Abstract
To explore the potential for monoclonal antibodies as a treatment for immune thrombocytopenia (ITP) and to further explore their mechanisms of action, we tested 8 monoclonal CD44 antibodies in murine ITP and found 4 antibodies that could successfully ameliorate ITP; 2 of these antibodies function at a full 3-log fold lower dosage compared with IVIg. Further characterization of the 2 most successful antibodies (5035–41.1D and KM114) demonstrated that, similar to IVIg: (1) the presence of the inhibitory IgG receptor FcγRIIB was required for their ameliorative function, (2) complement-deficient mice responded to anti-CD44 treatment, and (3) human transgenic FcγRIIA-expressing mice also responded to the CD44 therapeutic modality. Dissimilar to IVIg, the Fc portion of the CD44 antibody was not required. These data demonstrate that CD44 antibodies can function therapeutically in murine ITP and that they could potentially provide a very-low-dose recombinant therapy for the amelioration of human ITP.
Introduction
Intravenous immunoglobulin (IVIg) is used to treat autoimmune diseases, such as immune thrombocytopenia (ITP). IVIg is a limited resource, and its dosage and cost are both high. Although considered safe, it will always carry a theoretical risk of transferring infectious disease. Thus, it would be highly desirable to improve the efficacy of IVIg or develop monoclonal antibodies capable of mimicking the clinical effects of IVIg. Work by others has successfully improved the efficacy of IVIg in murine models of autoimmunity: It has been shown that enriched sialylated IVIg has a therapeutic effect at a 1-log-fold lower dosage than IVIg1 and a sialylated recombinant Fc fragment of IgG functions successfully at a 2-log fold lower dosage than IVIg.2
CD44 is a widely expressed cell surface polymorphic glycoprotein. It is involved in many processes, including tumor metastasis and inflammation. CD44 antibodies have been successfully used to treat several murine autoimmune disease models, including inflammatory arthritis and experimental autoimmune encephalomyelitis, although their mechanism of action remains unclear. CD44 antibodies can inhibit CD4 T-cell proliferation,3 leukocyte recruitment,4 leukocyte migration,5 and leukocyte rolling6 and induce apoptosis in leukemic cells.7 Despite these functions of CD44 antibodies, in this report we find that CD44 antibody KM114 ameliorates thrombocytopenia in SCID mice; this suggests that the rapid effects of this antibody are not primarily functioning via interaction with CD44 on B or T cells in this passive ITP model.
Here we show that select CD44 antibodies can function therapeutically in murine ITP in an FcγRIIB-dependent, complement-independent manner and only require the F(ab′)2 portion. As this murine model of ITP has successfully predicted responses to another agent,8 we speculate that antibodies to CD44 could potentially provide a very-low-dose recombinant therapy for the treatment of ITP.
Methods
Mice and reagents
C57BL/6 mice, CD44-deficient mice (B6.129(Cg)-Cd44tm1Hbg/J), C3-deficient mice (B6.129S4-C3tm1Crr/J) and FcγRIIB-deficient mice (B6;129S-Fcgr2btm1Ttk/J) were purchased from The Jackson Laboratory. IVIg was either Gamimune, 10% (Bayer) or Gamunex 10% (Talecris Biotherapeutics). Mice expressing the human FcγRIIA transgene were generated as previously described.9 The integrin αIIb antibody (clone MWReg30), the anti-red blood cell (RBC) antibody (clone TER119), and the CD44 monoclonal antibodies KM114 and IM7 were from BD Biosciences, KM81, KM201, CD44v4 (10D1), CD44v6 (9A4), and 5035-41.1D were purchased from Cedarlane, and IRAWB14 was kindly provided by Dr Katalin Mikecz (Rush University Medical Center, Chicago, IL). The F(ab′)2 fragment of antibody KM114 was produced and purified using a Pierce F(ab′)2 Preparation Kit (Thermo Scientific) according to the manufacturer's instructions. All mice experiments were approved by the St Michael's Hospital Animal Care Committee.
Induction and reversal of ITP
Murine thrombocytopenia was induced as described.10 Platelet counts were determined using a Beckman Z2 Coulter Counter (Beckman Coulter) as described.11 For antibody-mediated inhibition of thrombocytopenia, mice were injected intravenously with 50 μg CD44 antibody or TER-119 antibody in a volume of 200 μL or intraperitoneally with 50 mg (∼ 2 g/kg) IVIg in a volume of 500 μL 24 hours before the induction of ITP. For more detail, see supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
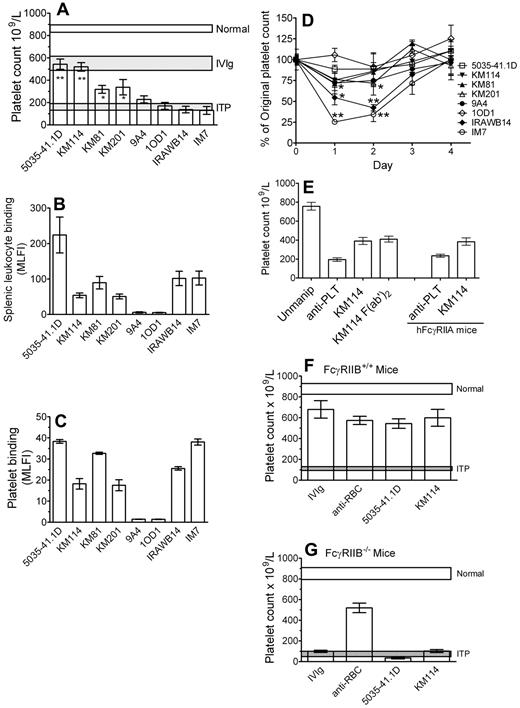
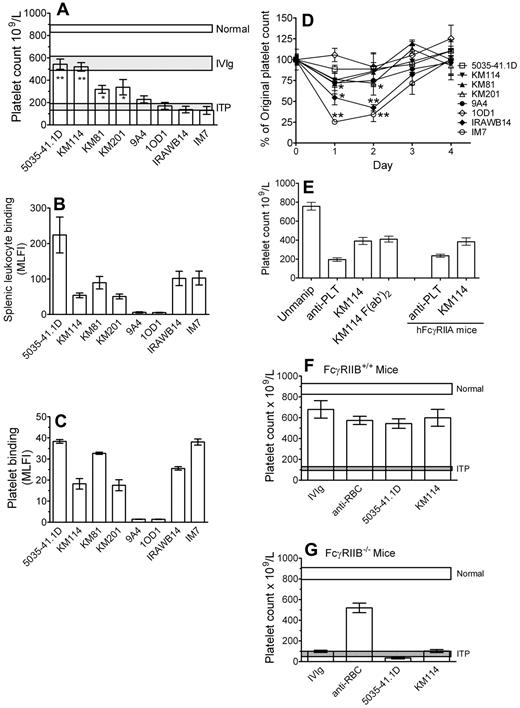
CD44 antibodies have been successfully used to treat murine autoimmune disease models.4,12-16 To determine whether CD44 antibodies could be used as an IVIg replacement therapy in the treatment of a murine model of ITP,17 we characterized 8 monoclonal CD44 antibodies of different specificity and isotype for their ability to reverse thrombocytopenia mediated by antiplatelet antibody, bind leukocytes and, as CD44 is expressed on murine platelets, to bind murine platelets, and induce thrombocytopenia (Table 1). Four of the 8 antibodies could significantly protect mice from antibody-induced thrombocytopenia at a 3-log-fold lower dosage than IVIg (Figure 1A columns 1-4). Two of these antibodies, 5035-41.1D and KM114, were as successful as IVIg in their efficacy at this 3-log fold lower dosage.
Antibodies to CD44 can ameliorate murine ITP and do so in a manner dependent on the presence of the inhibitory FcγRIIB but independent of the Fc region of IgG. (A) Mice were injected with 50 μg CD44 antibody (5035-41.1D, KM114, KM81, KM201, 9A4, IOD1, IRAWB14, or IM7) or 50 mg IVIg (∼ 2 g/kg of body weight). Twenty-four hours later, mice were injected with 2 μg antiplatelet antibody to induce thrombocytopenia. Mice were bled after a further 24 hours, and the platelet count was determined. Horizontal bars represent the range of the mean platelet count (± 1 SEM) of naive, antiplatelet antibody injected mice, and IVIg treated mice. n = 9 mice for each group from 3 independent experiments. *P < .01 versus ITP. **P < .001 versus ITP. (B) CD44 binding to splenic leukocytes was assessed as follows: RBC-depleted mouse splenic leukocytes were incubated with the indicated CD44 antibody followed by the appropriate fluorescein isothiocyanate-labeled secondary anti-IgG and assessed for mean log fluorescence intensity (MLFI) by flow cytometry. The values are the mean of 2 independent experiments. (C) To determine the extent of reactivity of murine platelets with the CD44 antibodies used in this study, murine platelets were incubated with the indicated CD44 antibody followed by appropriate fluorescein isothiocyanate-labeled secondary anti-IgG and assessed for MLFI by flow cytometry. The values are the mean of 4 independent experiments. (D) Mice were injected with 50 μg of the indicated CD44 antibodies to assess the ability of each antibody to induce thrombocytopenia in the absence of antiplatelet antibody. Platelet counting was evaluated before injection and after 24, 48, 72, and 96 hours. n = 6 mice for each group from 2 independent experiments. *P < .05 versus prebleed values. **P < .001 versus prebleed values. (E) C57BL/6 mice (columns 1-4) were treated and bled and platelets counted as in panel A, except that mice were treated with either 50 μg intact KM114 or 37 μg F(ab′)2 KM114 (the equivalent molar concentration). n = 6 mice for each group from 3 independent experiments. Mice expressing human FcγRIIA (columns 5 and 6) were injected with antiplatelet antibody alone or antiplatelet antibody plus KM114 as in panel A and platelets were counted as in panel A. n = 6 mice for each group from 2 independent experiments. (F) Wild-type mice (FcγRIIB+/+) or (G) mice genetically deficient in the inhibitory Fcγ receptor RIIB (FcγRIIB−/−) were injected with 50 mg IVIg, 50 μg anti-RBC monoclonal antibody (TER119), or 50 μg of the indicated CD44 antibodies (KM114 or 5035–41.1D). Twenty-four hours later, all mice were given 2 μg antiplatelet antibody to induce thrombocytopenia. After a further 24 hours, mice were bled for platelet counting. Normal and ITP are described as in panel A. n = 9 mice for each group from 3 independent experiments.
Antibodies to CD44 can ameliorate murine ITP and do so in a manner dependent on the presence of the inhibitory FcγRIIB but independent of the Fc region of IgG. (A) Mice were injected with 50 μg CD44 antibody (5035-41.1D, KM114, KM81, KM201, 9A4, IOD1, IRAWB14, or IM7) or 50 mg IVIg (∼ 2 g/kg of body weight). Twenty-four hours later, mice were injected with 2 μg antiplatelet antibody to induce thrombocytopenia. Mice were bled after a further 24 hours, and the platelet count was determined. Horizontal bars represent the range of the mean platelet count (± 1 SEM) of naive, antiplatelet antibody injected mice, and IVIg treated mice. n = 9 mice for each group from 3 independent experiments. *P < .01 versus ITP. **P < .001 versus ITP. (B) CD44 binding to splenic leukocytes was assessed as follows: RBC-depleted mouse splenic leukocytes were incubated with the indicated CD44 antibody followed by the appropriate fluorescein isothiocyanate-labeled secondary anti-IgG and assessed for mean log fluorescence intensity (MLFI) by flow cytometry. The values are the mean of 2 independent experiments. (C) To determine the extent of reactivity of murine platelets with the CD44 antibodies used in this study, murine platelets were incubated with the indicated CD44 antibody followed by appropriate fluorescein isothiocyanate-labeled secondary anti-IgG and assessed for MLFI by flow cytometry. The values are the mean of 4 independent experiments. (D) Mice were injected with 50 μg of the indicated CD44 antibodies to assess the ability of each antibody to induce thrombocytopenia in the absence of antiplatelet antibody. Platelet counting was evaluated before injection and after 24, 48, 72, and 96 hours. n = 6 mice for each group from 2 independent experiments. *P < .05 versus prebleed values. **P < .001 versus prebleed values. (E) C57BL/6 mice (columns 1-4) were treated and bled and platelets counted as in panel A, except that mice were treated with either 50 μg intact KM114 or 37 μg F(ab′)2 KM114 (the equivalent molar concentration). n = 6 mice for each group from 3 independent experiments. Mice expressing human FcγRIIA (columns 5 and 6) were injected with antiplatelet antibody alone or antiplatelet antibody plus KM114 as in panel A and platelets were counted as in panel A. n = 6 mice for each group from 2 independent experiments. (F) Wild-type mice (FcγRIIB+/+) or (G) mice genetically deficient in the inhibitory Fcγ receptor RIIB (FcγRIIB−/−) were injected with 50 mg IVIg, 50 μg anti-RBC monoclonal antibody (TER119), or 50 μg of the indicated CD44 antibodies (KM114 or 5035–41.1D). Twenty-four hours later, all mice were given 2 μg antiplatelet antibody to induce thrombocytopenia. After a further 24 hours, mice were bled for platelet counting. Normal and ITP are described as in panel A. n = 9 mice for each group from 3 independent experiments.
The relative binding of the antibodies to leukocytes was not related to their ability to ameliorate ITP (Figure 1B); 5035-41.1D bound leukocytes well and was therapeutic, yet KM114 and KM81, which were therapeutic, bound leukocytes less than IRAWB14 and IM7, which had no therapeutic effect at all. Figure 1C displays in vitro antibody binding to mouse platelets and reveals that, although the majority of the CD44 antibodies bound platelets, the most successful ITP-treating antibody, 5035-41.1D, also displayed more antibody binding than the others. As the majority of the CD44 antibodies bound platelets, we next questioned the ability of the antibodies to induce thrombocytopenia (Figure 1D). Two of the antibodies that were not therapeutic (IRAWB14, IM7) induced highly significant thrombocytopenia. Although KM201 did induce a statistically significant thrombocytopenia (P < .05), it was mild compared with IRAWB14- or IM7-induced thrombocytopenia, and it significantly protected mice from ITP. The 2 other nontherapeutic antibodies (9A4 and 10D1), which showed poor platelet binding, did not induce thrombocytopenia. Thus, it appears as if CD44 antibodies that can bind platelets well and induce thrombocytopenia are poor therapeutic antibodies in this model. As human platelets do not express CD44, this would not be an issue for humans. The ability of some antibodies and not others to ameliorate ITP was not related to antibody isotype (ie, 5035-41.1D, KM114, 9A4, and 10D1 are all IgG1, yet only 5035-41.1D and KM114 are therapeutic; KM81 and IM7 are both IgG2b yet KM81 is therapeutic, whereas IM7 was without effect). The fine antigenic specificity of the antibodies may not be a major factor in their therapeutic ability as antibodies, which bound all isoforms of CD44 were either successful (5035-41.1D, KM114, KM81, and KM201) or unsuccessful (IM7 and IRAWB14). Antibodies 10D1 and 9A4, which bind to variants of CD44, (CD44v4 and CD44v6, respectively) were nontherapeutic. It is of interest to note that 3 of the 4 therapeutic antibodies (KM114, KM81, and KM201) all bind epitopes in the hyaluronan-binding region. It is also of interest that IM7 has been shown to function successfully at treating murine autoimmune disease models, such as arthritis, experimental autoimmune encephalitis, and insulin-dependent diabetes mellitus,4,12-16 yet we found it to be unsuccessful in ameliorating the thrombocytopenia seen in this passive model of murine ITP. It is therefore possible, or perhaps probable, that different CD44 antibodies may work by disparate mechanisms in different autoimmune diseases.
To determine whether the observed protective effect was dependent on the Fc portion, an F(ab′)2 fragment of KM114 was used. For IVIg, an F(ab′)2 fragment does not ameliorate murine ITP.17 Here, we demonstrate that an F(ab′)2 fragment of CD44 antibody KM114 successfully ameliorates thrombocytopenia (Figure 1E, columns 3 vs 4) and thus, unlike IVIg, would not involve interaction with an activating FcγR. This also indicates that the KM114 antibody would not be directly engaging the inhibitory receptor FcγRIIB.
Human platelets express the activating Fcγ receptor FcγRIIA, and the presence of this receptor on platelets might affect the therapeutic response of CD44 antibodies. We therefore used mice expressing human FcγRIIA18 in the murine ITP model and found that CD44 antibody KM114 successfully treated thrombocytopenia in the presence of platelet expressed FcγRIIA (Figure 1E columns 5 and 6). This suggests that the presence of FcγRIIA on platelets does not prevent the therapeutic effect of CD44 antibody.
We next questioned whether the therapeutic antibodies functioned in a manner with similarities to IVIg. In C57BL/6 mice, IVIg requires expression of the inhibitory receptor FcγRIIB to function therapeutically in murine ITP19-21 Here, we tested the 2 most successful therapeutic CD44 antibodies (5035-41.1D and KM114) compared with IVIg and a therapeutic anti-RBC antibody (which ameliorates murine ITP independent of FcγRIIB11 ; Figure 1F). As expected, the anti-RBC antibody ameliorated ITP in FcγRIIB-deficient mice, whereas IVIg did not. Neither of the CD44 antibodies used was able to function in the absence of FcγRIIB (Figure 1G). This suggests that IVIg and CD44 antibodies share at least one common requirement in the amelioration of ITP.
We22 and others21 have shown that IVIg successfully ameliorates murine ITP in the absence of complement. We show here that KM114 can also ameliorate ITP in C3-deficient mice, ruling out a role for complement in the amelioration of ITP by antibody KM114 (supplemental Figure 1A). In support of this finding, the F(ab′)2 fragment of CD44 antibody KM114 (which cannot fix complement) functioned therapeutically in our model (Figure 1E), further ruling out a potential role for complement. In the ITP treatment protocol, CD44 antibody is given 24 hours before induction of thrombocytopenia; to ascertain whether CD44 antibodies would also be effective if given close to the time of induction of thrombocytopenia, we performed experiments with KM114 given 30 minutes after the injection of antiplatelet antibody and found this protocol to be successful at ameliorating thrombocytopenia (supplemental Figure 1B).
As human platelets do not express CD44, it could be argued that CD44 antibodies will not work in human ITP patients as platelet CD44 could, by itself, be the target of the CD44 antibody. Transfer experiments of platelets from CD44-deficient mice into wild-type mice treated with CD44 antibody revealed that KM114 was able to rescue both CD44+ and CD44− platelets from antiplatelet antibody-mediated clearance (supplemental Figure 1C).
CD44 antibodies have diverse effects on leukocytes, such as inhibition of proliferation, recruitment, migration, rolling, and induction of apoptosis.3-7 We have used SCID mice (which lack B and T cells) to ascertain whether or not the cells, which support these effects, might play a role in our model. We found that CD44 antibody KM114 ameliorates thrombocytopenia in SCID mice (supplemental Figure 1D), suggesting that the CD44 antibodies are not primarily functioning via interaction with CD44 on B or T cells in this passive ITP model.
Here, we demonstrate that antibodies directed to CD44 can potentially ameliorate murine thrombocytopenia, some as efficiently as IVIg at a 3-log-fold lower dosage and that they share some similar mechanistic pathways with IVIg. We speculate that CD44 antibodies may offer a potential replacement therapy for the treatment of ITP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Xi Chen, Mr Patrick J Mott, Dr Zhong-Wei Chai, Mr José A Guillen-Salgado, and Ms Joan Legarda for assistance and helpful suggestions, Dr Guangheng Zhu for technical assistance, and the St Michael's Research Vivarium staff.
This work was supported by a grant from the Canadian Blood Services–Canadian Institutes of Health Research Request For Proposals program.
Authorship
Contribution: A.R.C. designed research, performed experiments, analyzed data, and wrote the manuscript; S.S. designed research, performed experiments, and analyzed data; S.M. and S.J.S. performed experiments and analyzed data; M.P.R., P.A., and S.E.M. provided research tools and performed experiments; and A.H.L. designed research, analyzed data, obtained grant funding, and wrote the manuscript.
Conflict-of-interest disclosure: A.H.L. received honoraria from Baxter and LFB Biomédicaments. The remaining authors declare no competing financial interests.
Correspondence: Alan H. Lazarus, Transfusion Medicine Research, St Michael's, 30 Bond St, Toronto, ON, Canada M5B 1W8; e-mail: lazarusa@smh.ca.
References
Author notes
A.R.C. and S.S. contributed equally to this study and should be considered as co–first authors.