their distinctive morphologies and counted on day 7; mixed lineage colonies were counted on day 9. For each sample, n = 3, and each graph is a representative example taken from a pool of at least 4 experiments. Error bars indicate SEM; *P < .01 compared with uninduced controls.
Discussion
We developed a conditional shRNA-mediated knockdown system in ES cells to study the role of Smad1 in hematopoietic development throughout EB differentiation. The system allows us to study in vitro the function of a gene knockdown that is otherwise early embryonic lethal. It also provides an advantage compared with using extracellular inhibitors of BMP signaling, such as NOGGIN, because considerable regulatory potential may be revealed by distinct pathway-restricted SMAD genes. Previously we used a complementary ES-cell line for temporally controlled Smad1 expression. While Smad1 was sufficient to promote hemangioblast and hematopoietic fate, this only occurred if the induction was transient; unless induction of Smad1 expression was arrested, the expansion failed to occur. This intriguing result led us to hypothesize that prehematopoietic mesoderm is receptive to Smad1-mediated expansion, while the pool of committed progenitors is, in contrast, restricted by Smad1. In the present study, we provide direct evidence for this hypothesis. Knockdown of Smad1 on day 2, before hemangioblast commitment,6 severely limits the potential for hematopoiesis; this phenotype is the opposite of that seen for Smad1 forced expression. This deficit is likely because of a limited pool of epiblast-derived mesoderm precursors, as shown by the decreased levels of transcripts for ectoderm and mesoderm markers. There is instead a marked shift in these EBs toward primitive endoderm character. The mouse Smad1 knockout shows defects in extra-embryonic development, including overgrowth of the posterior visceral endoderm.15 Thus, at least some aspects of the mouse mutant appear to be recapitulated in the miSmad1ES-cell model. The gain in primitive endoderm at the expense of mesoderm could be caused in part by deregulated cross-talk between BMP and TGF-β signaling pathways. We found in Western blotting experiments that depletion of SMAD1 leads to a corresponding increase in the levels of nuclear SMAD2. It is well established that Smad2-dependent Nodal/ActivinA signaling promotes endoderm fate in EB cultures,30 although this promotes definitive endoderm rather than the primitive endoderm type that predominates in the Smad1 knockdown cultures. Interestingly, we found a very similar phenotype, with directed differentiation of CXCR4-negative, DPP4+ primitive endoderm, when Gata4 is expressed in EBs, starting at day 2.28 Gata4 levels are enhanced in the Smad1 knockdown cultures. Therefore, Smad1 might function by restricting the Gata4-dependent derivation of primitive endoderm, allowing normal germ layer specification to proceed.
In contrast, induction of Smad1 knockdown after germ layer specification and hemangioblast commitment, but before hematopoietic differentiation, results in an expansion of hematopoietic potential. Notably, the level of this expansion (5-fold) is equivalent to the level that expansion is blocked by continuous forced expression of Smad1 during this same developmental period (5-fold). This effect is unrelated to development of hematopoietic mesoderm or the hemangioblast. First, the timing of the effect is consistent with expansion of already committed progenitors, and is cell-autonomous, because Smad1 depletion in FLK1+ precursors is sufficient to cause the observed relative increase in hematopoietic progenitors. Second, it correlates with a significant increase in expression levels of hematopoietic-specific genes Gata1, Eklf, and Runx1, but not other regulatory genes including Scl and Lmo2 that are more associated with the earlier hemato-vascular progenitors. Because activation of Gata1 is necessary for erythroid survival and differentiation, and it has been reported that SMAD5 specifically up-regulates expression of both Gata1 and Eklf31,32 , we tested whether the depletion of Smad1 causes enhanced activation of Smad5. However, in contrast to SMAD2, levels of nuclear SMAD5 are unchanged by Smad1 depletion. In any event, the discrepancy between expression of Gata1 and Runx1 versus Scl and Lmo2 suggests an uncoupling of the transcriptional network responsible for the initial commitment of the hemato-vascular lineage.
We report an approximately 50% reduction of SMAD1 in cultured EBs, but this is not comparable with a heterozygous mutant ESC line or mouse, because quantification is a sum average of the population of cells at one snap-shot timepoint. The knockdown will vary in different cells at different timepoints, and we likely reduce SMAD1 below a functional threshold in some (but perhaps not all) progenitors during the course of our knockdown experiments. In this context, the results we present may underestimate the function for SMAD1 because with a greater knockdown, the changes in developmental potential might be even more dramatic.
Although we focused on erythroid regulatory genes, progenitors for other lineages, including myeloid cells are similarly enhanced. Our limited analysis showed increased levels of some relevant regulatory genes (Runx1), but not others (such as Pu.1). This might simply be because of relatively low levels of myeloid progenitors in the EB cultures (increasing the noise to signal ratio in whole EB samples) or to differences in timing for when the progenitors expand. Our studies suggest that cross-talk between BMP and TGF-β–like signaling components may influence embryonic hematopoiesis. This could occur, as we suggest, by relatively direct signaling component cross-talk, or Smad2 activity could be enhanced indirectly by Smad1 depletion. It may be interesting to explore whether this has clinical relevance for BM hematopoiesis, given that constitutive activation of Smad2 is associated with ineffective hematopoiesis in myelodysplastic syndrome.33
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ting-Chun Liu for her valuable technical contributions.
This work was supported by National Institutes of Health (NIH) grant R37HL056182 (T.E.). B.D.C is supported by NIH training grant T32HL083824.
National Institutes of Health
Authorship
Contribution: B.D.C. conceived the study, carried out the experiments, and wrote the manuscript; S.L. carried out experiments and provided technical expertise; and T.E. conceived the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Todd Evans, Department of Surgery, Weill Cornell Medical College, 1300 York Ave, LC-709, New York, NY 10065; e-mail: tre2003@med.cornell.edu.