In this issue of Blood, Navarrete et al report that upfront immunization of follicular B-cell lymphoma patients with bacterially produced Fab fragments induces immune responses; however, the influence on patient outcome is not yet known.1
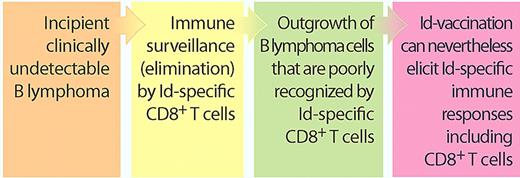
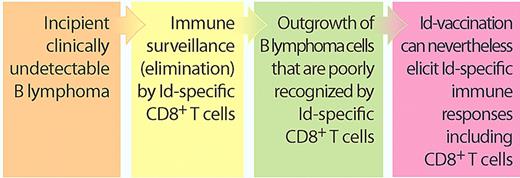
These results should be discussed together with a recent study in Blood by the Veelken laboratory.2 The authors suggest that B lymphomas escape killing by idiotype-specific CD8+ T cells, simply because the relevant parts of their immunoglobulin (Ig) V-region sequences bind poorly to the patient's own human leukocyte antigen (HLA) class I molecules.2 Taken together, these results indicate that even though the B lymphoma is relatively invisible to the patient's killer T cells, such cytotoxic cells can nevertheless be stimulated by vaccination to become responsive to lymphoma Ig (see figure).
B lymphomas escape Id-specific killer T cells: does this preclude Id vaccination? Recent in silico findings by Veelken and coworkers suggest that B lymphoma–derived Ig V-region Id peptides bind poorly to the patient's own HLA class I molecules, indicating that only lymphoma cells which escape immunosurveillance by Id-specific CD8+ T cells progress into clinical cancers.2 Nevertheless, they now show that Id vaccination of patients can elicit Id-specific responses including CD8+ responses.1 These results indicate that B-lymphoma patients are not totally devoid of lymphoma-reactive Id-specific CD8+ T cells and that these can be stimulated by vaccination with patient Fab fragments produced in bacteria. (Professional illustration by Paulette Dennis.)
B lymphomas escape Id-specific killer T cells: does this preclude Id vaccination? Recent in silico findings by Veelken and coworkers suggest that B lymphoma–derived Ig V-region Id peptides bind poorly to the patient's own HLA class I molecules, indicating that only lymphoma cells which escape immunosurveillance by Id-specific CD8+ T cells progress into clinical cancers.2 Nevertheless, they now show that Id vaccination of patients can elicit Id-specific responses including CD8+ responses.1 These results indicate that B-lymphoma patients are not totally devoid of lymphoma-reactive Id-specific CD8+ T cells and that these can be stimulated by vaccination with patient Fab fragments produced in bacteria. (Professional illustration by Paulette Dennis.)
B-lymphoma cells express an exquisite tumor-specific antigen, monoclonal Ig, which contains unique antigenic determinants called idiotopes (Id) in their variable (V) regions. An immune attack against Id on the B-lymphoma cells could take 2 forms: (1) anti-idiotypic antibodies binding the surface Ig3,4 and (2) T cells recognizing short V region–derived Id peptides presented on the lymphoma's HLA molecules.5,6 Major efforts have been made by numerous investigators to generate individualized Id vaccines that elicit clinically meaningful immune attacks against lymphomas, but the results have been mixed.7
The finding that Ig V regions of lymphomas bind poorly to the patients' own HLA class I molecules, as analyzed by bioinformatics, indicates that emerging B-lymphoma cells must escape an otherwise effective immune attack by Id-specific CD8+ killer T cells. This finding extends previous observations demonstrating that Id-specific CD4+ T cells play a role in immunosurveillance of B lymphomas.5 Thus, clinical B lymphomas may already be immune-sculpted into having an invisibility cloak, which suggests that Id vaccination could be futile simply because the patient's B lymphoma has been selected to escape an immune attack. A counterargument is that Id vaccination could boost otherwise too weak T-cell reactivity so that it rises to clinically relevant levels. In support of this idea, Navarrete and coworkers report in this issue that upfront Id vaccination of untreated patients with B lymphomas can elicit both Id-specific CD4+ and CD8+ T-cell responses, consistent with an available repertoire of weakly Id-reactive T cells that apparently can be boosted by vaccination (see figure).
Most investigators in this field would be inclined to believe that Id-specific T cells serve to eradicate B-lymphoma cells. However, it might be that some types of Id-specific T cells could have the opposite role and, in fact, support the growth of lymphomas. Indeed, previous reports have shown that Id-specific Th2 cells could induce Id+ B lymphomas in mice,8 and that Id-specific CD4+ T cells may play a role in development of multiple myeloma in humans.9 With some hindsight, such findings are perhaps not so surprising as helper T cells are known to induce proliferation of B cells, which in turn could predispose for oncogenetic mutations.
In broader terms, the many distinct subsets of T cells could differ in their effect on B-lymphoma cells. Based on current immunologic wisdom, it is reasonable to suggest that Id-specific CD8+ T cells should kill B lymphomas, as suggested by the reports of Veelken and coworkers, while the roles of Th1, Th2, Th17, T follicular helper, and regulatory T cells remain to be firmly established. Given that different modes of Id vaccination could differ in their propensity to generate the various T-cell subsets, it is important to develop Id vaccines that elicit T-cell subsets with detrimental rather than supportive effects on B-lymphoma growth.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■