Abstract
The endothelium actively participates in neutrophil migration out of the vasculature via dynamic, cytoskeleton-dependent rearrangements leading to the formation of transmigratory cups in vitro, and to domes that completely surround the leukocyte in vivo. Leukocyte-specific protein 1 (LSP1), an F-actin–binding protein recently shown to be in the endothelium, is critical for effective transmigration, although the mechanism has remained elusive. Herein we show that endothelial LSP1 is expressed in the nucleus and cytosol of resting endothelial cells and associates with the cytoskeleton upon endothelial activation. Two-photon microscopy revealed that endothelial LSP1 was crucial for the formation of endothelial domes in vivo in response to neutrophil chemokine keratinocyte-derived chemokine (KC) as well as in response to endogenously produced chemokines stimulated by cytokines (tumor necrosis factor α [TNFα] or interleukin-1β [IL-1β]). Endothelial domes were significantly reduced in Lsp1−/− compared with wild-type (WT) mice. Lsp1−/− animals not only showed impaired neutrophil emigration after KC and TNFα stimulation, but also had disproportionate increases in vascular permeability. We demonstrate that endothelial LSP1 is recruited to the cytoskeleton in inflammation and plays an important role in forming endothelial domes thereby regulating neutrophil transendothelial migration. The permeability data may underscore the physiologic relevance of domes and the role for LSP1 in endothelial barrier integrity.
Introduction
Recruitment of circulating neutrophils from the bloodstream to sites of tissue injury and infection is the hallmark feature of the inflammatory response. This process involves multiple, interdependent, regulated molecular interactions between the neutrophils and the vascular endothelium.1,2 Initial tethering and rolling of leukocytes along the vessel wall is followed by firm adhesion to the vascular endothelium caused by chemokine activation of leukocyte integrins.3,4 The neutrophils then crawl to sites where they migrate through the vascular endothelium,5 a process known as transendothelial migration or diapedesis. A growing body of literature suggests that transendothelial migration is an interactive process between leukocytes and endothelial cells, in which endothelial cells are not passive bystanders but rather active participants that regulate this process.6-9 There is also evidence that leukocyte adhesion molecules binding to endothelial adhesion molecules2,10 induce intracellular signaling, stimulating the endothelial cytoskeleton and regulatory proteins to initiate transendothelial migration.1,11,12
One such regulatory protein in endothelial cells is leukocyte-specific protein 1 (LSP1), which was initially characterized in lymphocytes and thymocytes.13,14 LSP1 has now been identified in monocytes, macrophages, dendritic cells, Langerhans cells, and neutrophils15-17 and in both murine and human endothelium.18 LSP1 is a cytoplasmic intracellular Ca2+- and F-actin–binding protein that interacts with the cytoskeleton in leukocytes.19,20 Strategically positioned between the plasma membrane and the cytoskeleton, neutrophil LSP1 may transmit signals that contribute to cell polarization and cell motility.19 By contrast, endothelial LSP1 but not leukocyte LSP1 is found primarily in the nucleus.18 LSP1 is a major substrate of the mitogen-activated protein kinase (MAPK)–activated protein (MAPKAP) kinase 2 in the p38 MAPK pathway as well as a substrate for protein kinase C (PKC21,22 ). Intriguingly, MAPK-activated protein kinase 2 is also segregated to the nucleus and transported to the cytoplasm on stimulation.23 Both p38 and PKC pathways have been implicated in alterations of the endothelial cytoskeleton.24,25
Numerous studies have documented the importance of the endothelium and the endothelial cytoskeleton in the formation of docking structures or transmigratory cups during transendothelial migration in vitro.6,7,26,27 These endothelial structures are projections that move up the side of the leukocyte, suggesting a very active, dynamic role of the endothelium in the leukocyte transmigration. Investigating neutrophil transmigration in vivo, we reported that these transmigratory cups extended all the way to the top of neutrophils, forming domes so that we could clearly see the endothelium, both lumenally and ablumenally, with respect to the neutrophil.8 This was not phagocytosis (also known as emperiopolesis) by the endothelium as the neutrophil never entered the intracellular compartment of the endothelium; rather, it was a covering and sealing of the neutrophil away from the mainstream of blood. The seal would allow the neutrophil to penetrate the ablumenal portion of the endothelium without creating a direct conduit between the intravascular and extravascular space.
In vivo, endothelial but not neutrophil LSP1 was able to regulate neutrophil transendothelial cell migration.18 Loss of LSP1 in the endothelium resulted in the most profound inhibition in transmigration that we have ever reported.18 Because endothelial LSP1 was so critical to the emigration process18 and could bind F-actin, we tested the hypothesis that endothelial LSP1 left the nucleus and associated with the cytoskeleton, allowing the formation of domes in vivo during transendothelial migration. Our data show that in response to an inflammatory stimulus, endothelial LSP1 was indeed recruited from the nucleus as well as cytosol to the cytoskeleton. Moreover, endothelial domes were important during transendothelial migration in vivo; Lsp1−/− endothelium (in LSP1 knockout and chimeric animals lacking LSP1 only in the endothelium) often failed to construct these structures. Finally, disproportionately increased permeability despite reduced numbers of transmigrating neutrophils underscores the physiologic relevance of domes and the role for LSP1 in endothelial barrier integrity.
Methods
Antibodies
Mouse anti–human LSP1 mAb (Clone 16), rat mAb RB6-8C5 against mouse Ly-6G (Gr1), mAb RB6-8C5 against mouse Ly-6G conjugated to fluorescein isothiocyanate (FITC) or phosphatidylethanolamine (PE) and rat mAb against mouse PECAM-1 (Clone 390, which has been previously reported not to interfere with leukocyte recruitment28,29 ) were purchased from eBioscience. Other antibodies are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Animals
WT 129/SvJ and WT C57BL/6 mice were purchased from The Jackson Laboratory. Jongstra-Bilen and colleagues (Toronto General Hospital, ON) kindly supplied the Lsp1−/− mice, which were generated on the 129/SvJ background by homologous recombination as previously described and bred onto a C57BL/6 background.30 Both backgrounds behaved similarly in experiments. Lys-EGFP mice in which the enhanced GFP gene was knocked into the murine lysozyme M (lys) locus, were kindly provided by Thomas Graf (Albert Einstein College of Medicine, Bronx, NY) and generated as previously described.31 Bone marrow (BM)–chimeric mice were generated following standard protocols in our laboratory.18 All mice were bred at the University of Calgary animal center and used in experiments when they were between 8 and 16 weeks of age for studies involving neutrophil recruitment. All animal protocols and procedures were approved by the University of Calgary Animal Care Committee and conformed to Canadian Council for Animal Care guidelines.
Intravital microscopy
A mixture of 10 mg/kg xylazine (Bayer Inc Animal Health) and 200 mg/kg ketamine hydrochloride (Rogar/STB Inc) was injected intraperitoneally to anesthetize male mice. In all protocols, the left jugular vein was cannulated to administer additional anesthetics or antibodies. The mouse cremaster muscle was used to study neutrophil recruitment as previously described.18
Detection of endothelial domes by 2-photon microscopy
Our 2-photon system consisted of an FV300 laser scanning confocal unit (Olympus) that was modified in house to allow for multiphoton imaging (see Supplemental methods). For visualization the cremaster blood vessels were labeled with anti–mouse PECAM-1 Ab coupled to Alexa Fluor 594 or 488 (each 70 μg/animal). In some instances the endothelium was labeled with soybean agglutinin (SBA)–lectin coupled to Alexa Flour 555. Infiltrating neutrophils were labeled with anti–mouse Gr-1 coupled to Alexa 488 or 594 (each 50 μg/animal). To investigate endothelial domes under WT conditions, lys–enhanced green fluorescent protein (EGFP) mice were used and the vasculature was labeled with anti mouse PECAM-1 Ab coupled to Alexa Fluor 594 (70 μg/animal). The cremaster was imaged using 800 nm excitation at approximately 50 mW total power. To quantify endothelial domes, events were counted in a given vessel in the field of view along 100 μm of the venule and expressed as percentage, per event, of adherent cells.
Induction of neutrophil recruitment in cremaster muscle
To induce an inflammatory stimulus, the cremaster muscle was superfused with 5nM KC (R&D Systems, MN,). In some experiments, recombinant mouse TNFα (0.5 μg; R&D Systems) or IL-1β (50 ng; R&D Systems) in 200 μL of saline was injected intrascrotally before the experiment. The neutrophil rolling flux, rolling velocity, adherence, and emigration were measured in the cremasteric venule from 4 hours to 5.5 hours after the TNFα injection at 10-minute intervals. In the case of KC, the above parameters were measured every 10 minutes for 90 minutes after the start of the superfusion.
Microvascular permeability measurement
Whole-mount staining of cremaster muscle tissue
WT animals were injected with either 0.5 μg TNFα or 50 ng IL-1β in 200 μL of saline intrascrotally 3 hours before tissue acquisition. Preparation and staining of the cremaster muscle was done as described in Supplemental methods.
TEM
In some instances, after superfusion with 5nM KC, the cremaster preparation was separated and prepared for transmission electron microscopy (TEM) analysis as described in Supplemental methods.
Human umbilical vein endothelial cell isolation
Human umbilical vein endothelial cells (HUVECs) were harvested and cultured from fresh human cords as previously described.34
Subcellular fractionations
Subcellular fractionations were recovered using the Calbiochem ProteoExtract Subcellular Extraction Kit. Confluent HUVEC monolayers grown on gelatin in T75 flasks were used. As per kit protocol, the confluent monolayers were subjected to the buffers (I-IV) in sequential manner with the required protease inhibitor cocktails and enzymes. Fraction I (cytosol) Fraction II (membrane/organelle), Fraction III (nuclear material), and Fraction IV (cytoskeleton) were collected. Seventy-five microliters of the supernatant was removed, and 25 μL of 4× sample buffer was added to the samples. The samples were sonicated to dissolve the pellets and then loaded onto a 10% sodium dodecyl sulfate (SDS) gel for Western blotting.
Western blotting
Whole-cell lysates and cellular fractionations prepared from confluent HUVECs were subjected to electrophoresis on a 10% SDS–polyacrylamide gel, transferred to a nitrocellulose membrane, and blotted using mAbs and polyclonal Abs against specific proteins. HUVEC fractionations from the same day were tested on different gels. After washing, the membranes were incubated with a secondary Ab conjugated to horseradish peroxidase and developed using enhanced chemiluminescence reagents, with subsequent exposure to X-ray film for the desired amount of time. As there was still some variability of LSP1 expression in different HUVEC isolations, we compensated for this variability with densitometry on each of the blots using ImageJ software (v1.41o; National Institutes of Health; http://rsb.info.nih.gov/ij/). LSP1 expression levels in total lysate were also corrected against β-actin.
Statistical analysis
The data are expressed as means ± SEM. For comparing differences within 2 groups, a Student t test with Bonferroni correction was used. Analysis of variance was used for statistical analysis for the differences between more than 2 groups. A P value of less than .05 was considered statistically significant.
Results
Endothelial LSP1 associates with the cytoskeleton after stimulation with inflammatory mediators
In previous studies we have shown that LSP1 is expressed not only in leukocytes, but also in mouse and human endothelial cells.18 Using biochemical techniques, we further investigated in this study the overall expression and subcellular localization of endothelial LSP1 after stimulation. HUVEC cell lysates showed significantly increased expression of total LSP1 after stimulation with TNFα, whereas treatment with IL-8 (the human analog of murine KC) for either 30 minutes or 4 hours did not affect LSP1 (Figure 1A). Next, we subjected cultured adherent HUVECs to proteome extraction and isolated the different subcellular fractions, including the cytosol, membrane/organelles, nuclear material, and cytoskeleton. Under basal conditions, LSP1 signal was predominantly expressed in the cytosol and nuclear fraction, and to a much lesser degree in the organelle/membrane and cytoskeletal fractions (Figure 1B). On TNFα stimulation, there was a very noticeable increase in LSP1 in the cytoskeletal region, but not in the nuclear region. Treatment with leptomycin B (LMB, a general nuclear export inhibitor) increased the expression of LSP1 in the nuclear fraction, demonstrating that LSP1 was not able to shuttle out of the nucleus. Moreover, the cytoskeletal-associated LSP1 was strongly inhibited with the LMB inhibitor (Figure 1B). This clearly suggests that the cytoskeletal LSP1 was derived in a large part from the nucleus. Interestingly, LSP1 was also noted in the cytosol, but levels did not change on treatment with TNFα, suggesting a second pool of LSP1 outside the nucleus that may serve another function (Figure 1B).
Total cell lysates and subcellular proteome extraction of HUVECs stimulated with TNFα. (A) Representative Western blot of total cell lysates stimulated with TNFα and IL-8 and densitometry. HUVEC monolayers were stimulated for 30 minutes or 4 hours with IL-8, or for 4 hours with TNFα. (B) Rrepresentative Western blot of subcellular proteomic extraction. HUVEC monolayers were stimulated and treated with or without LMB (nuclear export inhibitor). Fractions were blotted for LSP1 (arrows), pan-cadherin (membrane marker), ATF2 (nucleus), and actin (cytoskeleton). Densitometry of the Western blots in panel A (n = 3) is relative to total actin (as standardization). Error bars indicate SEM. *P < .05.
Total cell lysates and subcellular proteome extraction of HUVECs stimulated with TNFα. (A) Representative Western blot of total cell lysates stimulated with TNFα and IL-8 and densitometry. HUVEC monolayers were stimulated for 30 minutes or 4 hours with IL-8, or for 4 hours with TNFα. (B) Rrepresentative Western blot of subcellular proteomic extraction. HUVEC monolayers were stimulated and treated with or without LMB (nuclear export inhibitor). Fractions were blotted for LSP1 (arrows), pan-cadherin (membrane marker), ATF2 (nucleus), and actin (cytoskeleton). Densitometry of the Western blots in panel A (n = 3) is relative to total actin (as standardization). Error bars indicate SEM. *P < .05.
In leukocytes, LSP1 is a substrate of MK2,35 a signaling molecule downstream of p38 MAPK. Under basal conditions MK2 predominantly localizes to the nucleus, but on cell activation it moves into the cytosol with bound p38 MAPK. To determine whether endothelial LSP1 is also under the regulation of the p38 MAPK pathway, we exposed the HUVEC monolayers to SKF86002, an inhibitor of p38 MAPK, and examined LSP1 translocation in response to TNFα. In the presence of SKF, there was no difference in the expression of LSP1 in the nuclear fraction. Expression of LSP1 in the cytoskeletal fraction was reduced (supplemental Figure 1). To confirm the activation of the p38 MAPK pathway, we examined phosphorylation of MK2. TNFα treatment induced the phosphorylation of MK2, and pretreatment with the SKF inhibitor blocked this phosphorylation of MK2 in the nucleus; no MK2 could be found in the cytoskeleton. Total p38 did not change under these conditions (data not shown).
Endothelial LSP1 is necessary for endothelial domes during neutrophil diapedesis
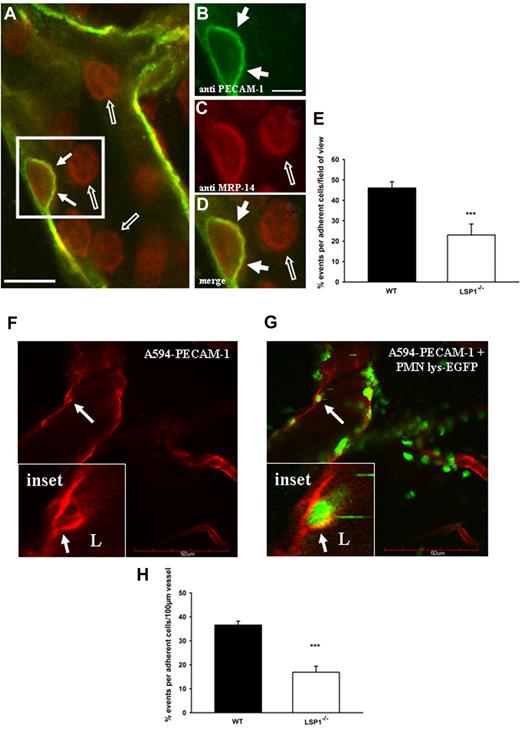
Three approaches were taken to analyze the role of LSP1 in dome formation in vivo: first, whole-mount staining of cremaster muscle tissue; second, 2-photon microscopy; third, transmission electron microscopy. As seen in Figure 2, whole mounts 3 hours after TNFα or IL-1β stimulation revealed endothelial domes in WT mice (filled arrows in Figure 2A, B, D; cremaster muscle treated with cytokine). Endothelial PECAM-1 surrounded a single adherent neutrophil (Figure 2B) and colocalized with the neutrophil marker (anti–Myeloid-related protein [MRP]–14). Most neutrophils were rounded, presumably rolling or free in circulation, and did not show PECAM-1–positive domes (open arrows in Figure 2A-D). As the formation of domes is a very dynamic and rapidly occurring process, few neutrophils inside the vessel of the whole mount were covered with domes at the time the tissue was fixed. Emigration was an equally rare event. Quantification of domes in Lsp1−/− animals showed fewer than half of these structures compared with WT animals (Figure 2E).
In vivo endothelial dome formation in WT mice visualized by whole-mount staining of the cremaster muscle and 2-photon microscopy. The cremaster of C57BL/6 WT mice was fixed in paraformaldehyde and stained for platelet endothelial cell adhesion molecule-1 (PECAM-1; green, Alexa 488) and MRP-14 (red, Alexa 568) 3 hours after application of IL-1β into the scrotum. (A) Overview merge of a postcapillary venule showing a migrating neutrophil covered by a dome (arrows in box; scale bar: 10 μm). (B-D) Magnifications of the area in panel A showing the single channels for PECAM-1 (B), MRP-14 (C), and the merge (D). The dome is highlighted by arrows. Presumably rolling and adherent cells not covered by a dome are highlighted by open arrows. Scale bar represents 5 μm. Image acquisition, panels A-D: Zeiss LSM510Meta confocal fluorescent microscope on Axiovert 200M (Zeiss), 40×/1.2 NA water C-Apochromat (Zeiss); DAKO fluorescent mounting medium; LSM510Meta photo-multiplier tubes (Zeiss); LSM image Examiner Version 4.0.0.241 (Zeiss). (E) Percentage quantification of the whole-mount staining based on the number of cells interacting with the vasculature/vessel in the field of view in WT vs Lsp1−/− animals. Eleven to 16 vessels per whole mount and per background (n = 5) were analyzed. ***P < .001. Error bars indicate SEM. For 2-photon microscopy, the cremaster of lys-EGFP mice was superfused with 5nM KC and the endothelium stained with anti–PECAM-1 Ab coupled to Alexa 594. (F) PECAM-1 positive stained endothelium demonstrating the formation of a dome (arrow). (G) Neutrophils (green) migrate and are encapsulated by endothelial domes (red) as highlighted by arrows. Insets represent magnifications of the dome and the encapsulated neutrophil from panels G and F. The dome reaches into the vessel lumen (L) and is highlighted by the arrow. Image acquisition, panels F-G: Olympus FV300 laser scanning confocal unit on Olympus BX61WI; Olympus 20×/0.95 NA water XLUMPLAN FI; bicarbonate superfusion buffer (132mM NaCI, 4.7mM KCI, 1.2mM MgSO4, Olympus Fluoview (FV300 O3D V5.0). (H) Percentage quantification of the 2-photon microscopy images in WT vs Lsp1−/− animals. Calculations were based on the number of cells interacting with the vasculature/vessel in the field of view (5- 8 vessels per mouse were observed; each group contained at least 5 animals). ***P < .001. Error bars indicate SEM. Scale bar represents 50 μm.
In vivo endothelial dome formation in WT mice visualized by whole-mount staining of the cremaster muscle and 2-photon microscopy. The cremaster of C57BL/6 WT mice was fixed in paraformaldehyde and stained for platelet endothelial cell adhesion molecule-1 (PECAM-1; green, Alexa 488) and MRP-14 (red, Alexa 568) 3 hours after application of IL-1β into the scrotum. (A) Overview merge of a postcapillary venule showing a migrating neutrophil covered by a dome (arrows in box; scale bar: 10 μm). (B-D) Magnifications of the area in panel A showing the single channels for PECAM-1 (B), MRP-14 (C), and the merge (D). The dome is highlighted by arrows. Presumably rolling and adherent cells not covered by a dome are highlighted by open arrows. Scale bar represents 5 μm. Image acquisition, panels A-D: Zeiss LSM510Meta confocal fluorescent microscope on Axiovert 200M (Zeiss), 40×/1.2 NA water C-Apochromat (Zeiss); DAKO fluorescent mounting medium; LSM510Meta photo-multiplier tubes (Zeiss); LSM image Examiner Version 4.0.0.241 (Zeiss). (E) Percentage quantification of the whole-mount staining based on the number of cells interacting with the vasculature/vessel in the field of view in WT vs Lsp1−/− animals. Eleven to 16 vessels per whole mount and per background (n = 5) were analyzed. ***P < .001. Error bars indicate SEM. For 2-photon microscopy, the cremaster of lys-EGFP mice was superfused with 5nM KC and the endothelium stained with anti–PECAM-1 Ab coupled to Alexa 594. (F) PECAM-1 positive stained endothelium demonstrating the formation of a dome (arrow). (G) Neutrophils (green) migrate and are encapsulated by endothelial domes (red) as highlighted by arrows. Insets represent magnifications of the dome and the encapsulated neutrophil from panels G and F. The dome reaches into the vessel lumen (L) and is highlighted by the arrow. Image acquisition, panels F-G: Olympus FV300 laser scanning confocal unit on Olympus BX61WI; Olympus 20×/0.95 NA water XLUMPLAN FI; bicarbonate superfusion buffer (132mM NaCI, 4.7mM KCI, 1.2mM MgSO4, Olympus Fluoview (FV300 O3D V5.0). (H) Percentage quantification of the 2-photon microscopy images in WT vs Lsp1−/− animals. Calculations were based on the number of cells interacting with the vasculature/vessel in the field of view (5- 8 vessels per mouse were observed; each group contained at least 5 animals). ***P < .001. Error bars indicate SEM. Scale bar represents 50 μm.
To visualize the dynamic formation of domes in vivo, neutrophil interactions with the endothelium of the microvasculature were visualized using 2-photon microscopy. Although both cytokines and chemokines induced dome-associated neutrophil adhesion and subsequent emigration, the chemokine KC induced more concentrated emigration in a shorter period of time, allowing observation of more frequent dome formations. Figure 2F shows the formation of a dome (arrows) around a lys-EGFP neutrophil (Figure 2G). Higher digital magnification reveals that the dome (arrows) is forming around the neutrophil (Figure 2G insets). On occasion, rolling neutrophils (open arrow in supplemental Figure 2B) could be seen rolling across a dome covering a neutrophil (filled arrow in supplemental Figure 2A-B insets). The dynamic process of a dome forming up the sides of an adhering neutrophil over time is shown in supplemental Figure 3. At the end stage of this dynamic process, the formed domes completely cover the transmigrating neutrophil (as seen in other in vivo experiments; arrows in supplemental Figure 4A-B insets). Quantification of the in vivo images showed that endothelial domes in Lsp1−/− animals were found at 40% frequency compared with WT animals (Figure 2H). For completeness, we also performed experiments where we injected TNFα into the scrotum to activate the cremaster vasculature. Whereas 4 hours after injection the majority of the neutrophils could be found outside the vessel, at an earlier time point (2 hours) neutrophils could be seen transmigrating. Similar to the results obtained with the chemokine superfusion, endothelial domes could be observed in WT animals, but were less frequently seen in Lsp1−/− animals (data not shown). From z-stacks we were also able to create a 3-dimensional image and a video of a migrating neutrophil, which enabled us to visualize the endothelial domes in more detail in z-stacks over time (supplemental Figure 5 and supplemental Video 1). The cells that appear blurry represent crawling or rolling neutrophils.
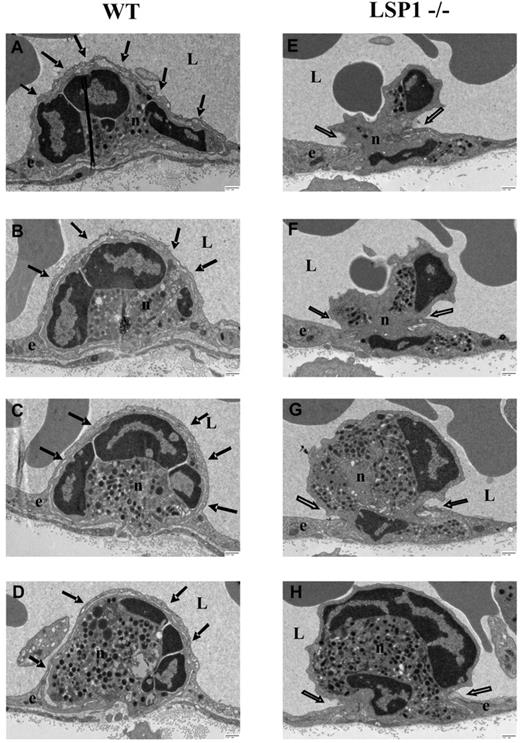
Using transmission electron microscopy, a striking difference was consistently observed between the WT and Lsp1−/− mice. Neutrophils in WT mice were completely covered by the endothelium, whereas in Lsp1−/− mice the endothelium often only covered the neutrophil pseudopod and did not climb up the sides of the extravasating neutrophil. Figure 3 shows electron micrographs and a cartoon representation of these endothelial domes (right panel). The WT neutrophils are completely covered by the endothelium, but endothelium was also below the neutrophil (Figure 3A arrows e1); in Lsp1−/− animals, the neutrophils have pushed very long pseudopods displacing the endothelium, yet neither an endothelial dome nor a clear docking structure can be seen (Figure 3B arrows). WT neutrophils had much smaller pseudopod extensions before the endothelium moved up the sides. Although we cannot absolutely state that eventually a dome-like structure did not form in Lsp1−/− mice, we never saw the huge pseudopods with no endothelium moving up the sides of WT neutrophils. Serial sections of transmigrating neutrophils demonstrated that the endothelium was indeed covering the whole cell body of the neutrophil whenever a serial section was taken (Figure 4A-D arrows; supplemental Figure 8) in WT animals, whereas in Lsp1−/− animals no endothelial dome structure could be observed (Figure 4E-H open arrows). Although in some serial sections domes could be observed while neutrophils were migrating in what appeared to be a transcellular fashion (Figure 4A-D arrows), in most cases formation of domes in WT animals were observed while the neutrophils were emigrating between 2 endothelial cells (Figure 3A arrows e2 and e3). In those situations, both endothelial cells contributed to the formation of the dome structure. Quantification of the transmission electron microscopy sections revealed that endothelial dome formation was reduced by more than half in Lsp1−/− animals compared with WT animals (Figure 3C). Whether the dome formed in LSP1−/− mice only after a very significant proportion of the neutrophil had invaginated the endothelium, or whether another protein can replace LSP1, remains unknown.
Electron micrographs of migrating neutrophils in WT and LSP1−/− mice. (A) WT; (B) Lsp1−/− mice. Sections (70 nm) were taken after KC superfusion (5nM) of the cremaster. Images and corresponding cartoons demonstrate endothelial dome formations (arrows) in WT mice (A) and the lack of such formations in LSP1 deficient mice (B arrow). Scale bars indicate 2 μm (A) and 1 μm (B). Image acquisition, panels A-B: Hitachi H-7000 transmission electron microscope; direct magnification: 3000× (A), 4000× (B); 16000 AMT camera, AMT Capture Engine software (V.600.128); images generated with Microsoft Office PowerPoint 2003 (SP3). Percentage quantification of the electron micrograph sections based on the adherent neutrophils per vessel that underwent transendothelial migration in WT vs Lsp1−/− animals (C). Thirty-eight to 42 vessel sections per background were analyzed. ***P < .001. Error bars indicate SEM. e1-e3 indicate endothelial cells; n, neutrophil; and L, vessel lumen.
Electron micrographs of migrating neutrophils in WT and LSP1−/− mice. (A) WT; (B) Lsp1−/− mice. Sections (70 nm) were taken after KC superfusion (5nM) of the cremaster. Images and corresponding cartoons demonstrate endothelial dome formations (arrows) in WT mice (A) and the lack of such formations in LSP1 deficient mice (B arrow). Scale bars indicate 2 μm (A) and 1 μm (B). Image acquisition, panels A-B: Hitachi H-7000 transmission electron microscope; direct magnification: 3000× (A), 4000× (B); 16000 AMT camera, AMT Capture Engine software (V.600.128); images generated with Microsoft Office PowerPoint 2003 (SP3). Percentage quantification of the electron micrograph sections based on the adherent neutrophils per vessel that underwent transendothelial migration in WT vs Lsp1−/− animals (C). Thirty-eight to 42 vessel sections per background were analyzed. ***P < .001. Error bars indicate SEM. e1-e3 indicate endothelial cells; n, neutrophil; and L, vessel lumen.
Serial sections from micrographs of migrating neutrophils in WT and Lsp1−/− mice. Sections were taken after KC superfusion (5nM) of the cremaster. Serial sections (each 70 nm) were cut 350 nm apart. Filled arrows demonstrate endothelial dome formations in WT mice (A-D), and open arrows point the lack of such formations in LSP1-deficient mice (E-H). Scale bar indicates 500 nm. e indicates endothelial cell; n, neutrophil; and L, vessel lumen. Image acquisition: Hitachi H-7000 transmission electron microscope; direct magnification: 6000×; 16000 AMT camera, AMT Capture Engine software (V.600.128); images generated with Microsoft Office PowerPoint 2003 (SP3).
Serial sections from micrographs of migrating neutrophils in WT and Lsp1−/− mice. Sections were taken after KC superfusion (5nM) of the cremaster. Serial sections (each 70 nm) were cut 350 nm apart. Filled arrows demonstrate endothelial dome formations in WT mice (A-D), and open arrows point the lack of such formations in LSP1-deficient mice (E-H). Scale bar indicates 500 nm. e indicates endothelial cell; n, neutrophil; and L, vessel lumen. Image acquisition: Hitachi H-7000 transmission electron microscope; direct magnification: 6000×; 16000 AMT camera, AMT Capture Engine software (V.600.128); images generated with Microsoft Office PowerPoint 2003 (SP3).
Endothelial LSP1 is important to maintaining physiologic barrier function in inflammation
The cremaster muscle of WT and LSP1-deficient mice was superfused with 5nM of KC. There was no difference in the rolling behavior of the neutrophils (Figure 5A) and slightly fewer adherent cells (Figure 5B), but a large decrease in emigration (Figure 5C) in LSP1−/− compared with WT mice. Unexpectedly, the increase in permeability was comparable in both WT and LSP1-deficient animals (Figure 5D) although LSP1-deficient mice had fewer neutrophils crossing the endothelial barrier (Figure 5C). The increased permeability could be reduced to baseline in Lsp1−/− and WT mice if neutrophils were depleted, suggesting that the neutrophils were causing the increase in permeability in both strains of mice (Figure 5E). Clearly, the increase in permeability per migrating neutrophil was significantly lower in WT than in LSP1-deficient mice after 60 minutes of neutrophil emigration (Figure 5F). Injection of TNFα 4 hours before the cremaster preparation showed similar results in neutrophil behavior and permeability measurements (supplemental Figure 6).
Microvascular permeability changes in cremasteric venules of WT and Lsp1−/− mice. Measurements were taken after KC superfusion of the cremaster muscle preparation. Besides equal rolling flux (A) and decreased adhesion (B), LSP1-deficient animals show impaired neutrophil transmigration (C), but equal microvascular leakiness (D) compared with WT animals. Depletion of neutrophils with anti Gr-1 Ab injection 24 hours before experiments shows a return of permeability to baseline levels (E). The amount of permeability normalized for number of emigrated neutrophils, as shown 60 minutes after KC superfusion (F). Each group contained at least 3 animals. *P < .05; ***P < .001. Error bars indicate SEM.
Microvascular permeability changes in cremasteric venules of WT and Lsp1−/− mice. Measurements were taken after KC superfusion of the cremaster muscle preparation. Besides equal rolling flux (A) and decreased adhesion (B), LSP1-deficient animals show impaired neutrophil transmigration (C), but equal microvascular leakiness (D) compared with WT animals. Depletion of neutrophils with anti Gr-1 Ab injection 24 hours before experiments shows a return of permeability to baseline levels (E). The amount of permeability normalized for number of emigrated neutrophils, as shown 60 minutes after KC superfusion (F). Each group contained at least 3 animals. *P < .05; ***P < .001. Error bars indicate SEM.
It is possible that the LSP1-adherent neutrophils are more activated and increase the release of mediators that induce permeability increases. We tested responses to KC in chimeric mice where WT neutrophils were induced to adhere to Lsp1−/− endothelium and Lsp1−/− neutrophils were induced to adhere to WT endothelium. Both sets of chimeric mice demonstrated identical leukocyte rolling flux (Figure 6A) and adhesion (Figure 6B) on KC superfusion of the muscle microvasculature. Chimeras that lacked LSP1 only in their leukocytes emigrated as effectively across the vasculature as WT mice (Figure 6C), and normal permeability changes occurred in response to KC administration, suggesting that adhesion per se of Lsp1−/− neutrophils was not sufficient to induce exaggerated increases in permeability. In contrast, WT leukocytes reconstituted in Lsp1−/− mice (that is, lacking LSP1 in endothelium) displayed much more subtle transendothelial migration (Figure 6C), but the permeability in animals lacking LSP1 only in the endothelium was significantly higher (Figure 6D). The permeability per migrating neutrophil was also only increased in the Lsp1−/− endothelium (Figure 6F). Obviously, the WT neutrophils attempting to cross the LSP1-deficient endothelium did so poorly and with increased permeability. The lack of LSP1 in the endothelium is clearly responsible for the permeability changes, which occur through the neutrophils. Depletion of the neutrophils eliminated increases in permeability in both strains of mice (Figure 6E).
Microvascular permeability changes in cremasteric venules of chimeric mice (Lsp1−/− into WT and WT into Lsp1−/−). Measurements were taken after KC superfusion of the cremaster muscle preparation. Besides equal rolling flux (A) and adhesion (B), WT into LSP-1 deficient animals (lacking LSP1 only in the endothelium) show impaired neutrophil transmigration (C) and increased microvascular leakiness (D) compared with Lsp1−/− into WT animals (lacking LSP1 only in the neutrophils). Depletion of neutrophils with anti Gr-1 Ab injection 24 hours before experiments shows a return of permeability to baseline levels (E). The amount of permeability normalized for number of emigrated neutrophils as shown 60 minutes after KC superfusion (F). Each group contained at least 3 animals. *P < .05; **P < .01; ***P < .001. Error bars indicate SEM.
Microvascular permeability changes in cremasteric venules of chimeric mice (Lsp1−/− into WT and WT into Lsp1−/−). Measurements were taken after KC superfusion of the cremaster muscle preparation. Besides equal rolling flux (A) and adhesion (B), WT into LSP-1 deficient animals (lacking LSP1 only in the endothelium) show impaired neutrophil transmigration (C) and increased microvascular leakiness (D) compared with Lsp1−/− into WT animals (lacking LSP1 only in the neutrophils). Depletion of neutrophils with anti Gr-1 Ab injection 24 hours before experiments shows a return of permeability to baseline levels (E). The amount of permeability normalized for number of emigrated neutrophils as shown 60 minutes after KC superfusion (F). Each group contained at least 3 animals. *P < .05; **P < .01; ***P < .001. Error bars indicate SEM.
Differential effects of endothelial LSP1 in different inflamed vascular beds
The emigration of neutrophils in the peritoneum is quite different from other organs using opaque sites. Indeed, we previously found that neutrophils emigrated more in Lsp1−/− mice than in WT mice.18 We therefore checked whether there were more domes in WT than in Lsp1−/− animals in the peritoneum. Although electron microscopy revealed (Figures 7A and B) endothelial domes (arrows) during diapedesis after 4 hours of peritoneal cytokine treatment, endothelial dome formation was less frequent in the peritoneal endothelium compared with cremaster endothelium. Importantly, dome formation in LSP1-deficient animals was not different from WT controls in peritoneum (Figure 7C). Domes could be seen in the peritoneum of Lsp1−/− animals surrounding neutrophils, but this was a rare event (Supplemental Figure 7). Vascular leakage in the peritoneum based on Evan blue dye injection showed no difference between LSP1-deficient and WT animals at an early time point (2 hours) after saline or cytokine injection (Figure 7D). At a later time point (4 hours), LSP1-deficient animals showed a slight increased permeability into the peritoneal cavity compared with WT mice, which was consistent with the greater number of emigrating neutrophils. Surprisingly, saline-treated LSP1-deficient animals showed an increased Evan blue leakage into the peritoneal cavity at 4 hours.
Endothelial dome formation and microvascular permeability changes in peritoneal venules of WT and Lsp1−/− mice. Measurements were taken 2 or 4 hours after IL-1β injection into the peritoneum. Image and corresponding image demonstrate endothelial dome formation (filled arrows) and the overlap of endothelial cell borders (open arrow) in Lsp1−/− mice (A-B) 4 hours after IL-1β injection. Scale bar indicates 500 nm. Image acquisition, panels A-B: Hitachi H-7000 transmission electron microscope; direct magnification: 4000×; 16000 AMT camera, AMT Capture Engine software (V.600.128); images generated with Microsoft Office PowerPoint 2003 (SP3). Percentage quantification of the electron micrograph sections based on the adherent neutrophils per vessel that underwent transendothelial migration with domes in WT vs Lsp1−/− animals in the cremaster and the peritoneum (C). Twenty to 25 vessel sections per background were analyzed. (D) WT or Lsp1−/− mice were injected with Evan blue and injected with saline or IL-1β as indicated; 2 or 4 hours after injection, the dye that leaked out into the peritoneum was quantified. *P < .05; ***P < .001. Error bars indicate SEM. e indicates endothelial cell; n, neutrophil; p, pericyte; and L, vessel lumen.
Endothelial dome formation and microvascular permeability changes in peritoneal venules of WT and Lsp1−/− mice. Measurements were taken 2 or 4 hours after IL-1β injection into the peritoneum. Image and corresponding image demonstrate endothelial dome formation (filled arrows) and the overlap of endothelial cell borders (open arrow) in Lsp1−/− mice (A-B) 4 hours after IL-1β injection. Scale bar indicates 500 nm. Image acquisition, panels A-B: Hitachi H-7000 transmission electron microscope; direct magnification: 4000×; 16000 AMT camera, AMT Capture Engine software (V.600.128); images generated with Microsoft Office PowerPoint 2003 (SP3). Percentage quantification of the electron micrograph sections based on the adherent neutrophils per vessel that underwent transendothelial migration with domes in WT vs Lsp1−/− animals in the cremaster and the peritoneum (C). Twenty to 25 vessel sections per background were analyzed. (D) WT or Lsp1−/− mice were injected with Evan blue and injected with saline or IL-1β as indicated; 2 or 4 hours after injection, the dye that leaked out into the peritoneum was quantified. *P < .05; ***P < .001. Error bars indicate SEM. e indicates endothelial cell; n, neutrophil; p, pericyte; and L, vessel lumen.
Discussion
Recent work has demonstrated that during emigration, the endothelium plays a critical role in forming docking structures by extending projections up the side of the neutrophil, presumably facilitating the emigration across the vessel wall.7,26,27,36 Herein, we observed that one side of the docking structure extended all the way over the neutrophil and covered the cell in vivo. Numerous other in vivo studies have seen these domes,8,37-39 but this has not been noted in vitro despite detailed presentation of emigrating neutrophils across endothelium7,9,36,40 This raises, however, the possibility that in vitro, the endothelium is restricted to making only docking structures, perhaps because of the absence of a critical factor found in vivo (such as shear or hydrostatic pressure), or that the dome formation fails to form because of the absence of basement membrane and other key components. It is probable that a source of additional membrane for the formation of docking structures and domes would be required. Indeed, Mamdouh and colleagues reported membrane compartments in the endothelium adjacent to junctions that were mobilized during paracellular and transcellular emigration processes.41-43 Perhaps there are more of these storage compartments in vivo than in vitro, limiting the degree of dome formation in the latter setting.
As the formation of the domes as well as the docking structures is very dynamic, the endothelium would require significant cytoskeletal involvement. Indeed, in this study, the endothelial F-actin binding protein, LSP1, which moves from the nucleus to the cytosol and is associated with the cytoskeleton on stimulation, was important for the formation of the dome structures. Although the reason for storage of proteins in the nucleus is unclear, such storage is not unprecedented. Indeed, numerous proteins, including MK2, a protein upstream of LSP1, have been found in the nucleus and are mobilized to the cytosol during activation.23 This could be a strategy to compartmentalize actin-binding proteins away from the target site until they are absolutely needed. Overproduction of LSP1 in patients with neutrophil actin dysfunction (NAD47/89) or forced overexpression in highly motile cells led to hair-like F-actin–rich projections with severe crawling defects.44,45 Clearly, fine regulation of LSP1 is required for proper cytoskeletal dependent effects. We report that lack of LSP1 also impairs motility in endothelium. Although in some cases LSP1-deficient endothelium still formed domes, this may be more active penetration by neutrophils then active covering by Lsp1−/− endothelium. In most cases the Lsp1−/− endothelium remained in the same plane along the entire vessel, and as such did not move up the sides of adhering neutrophils.
The less frequent dome formation in the Lsp1−/− endothelium was associated with very few neutrophils emigrating out of the vasculature. Although there was a decrease in adhesion of approximately 30%, the emigration was inhibited by more than 90%, suggesting that a simple reduction in adhesion could not explain the reduced emigration. Moreover, when chimeric mice were made, identical levels of adhesion were noted but emigration was only impaired in the Lsp1−/− endothelium despite the presence of WT neutrophils. Although LSP1 is also found in neutrophils, LSP1-deficient neutrophils emigrated across WT endothelium unperturbed but WT neutrophils could not cross LSP1-deficient endothelium as shown in this study and elsewhere.18 This clearly suggests that impairment in the endothelium was mediating the reduced emigration. In this study, LSP1-deficient endothelium failed to project up the sides of the neutrophils and cover the neutrophils with domes. In fact, we found unusually large pseudopods penetrating the endothelium in the absence of any change in endothelial structure (see Figures 3B and 4E-H). Previous work by Carman et al reported that inhibition of SNARE protein–containing membrane fusion complexes were required for the permissive structures created by endothelium to permit emigration.36 Inhibition of these structures in the study by Carman and colleagues,36 much like inhibition of LSP1 in our paper, resulted in impaired emigration, highlighting the key contribution by the endothelium.
Our data suggest that the neutrophil is dependent on the endothelium for emigration. Interestingly, it has been reported that beads approximately the size of neutrophils could be enveloped by endothelium and would be released on the other side of the barrier, consistent with the view that the endothelium can move even inert objects from the lumenal to the ablumenal side.46 Indeed, once the dome is formed over the top of the neutrophil, the endothelium beneath the neutrophil could then retract, allowing easy passage for the neutrophil. By extension, the neutrophil may not be permitted to move across the ablumenal endothelium unless the dome formation is complete. Although the concept of one cell taking up another cell by invasion has been demonstrated during epithelial cell cancer (entosis or emperiopolesis),47 it is important to note that at no time did we see neutrophils inside the cytosol of the endothelium, that is, no emperiopolesis. We always saw a double membrane with gaps around the neutrophil. One reason endothelial domes may occur is to prevent the neutrophil from creating significant breaches in the endothelial barrier, thereby preventing excess fluid and protein from leaking into the interstitium. Indeed, the net positive hydrostatic pressure inside the postcapillary venule would force intravascular fluid, proteins, and particles to move out of the vasculature. One could imagine that the dome might function like an air-lock type seal (such as in a submarine), which prevents major leakage by first closing the top portal and then opening the bottom portal, allowing only a minimal amount of protein and fluid leakage out of the vasculature. Although there is growing acceptance of the view that a neutrophil could migrate either at endothelial junctions or directly through the endothelium,1,2,48 one might imagine significant disruption of the endothelial barrier if the neutrophil forced its way straight through the body of the endothelium. However, with the formation of a dome, subsequent transcellular or paracellular emigration could occur with limited vascular disruption. Domes could serve to limit vascular leakage regardless of the route of emigration. Indeed, we observed endothelial dome formation during transcellular and paracellular diapedesis events.
Study limitations
Although it is tempting to conclude that the lack of domes in Lsp1−/− mice explains the disproportional increase in permeability, it is plausible that excessive soluble factors released by Lsp1−/− adherent and emigrating neurophils could contribute to increase the permeability.49 However, when Lsp1−/− neutrophils were stimulated to cross the WT endothelium, excessive permeability increases were not seen. Nevertheless, it is also plausible that regardless of the neutrophil genotype, the Lsp1−/− endothelium delays or prevents emigration, causing the neutrophil to become overly activated and thereby increasing permeability. Previous results have shown that histamine, a mediator that increases permeability by retracting the endothelium independent of neutrophils, caused lesser permeability increase in LSP1-deficient animals.18 Clearly, LSP1 may also affect retraction of the endothelium by the cytoskeleton, further supporting a role for LSP1 regulating cytoskeletal events in endothelium. Our data support LSP1 as a new player during regulation of fluid and protein leakage out of the inflamed blood vessel.
Serial sections were performed in an attempt to show that the dome covered the entire neutrophil. Although we could never obtain serial sections of the entire neutrophil, none of the serial sections ever revealed that that the covering was discontinuous at any point over the neutrophil, suggesting that a true dome was formed by the endothelium, and that this dome was always followed by the neutrophil emigrating across the underlying endothelium, as we were able to observe using our 2-photon microscopy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Carla Badick and Lori Zbytnuik for their expert assistance in animal care, and Pina Colarusso for the management of the imaging facility and her help in the development of the imaging assays.
This work was supported by the Canadian Heart and Stroke Foundation and by an equipment and infrastructure grant from the Canadian Foundation for Innovation and the Alberta Science and Research Authority. B.P. is supported by a postdoctoral fellowship from the Alberta Heritage Foundation for Medical Research (AHFMR; CA#2997) and by the Immunology Training Program of the Canadian Institutes of Health and Research (CIHR) at the University of Calgary. P.K. is a Canada Research Chair and an Alberta Heritage Foundation for Medical Research Scientist and the Calvin, Phoebe and Joan Snyder Chair in Critical Care Medicine. H.L., S.B., and D.V. are supported by SFB 492 of the Deutsche Forschungsgemeinschaft and by the Max-Planck-Society.
Authorship
Contribution: B.P., J.K., E.M.L., H. L., S.A.P., and K.D.P., performed, designed, and analyzed experiments; P.K. initiated the study, designed, and supervised the research project. All experiments were done in the laboratory of P.K. or connected core facilities (except whole-mount staining of the cremaster, done by H.L. and S.B. in the laboratory of D.V.); S.B., D.V., and S.M.R. provided valuable reagents; M.P. and K.D.P. contributed to data interpretation; and B.P. and P.K. contributed to data analysis and interpretation and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Paul Kubes, University of Calgary, Immunology Research Group, Department of Physiology and Pharmacology, The Calvin, Phoebe, and Joan Snyder Institute for Infection, Immunity & Inflammation, 3280 Hospital Dr NW, Calgary, AB T2N 4N1, Canada; e-mail: pkubes@ucalgary.ca.
References
Author notes
B.P. and J.K. contributed equally to this work.