Abstract
Cytotoxic T lymphocytes (CTLs) suppress T cell responses directed against their antigens regardless of their own T cell receptor (TCR) specificity. This makes the use of CTLs promising for tolerance induction in autoimmunity and transplantation. It has been established that binding of the CTL CD8 molecule to the major histocompatibility complex (MHC) class I α3 domain of the recognizing T cell must be permitted for death of the latter cell to ensue. However, the signaling events triggered in the CTL by this molecular interaction in the absence of TCR recognition have never been clarified. Here we use single-cell imaging to study the events occurring in CTLs serving as targets for recognition by specific T cells. We demonstrate that CTLs actively respond to recognition by polarizing their cytotoxic granules to the contact area, releasing their lethal cargo, and vigorously proliferating. Using CTLs from perforin knockout (KO) mice and lymphocyte specific kinase (Lck) knockdown with specific small interfering RNA (siRNA), we show that the killing of the recognizing CD8 T cell is perforin dependent and is initiated by Lck signaling in the CTL. Collectively, these data suggest a novel mechanism in which the entire cascade generally triggered by TCR engagement is “hijacked” in CTLs serving as targets for T cell recognition without TCR ligation.
Introduction
CTLs recognize and kill target cells with marked specificity. This specificity is conferred on them by their TCR, which recognizes peptides in the context of target cell major histocompatibility complex class I (MHC-I).1-3 However, CTLs can induce death in a manner that does not involve their TCR. This occurs when CTLs suppress immune responses directed against their antigens in an activity coined “veto.”4 This type of CTL activity is also of a specific nature, as only T cells carrying TCRs capable of recognizing MHC-peptide (MHC-p) complexes displayed by the CTL are killed.4,5
This unique type of CTL activity has been heavily studied in the context of transplantation5-10 because CTLs can eliminate alloreactive T cells directed against them, and consequently against tissues carrying identical MHC-p complexes, without harming beneficial T cells directed against pathogens, thus inducing specific tolerance toward transplanted tissue.6,11 However, veto activity in CTLs is not necessarily limited to the allogeneic setting. Indeed, suppression of specific antipeptide responses by peptide-presenting CTLs has been demonstrated in the syngeneic setting.12 Thus, it has been suggested that CTLs may be important for maintaining self-tolerance by suppressing autoreactive T cell responses.8,12
In studies assaying this inhibitory CTL activity, it has been demonstrated that for killing to occur, the recognizing T cell must be allowed to contact the CTL.5,9,13-16 Uniquely, the CD8 molecule of the CTL must be allowed to engage nonpolymorphic residues of the α3 domain of the recognizing T cell MHC-I molecule.5,12,17 However, the direct consequence of this molecular engagement has remained unclear. It has been suggested that CD8 binding to the MHC-I α3 domain may elicit a signaling cascade in the recognizing T cell, culminating in its apoptosis.17 This was supported by the observation that apoptosis may be induced in splenocytes in the absence of CTLs by coapplication of antibodies to CD3 and their MHC-I α3 domain,17 allowing for the interpretation that the sole function of the CTL in this type of interaction is to present MHC-p and CD8 molecules to the recognizing T cell.
Conversely, it has been suggested that a signaling cascade in the CTL leading to an effector response might be initiated upon binding of the recognizing T cell to the CTL.11,18,19 This hypothesis is supported by the failure of cells lacking the CD8 cytoplasmic tail, yet expressing the transmembrane and extracellular domains, to eliminate alloreactive T cells in vivo.18 However, a direct link between CD8 engagement and CTL effector function has never been demonstrated in the context of this type of CTL activity. It has remained unclear whether a CTL operating in the absence of TCR specificity may undertake an active signaling-dependent role in the killing of recognizing T cells.
By directly targeting CTLs with TCR-transgeneic T cells in the absence of other cell populations, we were able to study events occurring in a CTL being recognized by a specific T cell. This approach allowed us to use single-cell imaging to visualize CTLs being targeted by specific T cells, revealing the cellular dynamics that occur between the two. Thus, we show that targeted CTLs respond actively by polarizing and secreting their cytotoxic granules, leading to the rapid lysis of the recognizing CD8+ T cell. In vivo, the ability of CTLs to suppress T cells directed against their antigens was dependent on this active granule-mediated response. Remarkably, CTL targeting induced in them a mitogenic signal leading to their increased survival and proliferation. The trigger for the targeted CTL response is shown to be ligation of the CTL CD8 molecule to the α3 domain of the recognizing cell MHC I, which induces Lck signaling, leading to cytotoxicity and mitogenic Erk phosphorylation.
Methods
Animals
FVB (H-2q), SJL (H-2s), DBA/2 (H-2d), CPt.C3-Faslgld (H-2d), and CB6 (H-2d/b) mice were obtained from The Jackson Laboratory. BALB/c (H-2d) perforin−/− mice were kindly provided by John T. Harty (University of Iowa). A breeding pair of 2C mice was kindly provided by Janko Nikolic-Zugic (Memorial Sloan-Kettering Cancer Center, NY). 2C lpr mice were derived as previously described.15 All mice were kept in small cages (5 animals in each cage) and fed sterile food and water. All studies were approved by the Weizmann Institute of Science Institutional Animal Care and Use Committee.
Antibodies
For imaging, anti–H-2Dd (BD Pharmingen) was conjugated to alexa 647 (Invitrogen). In addition, Alexa 647-anti–H-2Dd, alexa 488-anti–H-2Db (BioLegend), anti-perforin (clone PI-8, kindly provided by G. Berke of the Weizmann Institute), and anti–granzyme B (Santa Cruz Biotechnology) were used. The following materials were used for fluorescence-activated cell sorter (FACS) analysis: peridinin chlorophyll protein (PerCP)–anti-CD8α (Ly-2; BD Pharmingen); biotinylated 2C transgenic TCR 1B2 antibody (Ab; kindly provided by J. Nikolic-Zugic), stained with allophycocyanin (APC)–conjugated streptavidin (BD Pharmingen); Alexa-488 antiphospho-p44/42 mitogen-activated protein kinase (MAPK; [Erk1/2] [Thr202/Tyr204]; Clone E10; Cell Signaling); and antiphospho-Src (Tyr416; Cell Signaling Technology). For blocking CD8-MHC-Iα3 interactions, anti–H-2Db (clone 28-14-8, BD Pharmingen), which reacts with the H-2Db α3 domain,12 was used and controlled by an α2 domain-binding Ab (clone 28-8-6, BD Pharmingen). For lymphocyte function-associated antigen (LFA)–1 blocking experiments, anti–mouse CD11a (clone:M17/4, BioLegend) was used. For Western blotting, rabbit anti–mouse Lck antibody (no. 2752, Cell Signaling Technology) was used and controlled by mouse anti–mouse monoclonal anti–β-actin antibody (clone AC-74, Cell Signaling Technology).
Preparation of anti–third-party CTLs
Anti–third-party CTLs were prepared as previously described.6 For details, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Purification of 2C CD8 T cells
Spleens were obtained from 2C TCR transgenic mice aged 6 to 10 weeks. Red blood cells (RBCs) were lysed using cold ammonium-chloride potassium (ACK) buffer, and CD8 T cells were purified with BD IMag CD8 Magnetic Particles (BD Pharmingen).
Killing by targeted CTLs
Purified 2C CD8+ T cells were incubated with 2μM calcein-acetoxymethyl ester (AM; Invitrogen) in phosphate buffer solution (PBS) for 30 minutes at 37°C and washed twice with RPMI with 10% fetal calf serum. Anti–third-party CTLs were isolated from bulk culture and left untreated or treated with inhibitors (for details, see supplemental Methods). CTLs were then added at various ratios in triplicate to replicate 96-well flat-bottom plates (Falcon) containing 1.5 × 105 cells per well of calcein-loaded 2C CD8,+ incubated for 0 to 20 hours at 37°C and 5% CO2, immediately chilled, washed with calcium-free and magnesium-free PBS (Gibco), and stained for CD8 and the 2C transgenic TCR with the clonotypic 1B2 Ab. Cells were thoroughly vortexed and the frequency of calcein-positive 2C CD8+ T cells in each sample was determined by FACSCalibur (BD Biosciences).
Depletion was calculated using the following equation:
CTL viability and proliferation
For viability assays, CTLs were collected and plated at 1 × 106 cells, 5 × 105 cells, or 1 × 105 cells per well in 24-well flat bottom plates (Falcon). Zero, 5 × 105, or 9 × 105 purified 2C CD8+ cells per well were then added, respectively, to obtain a total of 1 × 106 cells per well. In parallel, CTLs were placed alone in untraversable transwells (0.4 μm) sharing medium with 2C/CTL cultures. The same process was repeated to determine the role of LFA-1, but was done in the presence of an LFA-1–blocking Ab. Likewise, in some experiments the targeting T cell was replaced with H-2b MHC-I monomers carrying the noncognate SIINFEKL peptide (National Institutes of Health Tetramer Core Facility) at 10−7mM. After 72 hours of culture, cells were stained with CD8 and H-2 antibodies and incubated with 7-aminoactinomycin-D (7AAD; Invitrogen) for 30 minutes at room temperature. FACS analysis was performed and absolute viable CTL numbers obtained. For proliferation assays, CTLs were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) at a concentration of 0.1μM per 107 T cells per mL (Invitrogen) for 10 minutes at 37°C. Labeling was quenched by adding medium with 10% fetal bovine serum (FBS). CTLs were then cultured as was done for the viability assays; after 72 hours, the CTL CFSE mean fluorescence intensity was determined with CellQuest software (Becton Dickinson and Company). For survival and proliferation in the syngeneic setting, see supplemental Methods.
Granzyme release
Granzyme activity was tested as described elsewhere.20 For details, see supplemental Methods.
CTL activity in vivo
The activity of CTLs in vivo was determined by their ability to confer tolerance toward allogeneic bone marrow grafts of the same H-2 background, as described elsewhere.6 To determine the role of the granule-mediated pathway in CTL protection, anti–third-party Balb/c perforin−/− CTLs were used, and their capacity to induce tolerance was compared with that of perforin-rich wild-type Balb/c CTLs.
Lck knock-down assay
Anti–third-party CTLs were transfected for sixty hours with Lck-specific siRNA using the ON-TARGETplus SMART pool (Category number L-043878-00-0010; Thermo Fisher Scientific) and controlled using a non-targeting pool. For further details, see supplemental Methods.
Image acquisition and analysis
For confocal fluorescence microscopy, images were taken with LSM 510 Laser Scanning Confocal Microscope (Carl Zeiss) and 100 X PLAN Apochromat objectives having a numerical aperture of 1.4. Acquisitions were analyzed using LSM (Carl Zeiss) and Volocity (Improvision) software, and images were generated. For live-cell video microscopy, live cell images were obtained on a Deltavision Restoration microscope (Applied Precision Instruments) using a MicroMax 5 MHz cooled charge-coupled device (CCD) camera (Roper Scientific). Image sequences of the time-lapse recording were processed with SoftWoRx (Applied Precision) and videos created. For information on specimen preparation, see supplemental Methods.
Statistical analysis
Imaging observations from experiments consisted of counts of conjugated CTLs displaying a particular pattern (that is, granule polarization). The significance of differences in pattern between treatments was evaluated by analysis of variance (ANOVA) and Dunnett post hoc comparison using JMP 9 (SAS Institute) statistical software. Significance in nonimaging-related experiments was determined by 2-tailed Student t tests using Microsoft Excel software. CD8-pERK correlation was calculated using Matlab software Version 7.7 (Mathworks).
Results
Characterization of the model used for targeting CTLs
In previous studies, CTL veto activity was determined indirectly by assessing the inhibition of T cell responses against a stimulator cell population of an H-2 background identical to that of the CTLs. We sought to study CTL veto activity directly using 2C TCR transgenic CD8 T cells to target CTLs, in the absence of stimulator cells. 2C CD8 T cells are inherently alloreactive against cells of H-2d mouse strains (for instance, DBA, BALB/c). Accordingly, the CTLs targeted in this system were of an H-2d background. To eliminate alloreactive clones that may engage the allogeneic 2C cells via TCR recognition, H-2d CTLs were generated by stimulation against a third party (H-2q) under conditions of IL-2 deprivation so that only strictly anti–third-party CTLs survive.6 Nevertheless, to ascertain that no CTL activity originates from residual alloreactivity against the 2C cells, CTLs of a cross between H-2d and H-2b (H-2b is the 2C H-2) mice were extensively used (that is, F1 CTLs). To control for CTL activity due to nonspecific cell-cell interactions, we used anti–third-party CTLs on an H-2s background (SJL), which are unrecognizable by 2C CD8+ T cells.9,13
CTLs being targeted respond with granule-mediated cytotoxicity
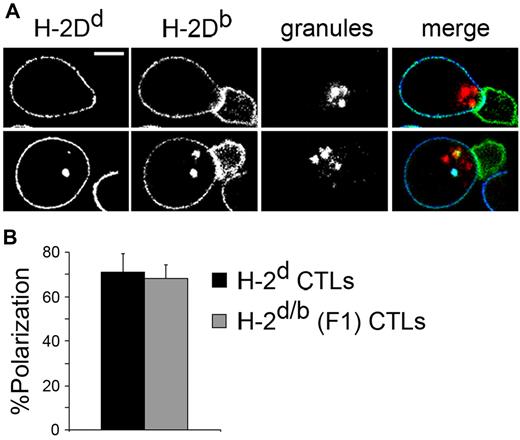
In our model, the 2C CD8+ T cells unidirectionally recognize the anti–third-party CTLs. However, when studying cell-cell conjugates of 2C CD8+ T cells with CTLs by confocal microscopy, we found that CTLs polarize their secretory granules toward the contact area with the recognizing 2C cell, indicating potential granule-mediated killing (Figure 1).
CTLs polarize cytotoxic granules toward the area of contact with recognizing CD8+ T cells in the absence of TCR specificity. (A) H-2d/b (F1) CTLs were labeled with LysoTracker Red for the detection of granules and targeted with 2C CD8+ T cells for 1 hour at 37°C. Cells were fixed, stained, and visualized with an LSM 510 Laser Scanning Confocal Microscope (Carl Zeiss) and 100× PLAN Apochromat objectives having a numerical aperture of 1.4. The F1 CTL appears on the left of each panel and stains positive for both H-2Dd (blue, merge) and H-2Db (green, merge). The 2C cell appears on the right and stains positive only for H-2Db. Granules appear in red in the merged image. Scale bar = 5 μm. (B) Quantitation of CTL granule polarization. Conjugates of H-2d/b (F1) or H-2d CTLs with 2C CD8+ T cells were prepared as in panel A and were evaluated for the presence of cytotoxic granule polarization. CTLs were scored as polarized when granules clearly accumulated in the area of contact. “%Polarization” refers to the percentage of conjugates in which the CTL is found polarizing its granules toward the contact area with the recognizing T cell. Values shown are the calculated means ± SD of 3 independent experiments, n > 70 in each.
CTLs polarize cytotoxic granules toward the area of contact with recognizing CD8+ T cells in the absence of TCR specificity. (A) H-2d/b (F1) CTLs were labeled with LysoTracker Red for the detection of granules and targeted with 2C CD8+ T cells for 1 hour at 37°C. Cells were fixed, stained, and visualized with an LSM 510 Laser Scanning Confocal Microscope (Carl Zeiss) and 100× PLAN Apochromat objectives having a numerical aperture of 1.4. The F1 CTL appears on the left of each panel and stains positive for both H-2Dd (blue, merge) and H-2Db (green, merge). The 2C cell appears on the right and stains positive only for H-2Db. Granules appear in red in the merged image. Scale bar = 5 μm. (B) Quantitation of CTL granule polarization. Conjugates of H-2d/b (F1) or H-2d CTLs with 2C CD8+ T cells were prepared as in panel A and were evaluated for the presence of cytotoxic granule polarization. CTLs were scored as polarized when granules clearly accumulated in the area of contact. “%Polarization” refers to the percentage of conjugates in which the CTL is found polarizing its granules toward the contact area with the recognizing T cell. Values shown are the calculated means ± SD of 3 independent experiments, n > 70 in each.
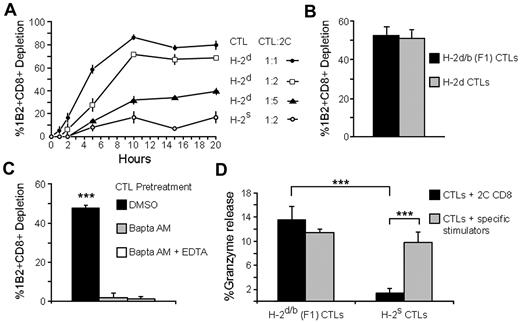
To evaluate whether granule-mediated killing indeed occurs, we initially checked whether rapid cytolytic killing results from this cell-cell interaction. To that end, 2C CD8+ T cells were loaded with calcein-AM, a vital dye that is released on cytolytic cell death, and incubated with anti–third-party H-2d CTLs at several CTL:2C ratios. After 1, 2, 5, 10, 15, and 20 hours of incubation, depletion of calcein-positive 2C cells was determined by FACS. At 5 hours, approximately half of the 2C cells that were incubated at the highest CTL-to-2C ratio were depleted, and those incubated at the intermediate CTL-to-2C ratio were also considerably depleted (P = .005, Figure 2A). 2C cell depletion was further increased at 10 hours, and then reached a plateau (Figure 2A). To control for nonspecific depletion of 2C cells, depletion by anti–third-party H-2s CTLs, which are of an irrelevant H-2 background, was evaluated. These CTLs were unable to induce substantial depletion, and exhibited very low depletion levels at all timepoints (Figure 2A).
CTLs kill recognizing CD8+ T cells in rapid, granule-mediated fashion. (A) Targeted CTL cytotoxic response. H-2d CTLs (directed against a third party) were targeted with 2C CD8+ T cells loaded with calcein, a probe that is lost on cell death. After 1, 2, 5, 10, 15, and 20 hours of incubation at 3 CTL-to-2C ratios (1:1, 1:2, and 1:5), depletion of calcein-positive 1B2+CD8+ 2C T cells was evaluated by FACS. 2C CD8+ T cells were incubated with identically treated H-2s CTLs as control. (B) Depletion of recognizing T cells by targeted H-2d/b (F1). F1 CTLs were targeted with 2C cells prepared as in A at a 1:1 ratio for 5 hours. Depletion was determined as in panel A and compared with the depletion induced by H-2d CTLs (anti–third-party) CTLs. (C) Dependence on intracellular Ca2+. CTLs were pretreated with BAPTA-AM and targeted with 2C CD8+ T cells. After 5 hours, 2C cell depletion was evaluated as in A and compared with the depletion induced by untreated CTLs. (D) Specific granzyme release associated with CTL activity. H-2d/b (F1) CTLs were targeted with 2C CD8+ T cells or incubated with the specific stimulator cells against which they had been generated for 4 hours, and granzyme release was evaluated by the BLT-esterase assay. 2C cells had no detectable granzyme secretion. Similarly treated H-2s CTLs did not release granzyme. Error bars in panels A-D represent SD from triplicate samples. Data shown are single experiments representative of 3 experiments. ***P < .001.
CTLs kill recognizing CD8+ T cells in rapid, granule-mediated fashion. (A) Targeted CTL cytotoxic response. H-2d CTLs (directed against a third party) were targeted with 2C CD8+ T cells loaded with calcein, a probe that is lost on cell death. After 1, 2, 5, 10, 15, and 20 hours of incubation at 3 CTL-to-2C ratios (1:1, 1:2, and 1:5), depletion of calcein-positive 1B2+CD8+ 2C T cells was evaluated by FACS. 2C CD8+ T cells were incubated with identically treated H-2s CTLs as control. (B) Depletion of recognizing T cells by targeted H-2d/b (F1). F1 CTLs were targeted with 2C cells prepared as in A at a 1:1 ratio for 5 hours. Depletion was determined as in panel A and compared with the depletion induced by H-2d CTLs (anti–third-party) CTLs. (C) Dependence on intracellular Ca2+. CTLs were pretreated with BAPTA-AM and targeted with 2C CD8+ T cells. After 5 hours, 2C cell depletion was evaluated as in A and compared with the depletion induced by untreated CTLs. (D) Specific granzyme release associated with CTL activity. H-2d/b (F1) CTLs were targeted with 2C CD8+ T cells or incubated with the specific stimulator cells against which they had been generated for 4 hours, and granzyme release was evaluated by the BLT-esterase assay. 2C cells had no detectable granzyme secretion. Similarly treated H-2s CTLs did not release granzyme. Error bars in panels A-D represent SD from triplicate samples. Data shown are single experiments representative of 3 experiments. ***P < .001.
To rule out any role for alloreactivity in the demonstrated rapid killing, 2C cell depletion was demonstrated with CTLs of an F1 origin (H-2d/H-2b), which are inherently nonreactive with the H-2b background of the 2C cells (Figure 2B). In addition, to be sure that CTL cytotoxicity is not only triggered but also delivered in a specific manner, experiments were also repeated in the presence of bystander CD8 T cells that lack antigen specificity for the CTL. Importantly, efficient killing was limited to the offending T cells only, without any cytotoxicity toward bystander T cells (supplemental Figure 1A).
The observed cytotoxic granule polarization and the rapid killing displayed by targeted CTLs indicate this activity is granule mediated. To address this possibility, we conducted a series of experiments.
Initially, dependence of rapid CTL killing on intracellular Ca2+ was evaluated, as it is well established that CTL granule secretion is highly dependent on intracellular Ca2+ levels.21 H-2d CTLs were preincubated with the intracellular Ca2+ chelator BAPTA-AM (N,N′-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-[2-[(acetyloxy)methoxy]-2-oxoethyl]]-bis[(acetyloxy)methyl]]ester) and targeted with calcein-loaded 2C CD8+ T cells. After 5 hours of culture, it was evident that BAPTA-AM pretreatment completely abolished CTL capacity to induce 2C cell depletion (Figure 2C). To determine whether granule constituents are indeed secreted on interaction with recognizing T cells, F1 CTLs were targeted with 2C CD8 T cells for 4 hours, and granzyme activity was measured in supernatants by the N-alpha-benzyloxycarbonyl-L-lysine thiobenzyl esterase (BLT-esterase) assay. Targeted CTLs secreted granzyme comparable with the levels observed when CTLs were allowed to interact with stimulator cells against which they had been generated (Figure 2D). The 2C cells themselves did not contribute to the observed granzyme activity, as incubation of the 2C cells with F1 stimulator cells did not generate any granzyme activity (not shown). Importantly, CTLs of the H-2s background undetectable by 2C cells did not exhibit any specific granzyme release (Figure 2D).
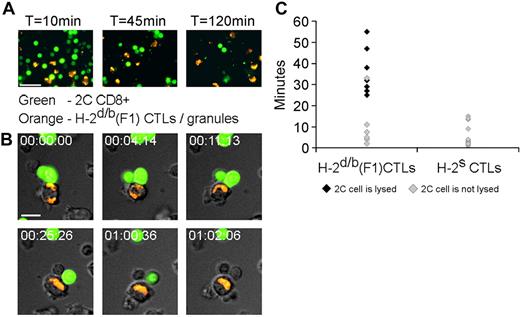
To study the dynamics of granule polarization and death induction, we followed single cell-cell interactions by live cell imaging. To this end, CTLs were loaded with LysoTracker Red (Invitrogen), which accumulates in granules and added to calcein-loaded 2C CD8+ T cells. We observed several instances of CTL/2C contacts that culminated in 2C cell lysis (Figure 3A). CTLs are highly motile and exhibit a random motion through which encounters with the immotile 2C cells are made possible. Lytic CTL/2C interactions are initiated when the CTL loses its elongated configuration to come to a stop as described for migrating T cells encountering their cognate antigen.22 Next, the CTL mobilizes its granules toward the cell-cell interface, in a manner akin to the observed when a CTL encounters its cognate target.23 Granule polarization is sustained over a lengthy period and culminates in 2C cell lysis as indicated by vital dye release. On lysis completion, CTLs abandon their polarized formation and resume movement throughout the microscopic field, as occurs in CTLs after death of their cognate target.24 Such a cell-cell interaction is shown in Figure 3B (see supplemental Videos 1-2).
CTL stopping, lengthy conjugate duration and sustained granule polarization precede death of the recognizing cell. Lysis of recognizing T cells by targeted CTLs as demonstrated by live cell video microscopy. Target H-2d/b (F1) CTLs were loaded with LysoTracker Red, which accumulates in granules (orange in appearance), and added to calcein-loaded 2C CD8+ T cells (green). Cells were then subject to live video microscopy with a Deltavision Restoration microscope (Applied Precision Instruments) using a MicroMax 5 MHz cooled CCD camera (Roper Scientific). Image sequences of the time-lapse recording were processed with SoftWoRx (Applied Precision). (A) Wide field microscopy demonstrating killing of recognizing T cells. F1 CTLs and 2C CD8+ cells were stained as described. After 2 hours of incubation, marked depletion of the recognizing 2C CD8+ cells was observed. (B) Snapshots depict the sequence of events occurring in the targeted CTL, culminating in lysis of the recognizing T cell. Time elapsed from the beginning of stable cell-cell contact is indicated. Top row: the CTL shifts its granules toward the contact area. Bottom row: sequential lysis of the 2 2C cells may be observed. Scale bar = 10μm. (C) The effect of specific recognition of CTL on conjugate duration and lysis. Calcein-loaded 2C CD8+ T cells were placed in culture with target H-2d/b CTLs or with unrecognizable H-2s CTLs and were then monitored by video microscopy. The durations of CTL-2C contacts were recorded and plotted. Data shown were acquired from several microscopic fields and are from a single experiment representative of 3 experiments.
CTL stopping, lengthy conjugate duration and sustained granule polarization precede death of the recognizing cell. Lysis of recognizing T cells by targeted CTLs as demonstrated by live cell video microscopy. Target H-2d/b (F1) CTLs were loaded with LysoTracker Red, which accumulates in granules (orange in appearance), and added to calcein-loaded 2C CD8+ T cells (green). Cells were then subject to live video microscopy with a Deltavision Restoration microscope (Applied Precision Instruments) using a MicroMax 5 MHz cooled CCD camera (Roper Scientific). Image sequences of the time-lapse recording were processed with SoftWoRx (Applied Precision). (A) Wide field microscopy demonstrating killing of recognizing T cells. F1 CTLs and 2C CD8+ cells were stained as described. After 2 hours of incubation, marked depletion of the recognizing 2C CD8+ cells was observed. (B) Snapshots depict the sequence of events occurring in the targeted CTL, culminating in lysis of the recognizing T cell. Time elapsed from the beginning of stable cell-cell contact is indicated. Top row: the CTL shifts its granules toward the contact area. Bottom row: sequential lysis of the 2 2C cells may be observed. Scale bar = 10μm. (C) The effect of specific recognition of CTL on conjugate duration and lysis. Calcein-loaded 2C CD8+ T cells were placed in culture with target H-2d/b CTLs or with unrecognizable H-2s CTLs and were then monitored by video microscopy. The durations of CTL-2C contacts were recorded and plotted. Data shown were acquired from several microscopic fields and are from a single experiment representative of 3 experiments.
To study the significance of specific CTL recognition with regard to cell-cell interactions, F1 (H-2d/b) CTLs were compared with CTLs of an H-2s background, which were unrecognizable by the 2C TCR. These CTLs were involved only in short-lived, superficial contacts that did not involve cell polarization (supplemental Video 3). Thus, recognition of the CTL by specific T cell is a prerequisite for CTL stopping, rounding, and granule polarization. Importantly, only contacts assisted by this stop signal are of long duration and culminate in 2C cell lysis (Figure 3C). The need for TCR-mediated recognition of the CTL may be rooted in augmentation of LFA-1–mediated adhesion secondary to TCR signaling.25,26 In support of this possibility, we found that killing was indeed dependent on integrin-mediated adhesion, being significantly diminished in the presence of LFA-1–blocking antibody (supplemental Figure 1B).
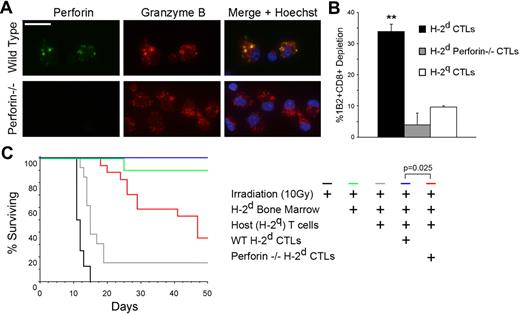
Among the cytotoxic constituents involved in this killing, we found a predominant role for perforin, as shown by pretreatment of the CTLs with an inhibitor of perforin-based cytotoxic activity (concanamycin A), and a minor role for the Fas/Fas ligand (FasL) pathway, as determined using Fas/FasL KO cells (supplemental Figure 2). To further confirm the central role of perforin in the rapid killing observed, anti–third-party H-2d perforin−/− CTLs (Figure 4A) were generated and targeted with 2C CD8 T cells. After 5 hours of culture, it was clearly evident that perforin−/− CTLs exhibited dramatically diminished killing capacity compared with wild-type CTLs (P = .0015, Figure 4B).
Targeted CTL activity is perforin dependent. (A) Perforin and granzyme content of anti–third-party H-2d CTLs. Wild-type H-2d CTLs (top row) and perforin−/− CTLs (bottom row) were fixed and stained intracellularly for perforin (green) and granzyme B (red). 4,6-diamidino-2-phenylindole (DAPI) staining of nuclei may be observed in merge (blue). Scale bar = 15μm. (B) The role of perforin in targeted CTL cytotoxicity. Perforin −/− H-2d CTLs were targeted with 2C CD8+ T cells. After 5 hours, 2C cell depletion was evaluated and compared with the depletion induced by wild-type CTLs, as well as by nonspecific CTLs. Error bars represent SD from triplicate samples. Data shown are single experiments representative of 3 experiments. **P = .0015. (C) The role of the granule-mediated cytotoxic pathway in the induction of tolerance toward MHC-mismatched bone marrow by targeted CTLs. In an established graft rejection model,6 supralethally irradiated C3H mice were reconstituted with host T cells and transplanted with allogeneic (H-2d), fully mismatched, T cell–depleted bone marrow either alone (n = 13) or supplemented with donor-type CTLs (H-2d, n = 6). For evaluation of the role of the granule-mediated pathway, H-2d perforin −/− CTLs were used (n = 17), and their capacity to induce tolerance was compared with that of perforin-competent wild-type CTLs. Data shown are from 2 experiments combined.
Targeted CTL activity is perforin dependent. (A) Perforin and granzyme content of anti–third-party H-2d CTLs. Wild-type H-2d CTLs (top row) and perforin−/− CTLs (bottom row) were fixed and stained intracellularly for perforin (green) and granzyme B (red). 4,6-diamidino-2-phenylindole (DAPI) staining of nuclei may be observed in merge (blue). Scale bar = 15μm. (B) The role of perforin in targeted CTL cytotoxicity. Perforin −/− H-2d CTLs were targeted with 2C CD8+ T cells. After 5 hours, 2C cell depletion was evaluated and compared with the depletion induced by wild-type CTLs, as well as by nonspecific CTLs. Error bars represent SD from triplicate samples. Data shown are single experiments representative of 3 experiments. **P = .0015. (C) The role of the granule-mediated cytotoxic pathway in the induction of tolerance toward MHC-mismatched bone marrow by targeted CTLs. In an established graft rejection model,6 supralethally irradiated C3H mice were reconstituted with host T cells and transplanted with allogeneic (H-2d), fully mismatched, T cell–depleted bone marrow either alone (n = 13) or supplemented with donor-type CTLs (H-2d, n = 6). For evaluation of the role of the granule-mediated pathway, H-2d perforin −/− CTLs were used (n = 17), and their capacity to induce tolerance was compared with that of perforin-competent wild-type CTLs. Data shown are from 2 experiments combined.
The granule-mediated pathway is essential to the targeted CTL response in vivo
To determine whether granule-mediated tolerization by targeted CTLs occurs in vivo, we used a well-established graft rejection model in which anti–third-party CTLs induce tolerance toward allogeneic bone marrow grafts of the same H-2 background.6 To that end, supralethally irradiated mice were reconstituted with host T cells and transplanted with allogeneic (H-2d) fully mismatched bone marrow either alone or with the protection of anti–third-party CTLs of the same MHC-I background (H-2d). To determine the role of granule-mediated pathway in CTL protection, anti–third-party H-2d perforin−/− CTLs were used, and their capacity to induce tolerance was compared with that of perforin–rich, wild-type CTLs. As observed in Figure 4C, mice receiving no CTL protection succumbed to graft rejection within 20 days after transplantation. All mice receiving wild-type, perforin-rich CTLs survived. Mice receiving perforin−/− CTLs were not fully protected from rejection, and exhibited significantly reduced survival (Figure 4C).
To evaluate whether CTLs respond similarly when targeted in the more physiologic, peptide driven-setting, we used a syngeneic cell-cell system in which OT-1 CD8+ T cells target SIINFEKL peptide-loaded H-2b CTLs. To provide further assurance that the targeted CTL response is not mediated by any form of TCR specificity, we used 2C mouse CTLs of the H-2b background, with TCR of an irrelevant specificity. 2C CTLs loaded with SIINFEKL peptide rapidly killed recognizing OT-1 CD8+ T cells. Killing was prevented by pre-incubating the CTLs with the degranulation inhibitor BAPTA-AM (supplemental Figure 3A).
CTLs resist apoptosis and proliferate in response to being targeted
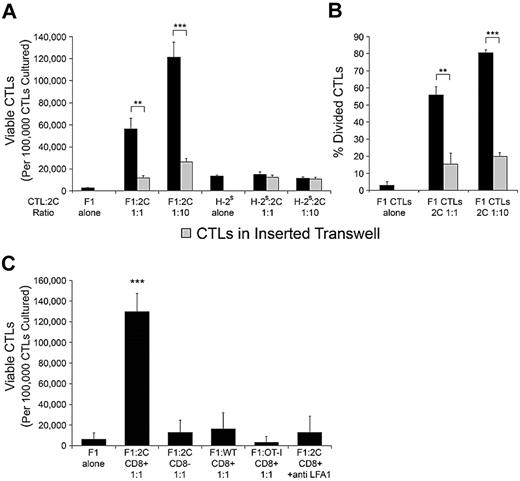
Because granule polarization and secretion are hallmarks of cell activation,27-29 we tested whether other activation hallmarks, such as survival and proliferation, occur in CTLs after targeting by specific T cells. To this end, H-2d/b (F1) CTLs were cultured either alone or with 2C CD8+ T cells at two CTLs-to-2C ratios (1:1 and 1:10). Viability was determined after 72 hours. The survival of CTLs was poor when they were cultured alone, but dramatically enhanced when cultured with 2C cells. Notably, CTL survival further increased when CTLs were cultured with a higher number of 2C cells (Figure 5A). This increase in viable CTL counts was at least partly due to increased proliferation, as shown by CFSE dilution in F1 CTLs (Figure 5B). Here, too, CTL proliferation further increased when CTLs were cultured with a higher frequency of 2C cells (Figure 5B). To test whether the observed benefit of 2C-CTL interaction to CTL survival and proliferation is due to specific recognition of the CTL, H-2s CTLs, which are unrecognizable to the 2C TCR, were cultured with 2C cells as described above. As shown in Figure 5A, 2C cells had no effect on H-2s CTL survival, as the number of viable CTLs was similar in their presence and their absence. Correspondingly, no proliferation of H-2s CTLs was observed (not shown). This mitogenic signal was dependent on integrin-mediated adhesion, being significantly diminished in the presence of LFA-1–blocking antibody (Figure 5C). In addition, wild-type CD8+ T cells, OT-1 CD8+ T cells, or 2C CD8-cells could not mimic the activity of 2C CD8+ T cells (Figure 5C). Clearly, TCR-driven recognition of the CTL, assisted by integrin-mediated adhesion, leads to this increase in survival and proliferation, but it was still uncertain whether this increase is due to soluble factors secreted by the cognate T cell on recognition of the CTL, or to a contact-dependent signaling cascade initiated in the CTL. To answer this question, F1 CTLs were placed in untraversable transwells and cultured in the vicinity of 2C-CTL interactions. The viability of CTLs cultured in the surrounding media of 2C-CTL interactions was significantly lower than that of CTLs cultured in contact with 2C cells at both ratios (P = .00566 and P = .00072, Figure 5A). Accordingly, transwell CTL proliferation was significantly diminished (P = .00218 and P < .001, Figure 5B). Similar survival and proliferation patterns were observed in CTLs targeted in the syngeneic peptide-driven system. Here too, the mitogenic signal could not be transmitted by soluble factors secreted in the process of targeting, but rather could only be transmitted by direct contact with the recognizing T cell (supplemental Figure 3B-C).
CTLs are induced to survive and proliferate when targeted. (A) Effect of targeting on CTL viability. H-2d/b (F1) CTLs or H-2s CTLs were placed in culture with 2C CD8+ T cells at a CTL-to-2C ratio of 1:1 or 1:10, or were incubated without 2C cells. In parallel, CTLs were placed alone in untraversable transwells and cultured in the surrounding media of respective CTLs interacting with 2C cells at both 1:1 and 1:10 CTL-to-2C ratios. After 72 hours of incubation, viable CTL counts were obtained by enumerating the number of 7AAD-excluding CTLs as determined by FACS. Values are presented as the number of viable CTLs per 105 CTLs cultured at time zero. (B) Effect of targeting on CTL proliferation. F1 CTLS were loaded with CFSE and placed in culture or in untraversable transwells as described in panel A. After 72 hours of incubation the percentage of divided CTLs was determined by CFSE dilution. (C) Specific TCR recognition and integrin-mediated adhesion are required to induce CTL survival. F1 CTLs were incubated as described in A with either 2C CD8+, 2C CD8−, OT-I CD8+, or wild-type C57B/6 CD8+ cells. Only the recognizing 2C CD8+ cells induced significant CTL survival and proliferation. The observed CTL response was inhibited on addition of 20ug/mL anti–LFA-1 antibody. Data in panels A-C are from a single experiment representative of 3 independent experiments. Error bars represent SD from triplicate wells. **P < .01, ***P < .001.
CTLs are induced to survive and proliferate when targeted. (A) Effect of targeting on CTL viability. H-2d/b (F1) CTLs or H-2s CTLs were placed in culture with 2C CD8+ T cells at a CTL-to-2C ratio of 1:1 or 1:10, or were incubated without 2C cells. In parallel, CTLs were placed alone in untraversable transwells and cultured in the surrounding media of respective CTLs interacting with 2C cells at both 1:1 and 1:10 CTL-to-2C ratios. After 72 hours of incubation, viable CTL counts were obtained by enumerating the number of 7AAD-excluding CTLs as determined by FACS. Values are presented as the number of viable CTLs per 105 CTLs cultured at time zero. (B) Effect of targeting on CTL proliferation. F1 CTLS were loaded with CFSE and placed in culture or in untraversable transwells as described in panel A. After 72 hours of incubation the percentage of divided CTLs was determined by CFSE dilution. (C) Specific TCR recognition and integrin-mediated adhesion are required to induce CTL survival. F1 CTLs were incubated as described in A with either 2C CD8+, 2C CD8−, OT-I CD8+, or wild-type C57B/6 CD8+ cells. Only the recognizing 2C CD8+ cells induced significant CTL survival and proliferation. The observed CTL response was inhibited on addition of 20ug/mL anti–LFA-1 antibody. Data in panels A-C are from a single experiment representative of 3 independent experiments. Error bars represent SD from triplicate wells. **P < .01, ***P < .001.
Targeted CTL activity is dependent on the Src family kinase Lck
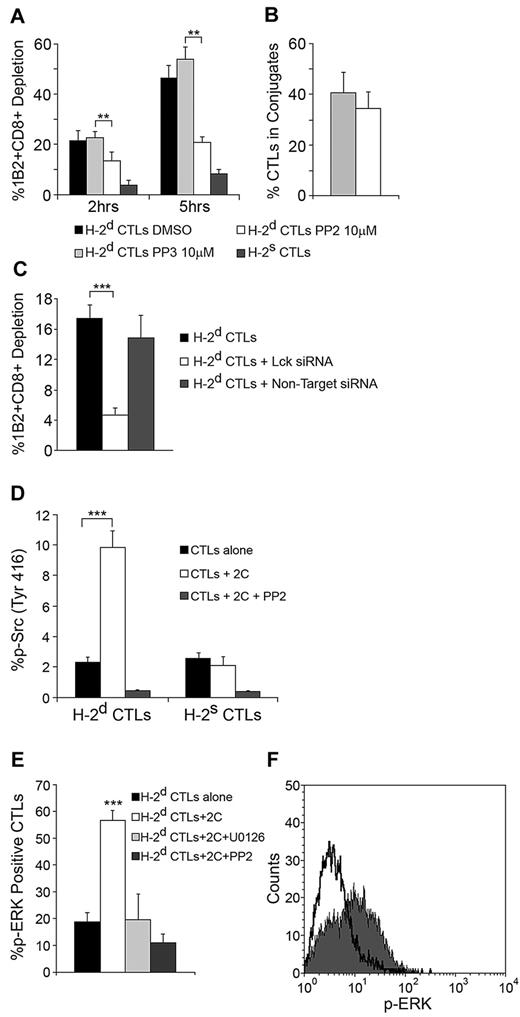
Signaling leading to cytoskeletal polarization, granule secretion, and proliferation in CTLs operating in the context of TCR specificity has been shown to be mediated via Src kinase activity. To evaluate Src kinase involvement in the targeted CTL response, CTLs were pretreated with the Src kinase inhibitor PP2. This significantly inhibited their capacity to kill recognizing 2C cells (P = .00782 at 2 hours, P = .001 at 5 hours Figure 6A). This effect was not due to inhibition of conjugate formation (Figure 6B).
CTL targeting induces Src Kinase signaling leading to cytotoxicity and Erk phosphorylation. (A) Src mediates the cytotoxic response of targeted CTLs. H-2d CTLs preincubated with PP2/PP3 for 1 hour were targeted with calcein-loaded 2C CD8+ cells (1:1 ratio). After 2 and 5 hours, 2C cell depletion was evaluated and compared with untreated CTLs. H-2s CTLs served as the control for nonspecific activity. (B) PP2 does not affect conjugate formation. Cells were fixed, differentially stained, and imaged 1 hour into culture. Conjugation was assessed counting conjugated CTLs per total CTLs in several microscopic fields. Error bars represent SD from triplicate datasets, n > 40 in each. (C) Lck is responsible for mediating the CTL response. H-2d CTLs were transfected with Lck siRNA. After 60 hours, CTLs were targeted with 2C CD8+ cells, and 2C cell depletion was compared with depletion induced by untreated CTLs or CTLs transfected with non-target siRNA. (D) Src activation-loop phosphorylation. H-2d CTLs were targeted with 2C CD8+ cells for 90 minutes and evaluated by FACS for activating Src kinase phosphorylation (Tyr416). H-2s CTLs controlled nonspecific phosphorylation. The effect of PP2 is shown. (E) Erk phosphorylation in targeted CTLs. CTLs were targeted as in panel D and evaluated for ERK phosphorylation. The effects of Erk inhibitor U0126 and Src inhibitor PP2 are shown. (F) Overlay of FACS plots representative of the Erk phosphorylation shown in panel E. Solid dark gray, targeted CTLs; black line, untargeted CTLs. Panels A-F: Data from single experiment representative of 3 experiments. Error bars represent SD from triplicate wells. **P < .01, ***P < .001.
CTL targeting induces Src Kinase signaling leading to cytotoxicity and Erk phosphorylation. (A) Src mediates the cytotoxic response of targeted CTLs. H-2d CTLs preincubated with PP2/PP3 for 1 hour were targeted with calcein-loaded 2C CD8+ cells (1:1 ratio). After 2 and 5 hours, 2C cell depletion was evaluated and compared with untreated CTLs. H-2s CTLs served as the control for nonspecific activity. (B) PP2 does not affect conjugate formation. Cells were fixed, differentially stained, and imaged 1 hour into culture. Conjugation was assessed counting conjugated CTLs per total CTLs in several microscopic fields. Error bars represent SD from triplicate datasets, n > 40 in each. (C) Lck is responsible for mediating the CTL response. H-2d CTLs were transfected with Lck siRNA. After 60 hours, CTLs were targeted with 2C CD8+ cells, and 2C cell depletion was compared with depletion induced by untreated CTLs or CTLs transfected with non-target siRNA. (D) Src activation-loop phosphorylation. H-2d CTLs were targeted with 2C CD8+ cells for 90 minutes and evaluated by FACS for activating Src kinase phosphorylation (Tyr416). H-2s CTLs controlled nonspecific phosphorylation. The effect of PP2 is shown. (E) Erk phosphorylation in targeted CTLs. CTLs were targeted as in panel D and evaluated for ERK phosphorylation. The effects of Erk inhibitor U0126 and Src inhibitor PP2 are shown. (F) Overlay of FACS plots representative of the Erk phosphorylation shown in panel E. Solid dark gray, targeted CTLs; black line, untargeted CTLs. Panels A-F: Data from single experiment representative of 3 experiments. Error bars represent SD from triplicate wells. **P < .01, ***P < .001.
As Lck is the major Src kinase in T cells, we hypothesized that it was responsible for mediating the observed killing response. To address this possibility, Lck knock down using Lck-specific siRNA was performed. As expected, Lck knock down significantly reduced the killing capacity of targeted CTLs (P = .00033, Figure 6C; see supplemental Figure 4A for Lck western analysis).
To further explore the role of Src signaling in the targeted CTL response, we evaluated Src phosphorylation in targeted CTLs directly by labeling them with an anti–phospho-Src antibody recognizing phosphorylation in the activation loop of the kinase domain. FACS analysis revealed a significant (P < .001) increase in Src phosphorylation in targeted CTLs (Figure 6D).
As the majority of known proliferative responses in T cells are mediated via MAPK/ extracellular signal-regulated kinase (ERK) pathway,30,31 we evaluated the participation of ERK in the observed response of targeted CTLs. To that end, targeted CTLs were labeled with a phospho-ERK1/2 antibody, and the level of phosphorylation was determined by FACS. Analysis revealed that targeting induced a vast increase in CTL ERK1/2 phosphorylation (P < .001, Figure 6E-F). Notably, phosphorylation was inhibited by the Src kinase inhibitor PP2, indicating that ERK phosphorylation is dependent on the observed Src kinase signaling (Figure 6E).
The CD8 molecule is key to the targeted CTL response
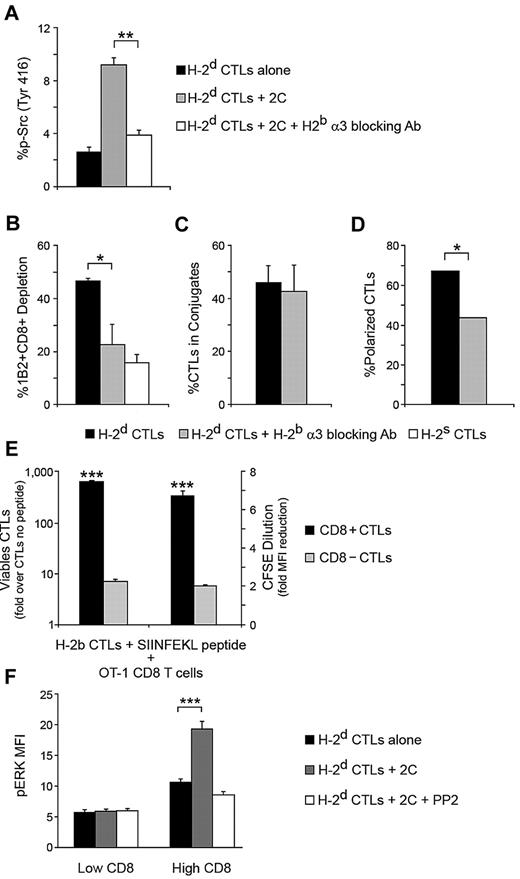
Through its physical association with CD8, the Src kinase Lck contributes to T cell activation, making CD8 ligation essential for many CTL responses, including granule-mediated killing.32-34 Having observed that Lck signaling is central in the targeted CTL response, we next evaluated the possibility that ligation of the CTL CD8 molecule is responsible for this circumstance. To that end, we evaluated Src phosphorylation in CTLs being targeted in the presence of an antibody that specifically blocks ligation of the CTL CD8 molecule to the α3 domain of the 2C cell MHC-I molecule. This antibody is directed against the H-2b α3 domain, and thus does not affect ligation of the 2C CD8 molecule. FACS analysis revealed that CD8 ligation is crucial for inducing Src kinase phosphorylation in the CTL, being significantly inhibited in the presence of the antibody (P = .0063, Figure 7A).
Targeted CTL activation is CD8 dependent. (A) CD8 mediates Src activation loop phosphorylation. H-2d CTLs were targeted with 2C CD8+ cells for 90 minutes, and Src phosphorylation was evaluated by FACS. The role of the CTL CD8 molecule in phosphorylation was determined by applying a blocking antibody specific for the 2C MHC-I α3 domain (H-2b). (B) Contribution of the CTL CD8 molecule to killing. Depletion of targeting 2C CD8+ cells was determined in the presence or absence of the blocking antibody from A. In A and B nonspecific CTL activity was controlled by H-2s CTLs. (C) Relevance of CD8 in adhesion. CTLs were targeted in the presence or absence of the blocking antibody from panel A, and adhesion was assessed by counting CTL-2C conjugates per total CTLs in several microscopic fields. (D) Polarization dependence on CD8. The effect of blocking CD8 ligation on CTL granule polarization toward conjugated 2C cells was evaluated by confocal microscopy. (E) CD8 contributes to CTL survival and proliferation. CD8+ and CD8− 2C CTLs (H-2b) were loaded with SIINFEKL peptide and targeted with OT-1 CD8+ cells. After 72 hours, survival and proliferation of the two CTL populations were evaluated by viable cell count and CFSE dilution. (F) ERK phosphorylation depends on CTL CD8 level. H-2d CTLs were targeted with 2C CD8+ cells for 90 minutes. The relationship between CD8 levels and targeting-induced ERK phosphorylation was determined by FACS analysis. In panels A-F, single experiments representative of 3 experiments are shown. SD is from triplicate wells/microscopic fields. *P < .05, **P < .01, ***P < .001; n > 50 (C); n > 100 (D).
Targeted CTL activation is CD8 dependent. (A) CD8 mediates Src activation loop phosphorylation. H-2d CTLs were targeted with 2C CD8+ cells for 90 minutes, and Src phosphorylation was evaluated by FACS. The role of the CTL CD8 molecule in phosphorylation was determined by applying a blocking antibody specific for the 2C MHC-I α3 domain (H-2b). (B) Contribution of the CTL CD8 molecule to killing. Depletion of targeting 2C CD8+ cells was determined in the presence or absence of the blocking antibody from A. In A and B nonspecific CTL activity was controlled by H-2s CTLs. (C) Relevance of CD8 in adhesion. CTLs were targeted in the presence or absence of the blocking antibody from panel A, and adhesion was assessed by counting CTL-2C conjugates per total CTLs in several microscopic fields. (D) Polarization dependence on CD8. The effect of blocking CD8 ligation on CTL granule polarization toward conjugated 2C cells was evaluated by confocal microscopy. (E) CD8 contributes to CTL survival and proliferation. CD8+ and CD8− 2C CTLs (H-2b) were loaded with SIINFEKL peptide and targeted with OT-1 CD8+ cells. After 72 hours, survival and proliferation of the two CTL populations were evaluated by viable cell count and CFSE dilution. (F) ERK phosphorylation depends on CTL CD8 level. H-2d CTLs were targeted with 2C CD8+ cells for 90 minutes. The relationship between CD8 levels and targeting-induced ERK phosphorylation was determined by FACS analysis. In panels A-F, single experiments representative of 3 experiments are shown. SD is from triplicate wells/microscopic fields. *P < .05, **P < .01, ***P < .001; n > 50 (C); n > 100 (D).
We then analyzed the role of CD8 ligation with regard to effector function and proliferation, first evaluating the killing capacity of targeted CTLs in the presence of the of H-2b α3 antibody. Blocking the CD8-MHC α3 interaction significantly diminished killing (P = .0104, Figure 7B). The percentage of CTLs participating in conjugates with 2C cells was not significantly altered in the presence of the blocking antibody (Figure 7C), indicating that the CD8-MHC interaction contributes to killing primarily by participating in CTL signaling, rather than by aiding adhesion. Indeed, confocal imaging of conjugates revealed that blocking CD8-MHC interaction significantly inhibited the observed CTL granule polarization toward the 2C cell (P = .0251, Figure 7D).
To determine the importance of CD8 ligation with regard to proliferation, we took advantage of the existence of a CD3+CD4−CD8− population among 2C CTLs.
CD8+ and CD8− 2C CTLs (H-2b) were targeted in the above-described SIINFEKL peptide-driven system by OT-1 CD8 T cells for 72 hours, after which CTL survival and proliferation were evaluated. Analysis revealed that survival and proliferation of CD8-negative CTLs in response to being targeted was significantly diminished (P < .001 for survival and proliferation) compared with CD8-positive CTLs (Figure 7E). While there is clear indication that the proliferative signal is transduced via CD8, presenting the CD8 ligand alone was not enough to induce survival or proliferation, as demonstrated by our experiments using MHC-I monomers (supplemental Figure 4B).
To further test the hypothesis that the signal for CTL activation and proliferation is mediated by the CD8 molecule, we used an approach recently described by Feinerman et al35 to assess the relation between CD8 expression intensity and the observed ERK phosphorylation in the targeted CTL. We found that “CD8–high” CTLs exhibit the highest degree of ERK phosphorylation in response to targeting with specific T cells, while ERK phosphorylation in “CD8 low” CTLs was similar to that found in untargeted CTLs (Figure 7F). Furthermore, using multivariate analysis adjusted for cell size and controlled by MHC-I level (supplemental Figure 4C-D), a positive correlation between the CTL CD8 level and the level of induced pERK was found (correlation coefficient = 0.322782 ± 0.0523, P < .001). Notably, even when the CD8 level was high, Src kinase signaling had to be allowed for Erk phosphorylation to ensue, as adding the Src kinase inhibitor PP2 completely abolished the observed increase in ERK phosphorylation in the targeted CTLs (Figure 7F), further indicating that CD8 induces ERK phosphorylation primarily via its observed triggering of Src kinase signaling (Figure 7A).
Discussion
Veto activity was defined in 1980 by Miller as the capacity to specifically suppress cytotoxic T cell precursors.4 This activity is possessed by several bone marrow cells14,36 and lymphocyte subpopulations,9 and was shown to induce transplantation tolerance across major histocompatibility antigens. Very strong veto activity was documented for CD8+ CTL lines or clones.8,37,38 Thus, cytotoxic T cells cannot only kill target cells for which they have receptor-mediated specificity, but also delete CD8+ T cells directed against them.4
Here, we find that the granule-mediated pathway plays an important role in CTL suppressor activity. Therefore, in the bone-marrow rejection model we use, perforin−/− CTLs exhibit markedly diminished tolerizing activity compared with wild-type CTLs. A role for perforin in overcoming graft-rejecting T cells has been established,39,40 but, these studies used broad-repertoire CD8 T cells, allowing for tolerization via allorecognition of the host T cells by the donor T cells. We show, that perforin-mediated tolerization by CTLs may occur in the absence of TCR involvement using CTLs with a TCR repertoire directed toward a third party.
Studies probing the mechanism by which “veto” cells tolerize T cell responses directed against them have suggested a role for FasL5,13 and TGFβ,11,41 and in prior research on the veto activity of anti–third-party CTLs, we have shown a role for Fas/FasL.5 In that study, recognizing T cells were stimulated with cognate stimulator cells throughout the tolerization process (> 72 hours), allowing for Fas expression. In our current experimental setting, naive CD8 T cells are used to target CTLs. Being naive, the cells do not express the Fas receptor, and therefore cannot be tolerized by the Fas/FasL pathway, showing the clear dependence on the granule-mediated pathway. In transplantation, we believe that both pathways contribute to tolerization; perforin is critical in the early stages of rejection, and the Fas/FasL pathway gains importance over time, as the host alloreactive T cells are activated by the graft and elevate Fas receptor levels on their membranes.
The involvement of the granule-mediated pathway was surprising, as it implies activation in the absence of TCR specificity. Detailed analysis of the events revealed that CTLs respond with granule polarization comparable with what has been reported for TCR-driven polarization (> 60%),42 cytolytic degranulation, and proliferation, an additional hallmark of activation. Mechanistically, we suggest a pivotal role for the CTL CD8 molecule in triggering this activity.
While the central role of the CTL CD8 molecule in the deletion of recognizing T cells has been demonstrated,5,12,17 its mode of action has never been clarified. We show that blocking the ability of the CTL CD8 coreceptor to interact with the cognate T cell MHC-I α3 domain reduces the observed granule-mediated killing of the latter. This was not due to obstructing adhesion, as conjugates formed normally, but was associated with a reduction in the polarization of granules toward the contact area with the recognizing cell. When focusing on the signaling events that occur in the targeted CTL, we found that Src kinase phosphorylation and ERK phosphorylation are essential components in the CTL response, and that they are completely dependent on CD8 ligation to the MHC-I α3 domain on the recognizing T cell. Lck is the major Src kinase in T cells, and we showed it to be crucial for rapid killing to occur.
The association of Lck with the intracellular portion of CD833 may explain how CD8 ligation leads to its activation and downstream signal transduction in the targeted CTL. In TCR recognition-mediated CTL activation, CD8 ligation to target MHC-I α3 has been shown to be essential for the activation of Lck.43,44 Likewise, it is established that Lck activity is necessary for granule exocytosis in CTLs.45,46 Moreover, it is known that TCR recognition-mediated killing of targets that are not recognized with high affinity depends on the ligation of CD8 to the target cell MHC-I α3 domain.47 Specifically, it is the granule-mediated cytotoxic pathway that is inhibited when CD8 ligation is blocked, demonstrating the important contribution of CD8 to granule-mediated killing in the presence of TCR recognition.48 CD8 ligation contributes to CTL activation by increasing the avidity of weak TCR-MHC-p interactions, but, importantly, also by facilitating Lck signaling, as activation is impaired in the absence of its cytoplasmic domain.49
We now demonstrate that in a cell-cell interaction consisting of a T cell being targeted by another T cell, CD8 ligation may induce activation in the absence of TCR specificity. Such a cell-cell interaction may serve as a unique system for studying coreceptor function in the absence of TCR engagement. Initiated through recognition of the target T cell by the cognate T cell TCR, this interaction benefits from dramatic augmentation of integrin-dependent adhesion provided by TCR signaling-dependent changes in LFA-1–intercellular adhesion molecule–1 associations.25,26 This adhesion may permit low-affinity interactions, such as CD8–MHC-I,50 to take place. Thus, T cells serving target for recognition enjoy the intermembrane intimacy that may be provided only as a result of TCR recognition, in the absence of their own TCR involvement. This TCR-mediated intimacy, we suggest, is key in targeted T cell activation. Our data showing that activation may not be triggered in the absence of integrin-mediated adhesion supports this idea. In the context of this intermembrane intimacy, we show that a signaling cascade is initiated in the targeted T cell. This cascade involves phosphorylation of classical mediators of T cell activation such as Src and ERK and culminates in outcomes similar to those observed in activation induced by TCR recognition, namely, cytoskeletal polarization, degranulation, and proliferation. The trigger for this cascade, we suggest, is the engagement of the CTL CD8 molecule with the recognizing T cell MHC-I α3 domain. Importantly, presenting CD8 ligand in the form of MHC-I monomers could not trigger activation, underlining the need for TCR-driven targeting. We therefore propose that in the context of a cell-cell interaction enjoying the avidity of unidirectional TCR-mediated recognition, CD8 ligation is sufficient to induce Lck phosphorylation and T cell activation. For a summary of events, see supplemental Figure 5.
With regard to the physiologic importance of this route to T cell activation, we show that CTLs may suppress peptide-specific T cell responses directed against them in the syngeneic setting. This type of activation may play a physiologic role in the elimination of autoreactive T cell clones, a suggestion gaining support from research showing granule-mediated killing of autologous lymphoid cells by CD4 regulatory T cells.51 The potential for 2-way cytotoxicity would seem to play a role in the outcome of any CTL attempt to tolerize an autoreactive armed CTL. In this regard, future studies on the time course of granzyme polarization in CTLs serving as targets of TCR-mediated CTL activation, are warranted. If a role in protecting self indeed exists, the observed proliferation of recognized CTLs could be advantageous in further suppressing an autoimmune response.
In addition, CTL-induced killing of recognizing T cells could play a role in terminating immune responses. Under conditions of inflammation, patrolling CTLs could present soluble peptides released from dying cells directly onto their MHC-I molecules, deleting T cells directed against them and thereby actively preventing further aggravation of the immune response. In this regard, it has been demonstrated that perforin and FasL are needed for the homeostatic regulation of graft-versus-host disease–inducing donor T cells.52 It should be interesting to examine whether veto activity mediates part of this regulation, perhaps by aberrant presentation of host antigen on CTLs, leading to elimination of graft-versus-host disease–inducing T cell clones recognizing them.
The possibility that the targeted CTL response constitutes a form of immune evasion under certain conditions should also be considered. This suggestion is in line with Green et al, who demonstrated that viruses might escape killing of infected cells using veto mechanisms for induction of apoptosis in recognizing CD8 T cells.19 Moreover, it has recently been shown that the granule-mediated pathway plays a central role in suppressing tumor cell clearance.53 A possible role for targeted CTL activation and degranulation in tumor evasion of T cell responses should be evaluated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health Grant PO1CA100265-01A1 (Project 5), European Commission LSHB-CT-2004-503319 (Allostem Project), the Gabriella Rich Center for Transplantation Biology Research, and Mrs E. Drake. O. M. was the recipient of EMBO fellowship ASTF.49.00-0.
National Institutes of Health
Authorship
Contribution: O.M., D.H. and Y.R. designed the research; O.M., D.H., A.L., E.S., E.O., Y.E. and R.A. performed the research; O.M., D.H., S.R.-Z., Y.E.A., M.L.D. and Y.R. analyzed the data; and O.M., D.H., M.L.D. and Y.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Yair Reisner, Chairman, Department of Immunology, Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: yair.reisner@weizmann.ac.il.
References
Author notes
O.M. and D.H. contributed equally to this work.