Abstract
Mammalian TOR (mTOR) regulates cell growth, proliferation, and migration. Because mTOR knock-outs are embryonic lethal, we generated a viable hypomorphic mouse by neo-insertion that partially disrupts mTOR transcription and creates a potential physiologic model of mTORC1/TORC2 inhibition. Homozygous knock-in mice exhibited reductions in body, organ, and cell size. Although reductions in most organ sizes were proportional to decreased body weight, spleens were disproportionately smaller. Decreases in the total number of T cells, particularly memory cells, and reduced responses to chemokines suggested alterations in T-cell homing/homeostasis. T-cell receptor-stimulated T cells proliferated less, produced lower cytokine levels, and expressed FoxP3. Decreased neutrophil numbers were also observed in the spleen, despite normal development and migration in the bone marrow. However, B-cell effects were most pronounced, with a partial block in B-cell development in the bone marrow, altered splenic populations, and decreases in proliferation, antibody production, and migration to chemokines. Moreover, increased AKTSer473 phosphorylation was observed in activated B cells, reminiscent of cancers treated with rapamycin, and was reduced by a DNA-pk inhibitor. Thus, mTOR is required for the maturation and differentiation of multiple immune cell lineages. These mice provide a novel platform for studying the consequences of constitutively reduced mTORC1/TORC2 activity.
Introduction
The mammalian target of rapamycin (mTOR) is part of a conserved pathway regulating fundamental physiologic functions, including nutrient sensing and metabolism, and cell growth, proliferation, and migration. mTOR forms 2 protein complexes: one with RAPTOR, mLST8(GβL), and PRAS40 to form TOR complex 1 (mTORC1) involved in phosphorylating S6K and 4EBP1,1,2 and a second with RICTOR, mLST8(GβL), SIN1, and PROTOR to form TOR complex 2 (mTORC2), which phosphorylates AKT on Ser473.2-4 In yeast, TOR controls cell proliferation and size.5,6 In Drosophila, inactivation of dTOR results in lethality and reduced embryo size.7,8 Genetically targeting the kinase domain of murine mTOR for inactivation results in embryonic lethality,9-11 although deletions in the C terminal portion yield mice that are normal and fertile.11 ENU-mutagenesis screens uncovered an additional embryonic lethal mutation of mTOR, resulting in flat-top embryos lacking telencephalons resulting from limited neuroectodermal cell proliferation.10,12 Knockouts of Raptor or Sin113 result in early embryonic lethality, whereas those of Rictor and mLST8(GβL) lead to late embryonic lethality and defective vascular development.
mTOR signaling/function has been deduced from studies with rapamycin, which associates with FKBP12,14 and together binds mTOR to destabilize mTORC1. Although originally thought to affect only mTORC1, long-term treatment with rapamycin can also affect mTORC2.15 Rapamycin and numerous rapalogs are potent immunosuppressants used in cancer chemotherapy and bone marrow (BM) transplantation.1 In the immune system, the effects of rapamycin have probably been most studied in T cells, although effects on B and dendritic cell activation/function have also been observed.16 A CD4+ T-cell conditional knock-out of mTOR results in increased differentiation of activated T cells into a T-regulatory cell (Treg) phenotype.17 Recently, adenosine triphosphate-competitive inhibitors of mTOR, Torin1,18 and PP242/PP30,19 have been found to inhibit both mTORC1/mTORC2 complexes and to inhibit growth of primary cells more effectively than rapamycin. These adenosine triphosphate-competitive inhibitors have shown activity toward B precursor acute lymphoblastic leukemia.20 However, because of the lethal effects of mTOR gene disruption, global effects of mTORC1/TORC2 inhibition on the immune system have not been well characterized.
Interestingly, different strains of mice carry polymorphisms of mTOR, with BALB/c and NZB mice having a polymorphism (cysteine instead of a conserved arginine) affecting R62821 in a region of the protein containing several HEAT domains, motifs often found in proteins involved in translation. We generated a mouse in which normal transcription of the BALB/c mTOR allele is disrupted by insertion of a neomycin cassette. This hypomorphic mTOR mouse has drastically reduced expression of mTOR yet is viable. We have used this model to examine the effects of constitutively reduced mTOR protein levels on immune cell development and activation, with particular emphasis on B and T lymphocytes. Our results confirm that mTOR plays a critical role in multiple arms of the immune system, affecting both homeostasis and function.
Methods
Generation and characterization of mice with neo-disrupted mTOR transcription
Recombineering techniques involving an 8.6-kb BamHI genomic fragment (exons 9-17) of mTOR isolated from a 129 BAC library were used to replace exon 12 with BALB/c sequences and to insert LoxP and Frt sites along with a neomycin cassette into intron 12 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).22,23 Detailed methods for generating, genotyping, and characterizing the RNAs and proteins, immunizations and measurements of antibody titers, preparation of cell populations, as well as subcellular localization, cell growth, nuclear factor-κB assays, and flow cytometric analyses are provided in the Supplemental data. Chemotaxis assays to S1P, SDF, TECK, MIP, and formyl-methionyl-leucyl-phenylalanine (fMLP) were performed as described24-27 ; additional details are provided in the supplemental data.
Results
Reduced mTOR produces small mice with disproportionately small spleens
Homologous recombination, used to engineer a single nucleotide change in exon 12 of mTOR, resulted in an amino acid change, R628C, on a 129xB6 background (supplemental Figure 1A). Neomycin cassette insertion in intron 12 of knock-in (KI) mice disrupted normal transcription of mTOR (supplemental Figure 1A-B), creating 2 artificial short RNA forms: a partial mTOR fused with neo (4.3 kb), neo only (1.3 kb; supplemental Figure 1B), and substantially reduced amounts of the full-length mTOR (9.2 kb) transcript. Western blots of lysates from various tissues yielded the expected 300-kDa protein; neither N-terminal nor C-terminal antibodies detected novel protein bands in KI versus wild-type (WT) mice. We and others have shown that the insertion of a neomycin cassette in an intron can partially disrupt gene expression.28,29
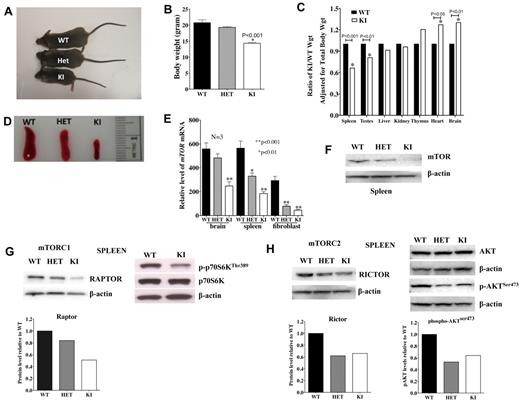
Mice homozygous for the neo-628C allele (KI) were viable; however, only approximately 30% of the expected KI homozygotes were recovered from matings of heterozygous mice. Moreover, KI mice were 25% to 30% smaller in body size and weight than their WT and heterozygous (HET) littermates (Figure 1A-B) but have survived beyond 1 year. Although most organs were proportionately sized, the KI spleens were disproportionately small (Figure 1C-D) relative to other organs.
mTOR expression affects body and organ weight as well as TORC1 and TORC2 activity. (A-B) Body sizes and weights of KI mice are less than that observed in age-matched WT and HET mice (N = 7 each). (C) KI organ weights are adjusted for body weight and shown as a proportion of the WT organ weight also adjusted for body weight. All data are from 7 mice (age, 57 days). (D) Spleens of KI mice are small and disproportionately reduced compared with other organs. (E) Relative mRNA expression of mTOR in brain, spleen, and fibroblasts of WT, HET, and KI mice (N = 3 each). (F) Protein levels of mTOR (C-terminal antibody) were lower in spleens of KI mice. Basal RAPTOR (G) and RICTOR (H) protein expression was reduced in spleens of HET and KI mice (top left: Western blot; bottom left: protein level relative to WT, normalized with β-actin). In KI splenocytes, lower mTOR levels resulted in less phosphorylation of TORC1 target p-p70SKThr389 (G, top right) and TORC2 target p-AKTSer473 (H, top right: Western blot; bottom right: protein level relative to WT, normalized with total β-actin).
mTOR expression affects body and organ weight as well as TORC1 and TORC2 activity. (A-B) Body sizes and weights of KI mice are less than that observed in age-matched WT and HET mice (N = 7 each). (C) KI organ weights are adjusted for body weight and shown as a proportion of the WT organ weight also adjusted for body weight. All data are from 7 mice (age, 57 days). (D) Spleens of KI mice are small and disproportionately reduced compared with other organs. (E) Relative mRNA expression of mTOR in brain, spleen, and fibroblasts of WT, HET, and KI mice (N = 3 each). (F) Protein levels of mTOR (C-terminal antibody) were lower in spleens of KI mice. Basal RAPTOR (G) and RICTOR (H) protein expression was reduced in spleens of HET and KI mice (top left: Western blot; bottom left: protein level relative to WT, normalized with β-actin). In KI splenocytes, lower mTOR levels resulted in less phosphorylation of TORC1 target p-p70SKThr389 (G, top right) and TORC2 target p-AKTSer473 (H, top right: Western blot; bottom right: protein level relative to WT, normalized with total β-actin).
mTOR mRNA expression was lower in multiple tissues, including brain, spleen, and fibroblasts of KI mice (Figure 1E). Lower expression was confirmed by Western analyses, revealing lower mTOR protein levels in spleen (Figure 1F), brain, liver, heart, kidney, thymus, pancreas, lung, ovary, and testis isolated from the KI mice (supplemental Figure 2A). Protein expression was also lower in fibroblasts of both HET and KI mice relative to WT (supplemental Figure 2B).
Reduced mTOR is associated with decreased mTORC1/TORC2 activity
To evaluate effects of decreased mTOR on mTOR signaling complexes, we examined protein levels of RAPTOR and RICTOR. Both were lower in splenocytes (Figure 1G-H) and fibroblasts (supplemental Figure 3A) from KI, compared with WT mice, although RAPTOR levels were generally more severely affected. To evaluate the effects of lower levels of mTOR and RAPTOR/RICTOR protein levels in the KI mice, phosphorylation of downstream targets was examined in spleens (Figure 1G-H), skin fibroblasts stimulated with insulin (supplemental Figure 3B-E), and thymic T lymphocytes stimulated through the T-cell receptor (TCR) (supplemental Figure 3F). Low mTOR resulted in lower phosphorylation of the mTORC1 target p-p70S6KThr389, which affects cell growth,1,2 and the mTORC2 target p-AKTSer473, which controls actin cytoskeleton and cell spreading2,30 (Figure 1G-H; supplemental Figure 3D-F). In contrast, pERK levels in T cells were not affected by low mTORC1/2 activities (supplemental Figure 3F). Despite a role for mTORC1 in the negative regulation of AKT,15 p-AKTThr308 levels in fibroblasts were the same as WT (supplemental Figure 3D). DEPTOR, a protein that associates with mTORC1 and mTORC2,31 was similar or slightly increased in KI versus WT fibroblasts (supplemental Figure 3G). In addition, lower mTOR activity resulted in lower viability/proliferation in KI compared with WT or HET MEFs (supplemental Figure 3H).
mTORC1/TORC2 signaling in activated lymphocytes
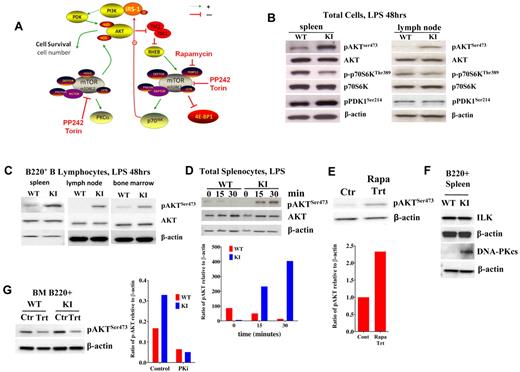
Because rapamycin is a potent immunosuppressant, it was particularly interesting that we observed the biggest difference in size in the spleen, a lymphoid organ, in the KI mice. To evaluate mTORC1 activity in lymphoid organs, we initially treated splenocytes and lymph node (LN) cells with lipopolysaccharide (LPS). A simplified scheme of identified mTOR signaling components is shown in Figure 2A. Lower p-p70S6K was observed relative to that induced in WT cells (Figure 2B). In dramatic contrast, mTORC2 activity was increased in the KI cells stimulated with LPS, as exhibited by the relatively high levels of pAKTSer473 seen in KI spleens and LNs (Figure 2B). pPDK1 levels were similar between KI and WT LNs but slightly higher in KI spleens (Figure 2B). The majority of cells isolated from the spleen and enriched by LPS are B lymphocytes. To determine whether increased phosphorylation of AKT occurred in B cells, we treated B220+ cells purified from spleens, LNs, and BM of KI and WT mice with LPS. All sources of purified B cells exhibited enhanced pAKTSer473 relative to WT (Figure 2C), even as early as 15 minutes after stimulation (Figure 2D) in total splenocytes. This mimics the enhanced pAKTSer473 expression seen in L363 myeloma cells after rapamycin treatment (Figure 2E). In contrast, stimulated thymocytes (supplemental Figure 3F) and peripheral T cells (data not shown) showed reduced pAKTSer473, demonstrating distinct regulation of pAKTSer473 in different tissues.
TORC1/TORC2 signaling in activated lymphocytes. (A) Schematic representing a simplified signaling pathway for TORC1/TORC2 (using ScienceSlides objects, Suite 2009). The dotted green line indicates that the signaling is not direct. (B) AKT and p70S6K phosphorylation in LPS-stimulated (48 hours) cells from spleens and LNs of KI versus WT mice. p-p70S6K levels remained low, as in unstimulated cells, whereas pAKT levels increased. (C) AKTSer473 phosphorylation in LPS-stimulated (48 hours) B220+ lymphocytes from spleens, LNs, and BM increased in KI mice compared with WT. (D) Time course (0, 15, and 30 minutes) of pAKTSer473 induction in splenocytes stimulated with LPS. (E) pAKTSer473 levels in control/untreated L363 myeloma cells versus cells treated with 10nM rapamycin for 24 hours. (F) ILK and DNA-PKcs in B220+ splenocytes stimulated with LPS after 24 hours. (G) pAKTSer473 induction (48 hours) in cells pretreated for 2 hours with 20μM of the DNA-PKcs inhibitor NU7026 and then stimulated with LPS.
TORC1/TORC2 signaling in activated lymphocytes. (A) Schematic representing a simplified signaling pathway for TORC1/TORC2 (using ScienceSlides objects, Suite 2009). The dotted green line indicates that the signaling is not direct. (B) AKT and p70S6K phosphorylation in LPS-stimulated (48 hours) cells from spleens and LNs of KI versus WT mice. p-p70S6K levels remained low, as in unstimulated cells, whereas pAKT levels increased. (C) AKTSer473 phosphorylation in LPS-stimulated (48 hours) B220+ lymphocytes from spleens, LNs, and BM increased in KI mice compared with WT. (D) Time course (0, 15, and 30 minutes) of pAKTSer473 induction in splenocytes stimulated with LPS. (E) pAKTSer473 levels in control/untreated L363 myeloma cells versus cells treated with 10nM rapamycin for 24 hours. (F) ILK and DNA-PKcs in B220+ splenocytes stimulated with LPS after 24 hours. (G) pAKTSer473 induction (48 hours) in cells pretreated for 2 hours with 20μM of the DNA-PKcs inhibitor NU7026 and then stimulated with LPS.
To determine whether another kinase capable of phosphorylating AKT on Ser473 was up-regulated after LPS under low mTOR conditions, we examined the levels of ILK and DNA-PKcs (PRKDC). Of these, only DNA-PKcs levels were up-regulated after LPS treatment in B cells (Figure 2F). Furthermore, pAKTSer473 levels in B cells pretreated with the DNA-PK inhibitor, NU7026, before LPS were decreased significantly in both WT and KI mice (Figure 2G).
Reduced mTOR alters T-cell differentiation and migration
mTOR inhibition with rapamycin has been shown to affect T lymphocyte differentiation/function.14 In our studies, we observed decreased absolute numbers of thymocytes and CD4+ and CD8+ cells in the spleens and LNs of KI mice (supplemental Figure 4A-C), despite normal to increased percentages of mature single positive (SP) mature CD4 and CD8 in the thymi and spleen (supplemental Figure 4A-B). Cell sizes (supplemental Figure 4D-F) of SP T cells were also smaller in all KI lymphoid tissues.
Thymic T cells from KI mice also exhibited migratory defects, displaying reduced migration to the chemokines TECK (CCL25) and SDF-1 (CXCL12; P < .01), but not to MIP3 (CCL19; P > .05; supplemental Figure 5A), despite relatively normal levels of the receptors for TECK (CCR9) and SDF-1 (CXCR4; data not shown). Lower mTOR levels could lead to down-regulation of the transcription factor KLF2, which drives expression of genes/proteins involved in entry, positioning, and egress, such as CD62L, CCR7, and S1P1, respectively.32,33 Nonetheless, KLF2 and CCR7 levels were equivalent in WT and KI thymi (data not shown), as were CD62L levels in SP splenic and LN T cells (supplemental Figure 5B-C), suggesting that altered expression of these surface markers did not contribute to altered cell trafficking. S1P1 expression was slightly lower in SP T cells from the KI spleen (supplemental Figure 5D), but not the thymus (data not shown), which could possibly affect splenic T-cell egress.
Activated and naive T cells express different adhesion molecules that may affect migratory patterns. CD44 (H-CAM) and CD62L (L-selectin) are adhesion molecules that have been associated with naive (CD44lo, CD62Lhi) and activated/memory (CD44hi, CD62Llo) T cells.34 CD4+ and CD8+ cells in the spleens (supplemental Figure 5B) and LNs (supplemental Figure 5C) of KI mice were predominantly CD44lo and CD62Lhi, suggesting a more naive, nonactivated T-cell population. Together, these data suggest that T cells from LNs and spleens of KI mice have alterations in activation, trafficking, or homeostasis and differentiation. Nonetheless, relatively normal thymic development was observed in the KI mice, as low mTOR expression did not grossly affect CD5, CD24, or TCR-β expression in CD4+ and CD8+ T cells from the thymus, except for a slightly larger population of CD8+ T cells with low TCR-β expression (supplemental Figure 5E). This is consistent with a relative increase in the percentage of immature CD8+ cells in KI mice (supplemental Figure 4A) and is similar to data reported for a T-cell conditional mTOR deletion.17
Reduced mTOR affects T-cell proliferation, survival, cytokine production, and differentiation into Tregs
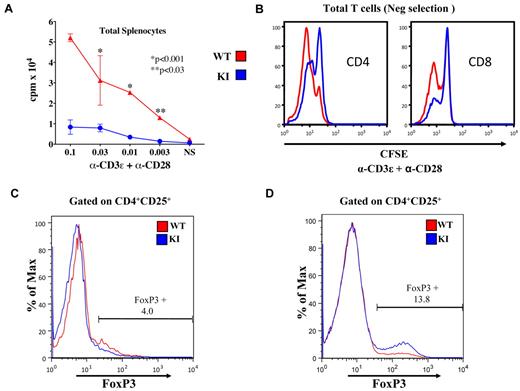
To examine functional responses, we evaluated proliferation rates and cell viability of KI splenocytes (Figure 3A; supplemental Figure 6A-B) in response to anti-CD3ϵ plus anti-CD28 costimulation. Cell expansion/proliferation was significantly lower in KI splenocytes. Because rapamycin affects antigen-presenting cells,16 we confirmed that there were intrinsic T-cell defects by stimulating purified splenic T cells with plate-bound anti-CD3ϵ plus anti-CD28. Again, although splenic T cells from the KI could proliferate, they did so less well than WT T cells (Figure 3B). Nonetheless, baseline apoptosis/death, with or without interleukin-7, was also decreased in KI T-cell populations, as measured by 7-amino-actinomycin D/annexin V staining after 48 hours (supplemental Figure 6B).
T-cell proliferation and differentiation in the spleen. (A) WT splenocytes stimulated with α-CD3ϵ + α-CD28 proliferated better than KI splenocytes as measured by tritiated thymidine incorporation (P < .001, **P < .03). This is representative of 3 experiments. (B) Stimulated WT (red) splenic T cells proliferated more rapidly than KI (blue) cells as evidenced by the lower carboxyfluorescein succinimidyl ester (CFSE) staining (72 hours). (C) FoxP3 expression in unstimulated CD4+, CD25+ cells was similar in WT (5.4%) and KI (4.0%) mice. (D) Increased percentages of FoxP3+ cells in purified KI splenic T cells stimulated with α-CD3ϵ/WT antigen-presenting cells for 3 days followed by culture in interleukin-2 for 3 days (WT 3.9% FoxP3+ vs KI 13.8% FoxP3+). Data are representative of 4 experiments.
T-cell proliferation and differentiation in the spleen. (A) WT splenocytes stimulated with α-CD3ϵ + α-CD28 proliferated better than KI splenocytes as measured by tritiated thymidine incorporation (P < .001, **P < .03). This is representative of 3 experiments. (B) Stimulated WT (red) splenic T cells proliferated more rapidly than KI (blue) cells as evidenced by the lower carboxyfluorescein succinimidyl ester (CFSE) staining (72 hours). (C) FoxP3 expression in unstimulated CD4+, CD25+ cells was similar in WT (5.4%) and KI (4.0%) mice. (D) Increased percentages of FoxP3+ cells in purified KI splenic T cells stimulated with α-CD3ϵ/WT antigen-presenting cells for 3 days followed by culture in interleukin-2 for 3 days (WT 3.9% FoxP3+ vs KI 13.8% FoxP3+). Data are representative of 4 experiments.
Consistent with reduced proliferation, there were defects in effector cytokine production by activated T cells as previously reported.17 Because T cells did not proliferate and expand into effector T cells, we examined KI splenocytes for evidence of differentiation into FoxP3 Tregs. Although FoxP3 expression was similar in naive (unstimulated) splenic CD4+ cells (Figure 3C), we observed an increased percentage of FoxP3 cells after stimulation of splenocytes with anti-CD3ϵ (data not shown) or purified CD4+ T cells stimulated with anti-CD3ϵ plus anti-CD28 in the presence or absence of WT antigen-presenting cells (Figure 3D). These results are consistent with the increased FoxP3 expression observed in stimulated T cells from CD4+ conditional mTOR knockouts.17
B-cell populations are altered in the spleen, LNs, and BM of mTOR KI mice
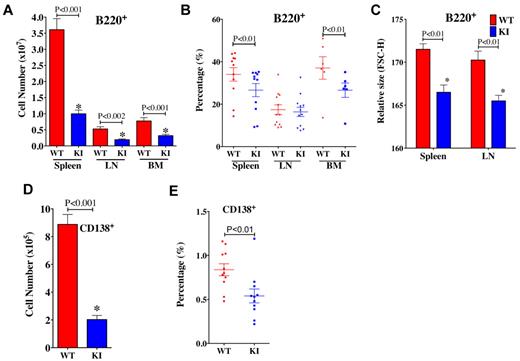
Although effects of rapamycin have focused on T cells, we observed more dramatic alterations in B-cell populations of KI mice. Both the numbers and percentages of B220+ B cells (Figure 4A-B) in spleen and BM were reduced in KI mice. The relative sizes (Figure 4C) of splenic and LN B220+ B cells of KI mice were also smaller than those of WT mice. Furthermore, the numbers (Figure 4D) and percentages of CD138+ plasma cells (Figure 4E) were also lower in KI spleens, suggesting alterations in B-cell differentiation/maturation. Together, these results suggest that reductions in mTOR levels affect B-cell development, differentiation, and/or homeostasis.
B-cell populations in lymphoid organs. (A) Total numbers of B220+ B cells are less in KI spleens, LNs, and BM compared with WT. (B) The frequency of B220+ cells was reduced in spleens and BM of KI mice, but not LNs. (C) The relative sizes of B cells in the spleen and LN were smaller in KI mice. (D) The total number of CD138+ plasma cells was also less in the spleens of KI mice. (E) The frequency of CD138+ cells was reduced in spleens of KI mice. For all panels, N = 6 to 12; age, 8 to 9 weeks.
B-cell populations in lymphoid organs. (A) Total numbers of B220+ B cells are less in KI spleens, LNs, and BM compared with WT. (B) The frequency of B220+ cells was reduced in spleens and BM of KI mice, but not LNs. (C) The relative sizes of B cells in the spleen and LN were smaller in KI mice. (D) The total number of CD138+ plasma cells was also less in the spleens of KI mice. (E) The frequency of CD138+ cells was reduced in spleens of KI mice. For all panels, N = 6 to 12; age, 8 to 9 weeks.
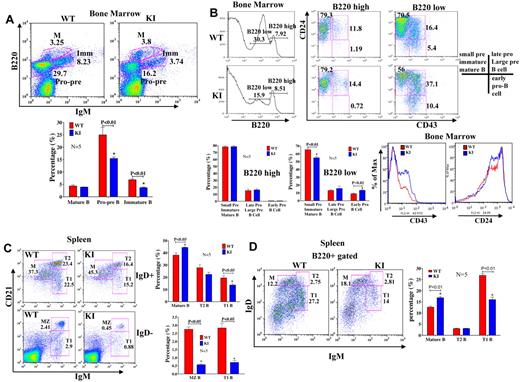
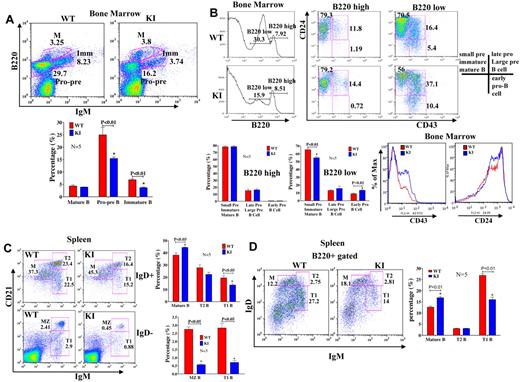
KI mice have a partial block in B-cell development in the BM
To evaluate whether reduced mTOR levels affected B-cell development, we looked at subpopulations of BM B cells to identify possible differences in development/differentiation. KI mice had fewer B220+ cells in the BM (Figure 5A-B). Cell surface marker analysis of B-cell populations suggested a partial block in B-cell development at the transition between the large to small pre-B cell stage (Figure 5A-B). There were also fewer pro-pre (B220+IgM−) and immature (B220+, IgM+) B cells in the BM of KI mice relative to WT (P < .01; Figure 5A-B). Analysis of B220lo versus B220hi populations of BM B cells for CD43 and CD24 expression (Figure 5B) revealed that KI mice showed decreases in B220lo, CD24+, CD43− and increases in B220lo, CD24+, CD43+ B cells, indicative of a block in B-cell development between large pre- and small pre-B cell stages. Blocks in BM B-cell development have been attributed to multiple causes, including decreased nuclear factor-κB activity35 and phospholipase C-γ2 levels.36 Nuclear factor-κB activation (p50, p65, and p52), as measured by enzyme-linked immunosorbent assay in nuclear extracts of LPS-stimulated B220+ BM cells, was not altered in KI mice (supplemental Figure 7A-C). Furthermore, IκB degradation did not show differences between WT and KI mice (supplemental Figure 7D), suggesting that this pathway is unlikely to account for the observed developmental differences. Phospholipase C-γ2 protein levels (supplemental Figure 7E) were only modestly lower in the spleens of KI mice and are unlikely to be a major factor contributing to the developmental block.
B-cell subpopulations are altered in the BM and spleen. (A) Cells isolated from BM of WT and KI mice (N = 5 each) were stained with antibodies to IgM and B220. Populations are labeled as follows: M indicates mature B cells; IMM, immature B cells; and Pro-pre, Pro/Pre B cells. There were fewer Pro/Pre and immature B cells in KI mice. (B) Cells isolated from the BM of WT and KI mice were stained with antibodies to B220, CD24, and CD43. Stages of B-cell development are based on the following: B220+CD24+CD43−: small pre-B, immature B, and mature B cells; B220+CD24+CD43+: late pro-B and large pre-B cells; B220+CD24−CD43+: early pro-B cells. Populations of CD43+ and CD24+ cells from the BM of WT and KI mice. There were more CD43+, CD24+ cells in the KI compared with WT mice. (C) Flow cytometric analyses revealed that mature B-cell populations were increased in KI spleens. (D) B220+ cells were also stained with IgM and IgD antibodies, and stages of B cells were identified by the following cell surface markers: M indicates mature B cells (B220+ IgDhighCD21+IgM−); T, transitional B cells (T1, B220+IgDloCD21loIgMhigh; T2, B220+IgDhighCD21highIgMhigh); and MZ, marginal zone B cells (B220+IgD−CD21highIgMhigh). Sample sizes for these experiments were 5 mice/group (ages, 8 to 9 weeks) and are representative of repeat experiments.
B-cell subpopulations are altered in the BM and spleen. (A) Cells isolated from BM of WT and KI mice (N = 5 each) were stained with antibodies to IgM and B220. Populations are labeled as follows: M indicates mature B cells; IMM, immature B cells; and Pro-pre, Pro/Pre B cells. There were fewer Pro/Pre and immature B cells in KI mice. (B) Cells isolated from the BM of WT and KI mice were stained with antibodies to B220, CD24, and CD43. Stages of B-cell development are based on the following: B220+CD24+CD43−: small pre-B, immature B, and mature B cells; B220+CD24+CD43+: late pro-B and large pre-B cells; B220+CD24−CD43+: early pro-B cells. Populations of CD43+ and CD24+ cells from the BM of WT and KI mice. There were more CD43+, CD24+ cells in the KI compared with WT mice. (C) Flow cytometric analyses revealed that mature B-cell populations were increased in KI spleens. (D) B220+ cells were also stained with IgM and IgD antibodies, and stages of B cells were identified by the following cell surface markers: M indicates mature B cells (B220+ IgDhighCD21+IgM−); T, transitional B cells (T1, B220+IgDloCD21loIgMhigh; T2, B220+IgDhighCD21highIgMhigh); and MZ, marginal zone B cells (B220+IgD−CD21highIgMhigh). Sample sizes for these experiments were 5 mice/group (ages, 8 to 9 weeks) and are representative of repeat experiments.
Reduced mTOR expression alters B-cell differentiation in the spleen
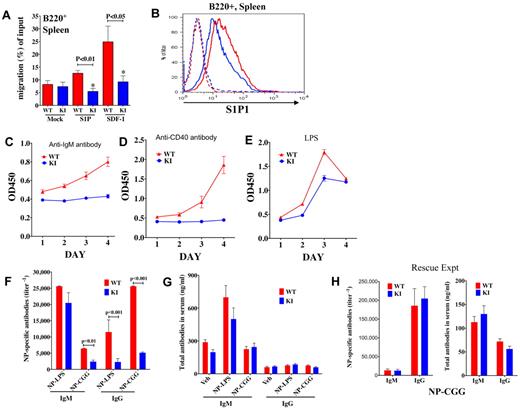
B cells develop in the BM and migrate to peripheral lymphoid organs. To determine whether the B cells that had bypassed the block in development in the BM and had migrated to the spleen would differentiate into the normal complement of B-cell subpopulations, we analyzed splenic B cells by flow cytometry. Analysis of cell surface markers on B220+ cells revealed that there were comparatively more mature (B220+, IgDhi, CD21+, IgM−) B cells in the spleens of KI mice relative to WT (Figure 5C-D; supplemental Figure 8A). Concomitant with the increased number of mature B cells, the KI mice had fewer transitional (T1: B220+, IgDlo, IgMhi, CD21lo, CD23−), marginal zone (MZ: B220+, IgMhi, IgDlo, CD21hi, CD23−), and follicular (FO: B220+, IgMlo, IgDhi, CD21int, CD23+; Figure 5C-D; supplemental Figure 8A-B) B cells in their spleens than WT mice (P < .01). Numbers of T2 (B220+, IgMhi, IgDhi, Cd21hi, Cd23+) B cells were not significantly different between KI and WT (Figure 5C-D). Increases in mature cell percentages could have resulted from reduced migration/egress from the spleen, increased survival, or may be secondary to reduced percentages of other cell populations. The ability of B220+ KI B cells to migrate to chemokines SDF-1 and S1P, which are critically involved in maintaining B-cell homeostasis and positioning in the spleen,33,37 was impaired (Figure 6A). In addition, expression of the S1P receptor, S1P1 (EDG1), was slightly decreased in the KI mice (Figure 6B). Therefore, trafficking and differentiation in the spleen are altered in KI mice.
B-cell migration, proliferation, and antibody production. (A) Migration (after 3 hours) of splenic B220+ cells from WT versus KI mice to chemokines S1P and SDF-1. (B) S1P1 (EDG1) receptor expression in splenic B220+ cells from KI (blue) and WT (red) mice. Dotted lines represent isotype controls. (C-E) Viability/cell growth curves in splenocytes of WT and KI mice were analyzed using WST-1. Viability/proliferation of total splenocytes stimulated with (C) α-IgM, or (D) α-CD40 was more compromised than proliferation of cells stimulated through the (E) TLR by LPS. (F) NP-specific IgM and IgG titers from sera of WT and KI mice immunized with either NP-LPS or NP-CGG. (G) WT and KI mice (n = 5 each) were immunized with either NP-LPS or NP-CGG, and total IgM and IgG antibody concentrations were examined in sera of mice at day 7 or day 14, respectively. (H) Crossing the KI mice to β-actin cre mice to produce KIneo− mice rescued antibody production in the neo-less KI mice. WT and KIneo− (neo-less) mice (N = 5 each) were immunized with NP-CGG and NP-specific antibody titers as well as total antibody concentrations measured in mouse sera at day 21; mice were given a boost at day 14.
B-cell migration, proliferation, and antibody production. (A) Migration (after 3 hours) of splenic B220+ cells from WT versus KI mice to chemokines S1P and SDF-1. (B) S1P1 (EDG1) receptor expression in splenic B220+ cells from KI (blue) and WT (red) mice. Dotted lines represent isotype controls. (C-E) Viability/cell growth curves in splenocytes of WT and KI mice were analyzed using WST-1. Viability/proliferation of total splenocytes stimulated with (C) α-IgM, or (D) α-CD40 was more compromised than proliferation of cells stimulated through the (E) TLR by LPS. (F) NP-specific IgM and IgG titers from sera of WT and KI mice immunized with either NP-LPS or NP-CGG. (G) WT and KI mice (n = 5 each) were immunized with either NP-LPS or NP-CGG, and total IgM and IgG antibody concentrations were examined in sera of mice at day 7 or day 14, respectively. (H) Crossing the KI mice to β-actin cre mice to produce KIneo− mice rescued antibody production in the neo-less KI mice. WT and KIneo− (neo-less) mice (N = 5 each) were immunized with NP-CGG and NP-specific antibody titers as well as total antibody concentrations measured in mouse sera at day 21; mice were given a boost at day 14.
Reductions in mTOR result in reduced B cell proliferation/viability in response to BCR or TLR signals
To evaluate functional responses of B cells, splenocytes were treated with B-cell mitogens, including anti-IgM, anti-CD40, and LPS. Proliferation rates/viability of KI splenocytes were significantly lower than those of WT mice. Signaling through the B-cell receptor (BCR) or CD40 (α-IgM, α-CD40) appeared to be more compromised than Toll-like receptor (TLR; LPS) signaling. Total splenocytes from KI mice did not proliferate in response to either α-IgM (Figure 6C) or α-CD40 (Figure 6D) but did show modest proliferation in response to LPS stimulation, a T-independent signal to the TLR (Figure 6E). Similar defects were observed using purified resting (CD43−) B cells (supplemental Figure 9A-C), although the defects were less pronounced. Together, these data suggest that reduced mTOR levels affect B-cell development as well as function.
Reductions in mTOR lead to reduced antibody production in response to both T-dependent and T-independent antigens
To evaluate whether mTOR-compromised mice could mount a robust antibody response to antigen, KI and WT mice were immunized with the T-dependent antigen nitrophenyl-chicken gamma globulin (NP-CGG) and the T-independent antigen nitrophenyl-lipopolysaccharide (NP-LPS). In both situations, IgM and specifically IgG NP-specific responses were lower in KI mice compared with WT (Figure 6F), despite similar amounts of total antibodies in their sera (Figure 6G), providing evidence for impaired B-cell function in vivo.
Neutrophil and monocyte abnormalities
Although CD11b+/GR1+ precursor populations in the BM were not different (supplemental Figure 10A), the percentages of Mac1+ and Gr1+ myeloid cells were lower in the spleens of KI versus WT mice (supplemental Figure 10B), suggesting abnormalities in neutrophil and monocyte homing and/or homeostasis. Neutrophils isolated from the BM of KI mice also showed lower mTOR protein levels compared with WT (supplemental Figure 10C). Nonetheless, the ability of these cells to migrate directionally in a gradient of fMLP was similar to WT neutrophils (supplemental Figure 10C; supplemental Video Sx). Thus, reduction of mTOR levels affects homeostasis of multiple immune cell lineages.
Altered phenotypes are rescued by removal of the neomycin cassette
To evaluate whether the described phenotypes were the result of the 628C polymorphism in the KI allele, the neo-mTOR KI mice were crossed to β-actin cre mice38 to remove the neo gene. Notably, mTOR protein levels were rescued (ie, normal) in the spleens of neo-less mice (supplemental Figure 11A). Mice and cells that expressed the 628C allele (but lacked the neo insertion: neo−) were all of normal size (supplemental Figure 11B-C), and the numbers and percentages of cells (supplemental Figure 11D-E) were not altered. Furthermore, B- and T-cell development (supplemental Figure 11F-G), viability/proliferation (supplemental Figure 11H), mTORC1 signaling to S6K, and mTORC2 signaling in activated cells (supplemental Figure 11I-J) were grossly normal. As documented in our earlier in vitro assays of the 628C versus 628R allele, there was less p4E-BP1Thr37/46 in BM from neo-less 628C mice compared with BM of WT littermates harboring the 628R allele. These data suggest that mice carrying the 628C allele do exhibit some dampened mTORC1 signaling with respect to 4E-BP1 (supplemental Figure 11K). Nonetheless, the neo-less mice appeared normal and, when challenged with NP-CGG, their antigen-specific IgM and IgG titers were the same as those in WT mice (Figure 6H). Table 1 is a comparison of phenotypes between mice with reduced mTOR (neo-containing) and normal mTOR levels (neo-less). These data imply that it is mainly the reduction in mTOR levels that led to the observed phenotypes in the neo-mTOR mice, and emphasize the importance of mTOR protein levels in determining homeostatic features of immune cells.
Discussion
The physiologic role of mTOR in cells of the immune system was evaluated in viable mTOR KI mice in which mTOR levels were constitutively reduced as a result of neo-disruption of transcription. There were reductions in body size, organ size (with spleens disproportionately affected), as well as lymphocyte sizes, numbers, proliferative responses, and antibody production. Rescue of these phenotypes via deletion of neo, which restored normal mTOR levels, suggested that it was the reduction in mTOR protein level, rather than the allelic variant of mTOR, that produced these phenotypes.
Our studies highlight how mTOR protein levels regulate B- and T-cell development/differentiation in the BM, spleen, and thymus and suggest that physiologic reductions in mTOR influence activation, migration, and homeostasis. In general, B cells were affected more than T cells. Some phenotypes in the mTOR-compromised mice mirror effects seen in rapamycin-treated cells, which are also smaller and less numerous than their nontreated counterparts.39,40 In addition, rapamycin has been shown to increase Akt phosphorylation in response to LPS41 and to decrease IgM production to formalinized Staphylococcus.42
Reduced mTOR protein levels did not profoundly affect thymic development of T cells; however, spleens of the mTOR-compromised mice exhibited several altered phenotypes, including reduced T-cell activation and differentiation. Although the numbers and cell sizes of the CD4 and CD8 T cells were reduced, the overall percentages of CD4 and CD8 cells were higher in the spleens. The lack of T cells populating the spleen does not appear to be an effect of cell death, as mTOR-compromised cells actually survived at least as well or better than WT cells, and responded with similar kinetics to interleukin-7. The T-cell populations in primary and secondary lymphoid organs were more naive, nonactivated T cells.
Although we did not observe increased numbers of Tregs in T cells isolated directly ex vivo from mice, when splenic T cells were activated or induced to differentiate after cross-linking of the TCR in KI mice, a higher proportion of cells differentiated into FOXP3 Tregs (iTregs). These data are consistent with induction of Tregs after rapamycin treatment,43,44 and in mTOR conditional knockouts of CD4+ T cells, which was attributed to hyperactive SMAD3 signaling, leading to enhanced expression of FOXP3.17 However, low S1P1 expression has also been correlated with the induction of Tregs, whereas high expression blocks the differentiation of CD4+, CD25+, FoxP3− precursors into Tregs,43,45 suggesting that there may be multiple effects of mTOR on iTreg differentiation. Our data continue to support the concept that reduced mTOR expression impairs differentiation/activation to other effector T-cell lineages.
Neutrophils provide a key link between innate and adaptive immune responses and are the first responders to microbial infection and tissue injury.46 Although Gomez-Cambronero showed that rapamycin treatment inhibits granulocyte-macrophage colony-stimulating factor and interleuin-8-induced human neutrophil migration in transwell assays,47 we did not observe defects in the ability of mTOR-compromised BM neutrophils to migrate to fMLP gradients. Although human and mouse neutrophils may have distinct requirements for mTORC signals, it remains to be determined whether the residual mTOR present in the KI neutrophils is enough to mediate chemotactic signals to fMLP.
Proliferation of all cell types examined, including B lymphocytes, were lower in the mTOR-compromised mice. In particular, mTOR levels played a significant role in modulating B-cell signaling through the BCR and TLR receptors, as well as CD40 ligation, with more pronounced effects in dampening BCR and CD40 signaling. Resting B cells from the mTOR KI mice showed modest proliferation in response to LPS, suggesting that their TLR signaling may be more effective than their BCR responses and, by extension, their innate immune responses more intact than their adaptive immune responses. Nonetheless, antigen-specific IgG antibody production was lower in the mTOR-compromised mice immunized with T-independent or T-dependent antigens. However, it is notable that IgM responses to NP-LPS (T-independent) were only slightly lower in KI mice.
Analysis of the B-cell populations in the BM suggested a partial block in B-cell development between the large pre-B and small pre-B cell stages. Once B cells made it past the observed restriction point in the BM and migrated to peripheral organs, there were significantly more mature and fewer transitional and marginal zone B cells in the spleen. S1P1-deficient B cells may exit from the BM but have difficulty being retained in marginal zones37 and exiting from secondary lymphoid organs.33 The low SIP1 levels seen in mTOR-compromised B cells may contribute to the shifts in B-cell populations in the spleen. In addition, new cells may not be generated as fast in the KI BM because of the block in B-cell development, and as the cells mature in the spleen, they may not be eliminated properly, potentially resulting from the observed increase in mTORC2 (p-AKT) activity.
In our studies, LPS-activated (but not unstimulated) B cells from spleen, LN, and BM of mTOR-compromised mice induced increased pAKTSer473 levels. This phenotype has been seen in tissue culture as well as in in vivo tumors treated with rapamycin. These data are in contrast to reduced pAKTSer473 levels seen in fibroblasts and T cells from these mice and suggest there are differences in AKT regulation that are mTOR-dependent and cell-type specific. The data for B cells are similar to that of skeletal muscle in which increased pAKTSer473 levels were seen in mice with muscle-specific inactivation of mTOR.48 Thus, in T cells and fibroblasts, the mTORC2 complex may provide the major kinase activity for pAKTSer473. Our data in LPS stimulated B cells, coupled with those of Janes et al showing that pAKT Ser473 is not entirely inhibited by PP24220 and those of Risson et al showing enhanced pAKT Ser473 in skeletal muscle,48 suggest that either another kinase may be more active or a phosphatase less active in regulating pAKTSer473 levels, from the mTOR-compromised mice. Data showing increased DNA-PKcs protein levels in response to LPS treatment coupled with the data showing decreased pAKTSer473 levels after pretreatment with the DNA-PKcs inhibitor NU7026 suggest that a kinase sensitive to low concentrations of this inhibitor contributes to the enhanced pAKTSer473 levels seen in KI B cells. Alternatively, pAKTSer473 up-regulation in the compromised mice may result from dampened S6K activity, recently shown to be a direct regulator of RICTOR49 and capable of altering pAKTSer473 levels.
Our mTOR mouse may phenocopy some aspects of long-term drug treatment with the new adenosine triphosphate competitive “active site” mTORC1/TORC2 dual inhibitors, such as Torin1,18 PP242,19 and AZD8055.50 Of particular interest is that both T and B cells as well as the spleens of mice given long-term treatment with PP242 were decreased in size, yet antigen-specific IgM production to NP-OVA was normal.20 The increases seen in p-AKTSer473 in activated B cells in these mice, coupled with the incomplete inhibition of p-AKTSer473 by PP242 treatment,20 suggest that long-term administration of these newer drugs may need to be assessed carefully in specific cell types.
The mTOR-compromised mouse provides a model for studying the in vivo role of mTOR protein levels in regulating physiologic and pathologic processes; it is particularly valuable for assessing immune cell activation and differentiation and the role of mTOR in immune responses. These mice also provide the opportunity to evaluate the complex effects of constitutive reductions in mTOR on whole animals and the distinct effects in different cell types on signal transduction pathways affecting and interacting with mTOR.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank colleagues Douglas Lowy, Jonathan Ashwell, Howard Young, and Glenn Merlino for their comments on the manuscript; Karen Reidy and Laura Roth for genotyping the animals; Rafael Casellas and Dennis Klinman for advice on immunizations; Michael Kuehl for providing myeloma cell lines; and Ke Zhang and Jennifer Cannon for important insights during the studies.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and the National Human Genome Research Institute.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
National Institutes of Health
Authorship
Contribution: S.Z. and J.A.R. designed and performed research, collected and analyzed data, and wrote the manuscript; M.J.-J., W.D., R.R., M.P., J.Z.W., K.S., J.P., V.B., and L.T. performed research; and C.A.P., J.Z.W., L.T., P.L.S., and B.A.M. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beverly A. Mock, Laboratory of Cancer Biology and Genetics, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bldg 37, Rm 3146, 37 Convent Dr, MSC 4258, Bethesda, MD 20892-4258; email: bev@helix.nih.gov.
References
Author notes
S.Z. and J.A.R. contributed equally to this study.