Abstract
Several novel targeted therapies have recently emerged as active in the treatment of non-Hodgkin lymphoma, including small molecules that inhibit critical signaling pathways, promote apoptotic mechanisms, or modulate the tumor microenvironment. Other new agents target novel cell surface receptors or promote DNA damage. Although most of these drugs have single-agent activity, none have sufficient activity to be used alone. This article reviews the utility and potential role of these new agents in the treatment of non-Hodgkin lymphoma with a specific focus on data that highlight how these agents may be incorporated into current standard treatment approaches.
Introduction
Malignant lymphoma is the 5th most common cancer in the United States, with the incidence increasing in the past 3 decades. Although some of the more aggressive forms of non-Hodgkin lymphoma (NHL) may at times be cured with combination chemoimmunotherapy, many patients with lymphoma eventually succumb to their disease. Initial attempts to improve the outcome of lymphoma patients were on the basis of intensifying therapy by adding additional chemotherapy agents or shortening the interval between doses. In aggressive lymphoma, these approaches initially improved survival and established CHOP chemotherapy (ie, cyclophosphamide, hydroxydaunorubicin [doxorubicin], Oncovin [vincristine], and prednisone/prednisolone) as the standard treatment approach. Further attempts to add additional drugs to the frontline combinations did not result in additional benefit. Intensifying CHOP by decreasing the time between doses added only a modest benefit in a subset of patients and significantly increased toxicity. In low-grade histology, intensifying therapy by adding additional chemotherapeutics did not improve survival at all. The significant advance in improving the survival of patients with B-cell NHL came with the inclusion of rituximab in treatment combinations for both low-grade and aggressive lymphoma subtypes. The use of chemoimmunotherapy in B-cell NHL has now become the standard of care.
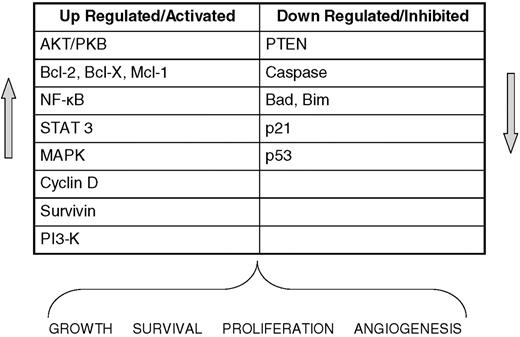
In recent years, advances in NHL have produced information critical to our understanding of cell growth, proliferation, and cell death in malignant cells. The intracellular machinery and signaling cascades that are active in lymphomas (Figures 1 and 2) have been dissected and reveal multiple potential targets for new agents.1 These advances in our understanding have spawned several clinical investigations of novel agents, several of which now appear to have clinically relevant single-agent activity in malignant lymphoma (Table 1). For the field of therapy to be advanced beyond current standards, novel agents need to be examined as additions to standard treatments and in unique combinations. Multiple new anti-CD20 antibodies are being developed to improve on the efficacy of rituximab. In this review, however, we will focus on novel agents, other than monoclonal antibodies, that have shown the most promise for future therapy of B-cell lymphoma.
Altered regulation in lymphoma. Key cellular components often dysregulated in malignant lymphocytes that lead to tumor growth, survival, and proliferation.
Altered regulation in lymphoma. Key cellular components often dysregulated in malignant lymphocytes that lead to tumor growth, survival, and proliferation.
Signaling pathways in malignant lymphoma. Several well-described pathways and potential targets for novel agents.
Signaling pathways in malignant lymphoma. Several well-described pathways and potential targets for novel agents.
Agents targeting the tumor microenvironment
Historically, the predominant approach to cancer therapy has been to develop agents that specifically inhibit the growth of the malignant cell. This selective inhibition has largely relied on the fact that the malignant cells proliferate faster than normal cells or express certain proteins more abundantly than nonmalignant cells. More recent data have shown that the tumor microenvironment may also be a valid therapeutic target because surrounding normal cells may provide support for the malignant cell. Investigators2,3 have shown that lymph nodes involved by malignant lymphoma commonly contain an admixture of non-neoplastic T cells, dendritic cells, macrophages, and stromal elements. They have found that CD4+ T cells (including follicular helper T cells4 and regulatory T cells,5 cytotoxic CD8+ T cells,6 macrophages,7 dendritic cells,8 and intratumoral microvessels9 ) all play a role in malignant cell growth. Targeting these supporting cells with the use of drugs that modify the immune response may provide a novel therapeutic opportunity.
Immunomodulatory drugs
The immunomodulatory (IMiD) class of agents has been already established as having a role in the treatment of myelodysplastic syndrome and multiple myeloma. Lenalidomide and pomalidomide are derived from the parent compound thalidomide and carry more potent activity (as measured in vitro inhibition of tumor necrosis factor-α [TNF-α]) as well as altered toxicity profiles.
Mechanism of action.
The exact mechanism of IMiD activity is unclear and may be different for different diseases. Much of what is known comes from data in myeloma cell lines. IMiDs have been shown to be antiproliferative, and in greater doses lenalidomide and pomalidomide can cause off-target myelosuppression. The direct antiproliferative effects may be caused by down-regulation of nuclear factor κB (NFκB)10 and direct stimulation of the intrinsic apoptotic pathway. IMiDs also inhibit the signal transducers and activators of transcription 3 (STAT3) and mitogen-activated protein kinase (MAPK) signaling pathways. Lenalidomide inhibits the Akt pathway11 and increases the expression the tumor suppressor gene p21.12 The IMiDs may also exert their effect by immune modulation. IMiDs stimulate T/natural killer (NK) cell activity in vitro and data suggest that this may be important in triggering tumor cell apoptosis.13 Lenalidomide has 2000 times more inhibitory effect on TNF-α secretion by monocytes in vitro than thalidomide,14 but an increase in TNF-α levels is seen in treated patients with multiple myeloma, presumably as the result of T-cell stimulation.15 IMiDs increase interleukin-2 (IL-2) and interferon-γ and down-regulate other cytokines, including IL-6, IL-8, IL-10, and platelet-derived growth factor.10
Use in lymphoma.
Lenalidomide has been extensively studied in the treatment of lymphoma and appears active in all subtypes. Wiernik et al16 published early results in relapsed aggressive NHL (including diffuse large B-cell lymphoma [DLBCL], mantle cell lymphoma [MCL], grade 3 follicular lymphoma [FL3], and transformed NHL): 49 patients enrolled; more than one-half had DLBCL; 35% overall (OR) and 12% complete (CR) responses were seen in this heavily pretreated population (median of 4 previous therapies). In a larger international trial (NHL-003), Witzig et al17 reported responses for multiple subtypes of aggressive histologies: all histologies (35%), DLBC (28%), MCL (42%), FL3 (42%), and transformed NHL (45%). The median duration of response was 11.6 months and was especially prolonged in FL3 and MCL subtypes. The most remarkable responses, however, are seen in MCL. The NHL-002 trial of single-agent lenalidomide in patients with relapsed aggressive NHL showed an OR of 53% and duration of response (DOR) of 13.7 months in the MCL subgroup.18 In the subgroup analysis from the NHL-003 trial, 54 patients with relapsed or refractory MCL with a median of 3 previous therapies had an OR of 43% (17% CR/unconfirmed CR [CR/CRu], 26% partial response [PR]).19 Even those having had previous therapy with bortezomib responded (9/17, overall response rate [ORR] 53%, CR 18%, PR 35%). Witzig et al20 demonstrated an OR of 26% (11/43) in indolent NHL with 2 CR, 1 CRu, and 8 PR. In a subgroup analysis,21 the OR in FL was 32% and in small lymphocytic lymphoma (SLL) was 22%. The single-agent activity in this relapsed setting has led to new trials in which lenalidomide has been added to combinations of active agents, as will be discussed in “The use of new drugs in rational combinations.”
IMiD toxicity.
The newer thalidomide analogs because of their chemical structure are presumed to be teratogenic, but this has not been proven. Regardless, great precautions must be taken to avoid exposing a fetus to these agents. Thrombosis, which has been a major concern in the multiple myeloma population, has not yet been shown to be a significant issue in the lymphoma studies, albeit that aspirin has been used frequently for prophylaxis. Unlike thalidomide, lenalidomide and pomalidomide cause myelosuppression that is dose dependent. In the NHL-003 study grade 3-4 adverse effects were neutropenia 27%, thrombocytopenia 15%, anemia 8%, fatigue 5%, and leukopenia 5%.
Targeting regulatory T cells with the use of denileukin diftitox
Denileukin diftitox (Dd) is a fusion protein composed of IL-2 binding sequences and active fragments of diphtheria toxin. It was approved for treatment of CD 25+ cutaneous T-cell lymphoma (CTCL) several years ago and is now being investigated in treatment of other lymphomas. Because CD25+ naturally occurring regulatory T-cells (Treg cells) are highly expressed in B-cell lymphoma and suppress other intratumoral immune cells,6 the use of Dd to deplete Treg cells may be of clinical benefit.
Mechanism of activity.
Dd was designed to target cells expressing CD25 (TAC), the high-affinity IL-2 receptor that is present on activated T cells, B cells, NK cells, and macrophages. Ex vivo studies suggest that Dd binds to high- and medium-affinity IL-2 receptors. The molecule is internalized and the toxin released, resulting in inhibition of protein synthesis and cell death.
Use in lymphoma.
Dd is approved for use in CD25+ CTCL that is refractory or persistent after previous therapy. Interestingly, patients whose CTCL was CD25− also had responses (31%) suggesting off-target effects. This agent was therefore also tested in B-cell lymphomas. Dang et al22 found good response rates (24.5%) in aggressive NHL subtypes, with responses seen in both CD25+ and CD25− tumors. Disappointingly, a phase 2 trial of Dd in indolent B-cell NHL showed a 10% ORR with only 3 PRs.23
Toxicity of Dd.
Vascular leak syndrome with edema and low serum albumin can occur, although this is uncommon and mild if patients are premedicated with antihistamines and corticosteroids. Transaminase elevation and fatigue can occur. Dd is not myelosuppressive and in combination with other drugs has not shown any unexpected toxicity.
Inhibiting signaling pathways
Multiple signaling pathways appear to play a significant role in lymphoma growth and survival (Figure 1). Tonic signaling through the B-cell receptor has been shown to play a role in B-cell lymphoma growth, as has constitutive activation of the NFκB and Jak/Stat pathways. Multiple cytokines and growth factors may activate intracellular signaling, resulting in increased proliferation and survival of lymphoma cells. Inhibiting these pathways may result in significant clinical benefit for patients.
B-cell receptor signaling
Spleen tyrosine kinase inhibitors.
Spleen tyrosine kinase (SYK) is known to play a crucial role in immune receptor signaling.24 Immune receptors, including the B-cell receptor (BCR), associate with transmembrane proteins that have cytoplasmic domains containing immunoreceptor tyrosine-based activation motifs. immunoreceptor tyrosine-based activation motif-mediated tonic BCR signaling is required for the survival of resting mature B cells and certain B-cell lymphomas. This signaling involves SYK, resulting in the expression of constitutively active SYK in B-cell lymphomas. Fostamatinib disodium is a tyrosine kinase inhibitor targeting SYK that inhibits lymphoma cell growth.
Mechanism of activity.
BCR signaling via SYK activation promotes cell survival and proliferation by affecting multiple pathways, including phosphatidylinositol 3-kinase (PI3K), AKT, MAPK, RAS, and mammalian target of rapamycin (mTOR). There is in vitro evidence that SYK expression is required for lymphoma survival and that inhibition leads to tumor regression in vivo.25 Fostamatinib disodium (R788) is oral prodrug of R406, which has been shown to downgrade BCR signaling via SYK inhibition and can lead to apoptosis in DLBCL cell lines.26
Use in lymphoma.
Fostamatinib disodium (R788) has shown promise in a phase 1/2 trial. Friedberg et al27 reported an ORR of 21% in a group of heavily pretreated patients with a variety of lymphoma subtypes. The median DOR was 4.3 months. Twenty-two percent of DLBCL patients and 55% chronic lymphocytic leukemia (CLL)/SLL patients responded.
Toxicity of SYK inhibitors.
Grade 3 + 4 fatigue and diarrhea occurred in more than 40% of patients, and neutropenia, anemia, and thrombocytopenia were observed in 31%, 27%, and 24% of patients in the phase 2 trial, respectively.
Bruton tyrosine kinase inhibitors
Bruton tyrosine kinase (Btk) is a member of the src-related Btk/Tec family of cytoplasmic tyrosine kinases and is required for BCR signaling. Btk plays a key role in B-cell maturation and is overexpressed in several B-cell malignancies. Activation of Btk triggers a cascade of signaling events that culminates in the generation of calcium mobilization and fluxes, cytoskeletal rearrangements, and transcriptional regulation involving NFκB. Moreover, Btk activation is tightly regulated by a plethora of other signaling proteins, including protein kinase C (PKC). PCI-32765, a potent, selective oral inhibitor of Btk, has shown clinical activity early phase trials in B-cell NHL.
Mechanism of activity.
PCI-32765 is an inhibitor of Btk with antineoplastic activity. It binds to and inhibits Btk activity, preventing B-cell activation and B-cell–mediated signaling as well as the growth of malignant B cells that overexpress Btk.
Use in lymphoma.
In a clinical trial of 29 patients (12 follicular, 7 CLL/SLL, 4 DLBCL, 4 mantle, 2 marginal zone lymphoma), patients received PCI-32765 at doses of up to 8.3 mg/kg/day. An overall response rate of 42% was observed; 1 CR (SLL), 7 PR (4 CLL/SLL, 2 MCL, and 1 FL). In pharmacodynamic studies Advani et al28 demonstrated complete occupancy of Btk by PCI-32765, with > 95% enzyme occupancy 4 hours after dose in all patients.
Toxicity of PCI-32765.
Most toxicities were reportedly < grade 2.
Janus kinase 2/STAT pathway
The Janus kinase 2 (JAK2)/STAT pathway plays an important role in the pathogenesis of hematologic malignancies. Activating mutations in the JAK2 gene has been reported in many myeloproliferative disorders but are rare in lymphoma. The STAT pathway, however, appears activated in lymphomas and may be suppressed by small molecule inhibitors such as SB1518.
Mechanism of activity.
SB1518 is an orally bioavailable inhibitor of JAK2 and the JAK2 mutant JAK2V617F with antineoplastic activity. SB1518 competes with JAK2 for ATP binding, thereby inhibiting JAK2 activation. It also inhibits the JAK-STAT signaling pathway and promotes caspase-dependent apoptosis. The JAK-STAT signaling pathway is a major mediator of cytokine activity, and inhibition of this pathway may suppress cytokines in the tumor microenvironment that promote tumor cell growth.
Use in lymphoma.
In a phase 1 study of SB1518 in patients with relapsed lymphoma, patients were treated at doses up to 400 mg orally daily.29 Eighteen patients are enrolled, and 3 patients at the 300-mg dose level demonstrated disease response (1 FL, 1 SLL, and 1 MCL). Eleven patients (73%) had stable disease. The effect of drug treatment on pJAK2, pSTAT3, and pSTAT5 was examined and SB1518 inhibited the JAK/STAT pathway as early as 4 hours after administration.
Toxicity of SB1518.
The common grade1-2 adverse effects were diarrhea and constipation in 40%, and 13% developed grade 3 neutropenia.
PI3K/AKT/mTOR pathways
PI3K inhibitors
Direct inhibition of PI3K can potentially lead to inhibition of AKT and mTOR, both of which are critical regulators of cell proliferation and growth.
Mechanism of activity.
PI3-kinase plays a key role in cell metabolism, proliferation, and survival, and it is often dysregulated in B-cell malignancy through BCR signaling or phosphatase and tensin homolog mutation. CAL-101 is a selective and potent inhibitor of the PI3-K isoform p110δ, which is predominant in hematologic cells. Studies confirm in vivo activity by down-regulation of AKT, a downstream target.
Use in lymphoma.
CAL-101 is an oral PI3K inhibitor that has entered phase 1 testing. Furman et al30 presented results in 57 patients (NHL, n = 29; CLL, n = 18; acute myeloid leukemia, n = 10) treated in standard dose escalation cohorts. Forty-nine percent had refractory disease. Responses were seen at all dose levels with objective responses in 9 of 15 indolent NHL, 6 of 7 MCL and, in CLL, 14 of 16 had reduced lymphadenopathy accompanied by increasing lymphocytosis.
Toxicity of CAL-101.
The dose-limiting toxicity was transaminitis.
AKT inhibitors
Perifosine is a novel oral agent in a new class of cancer therapies, the alkylphospholipids. It is a synthetic oral agent that blocks activation of AKT, a key intracellular kinase involved in cell survival and proliferation.
Mechanism of activity.
AKT is often constitutively activated in lymphomas as well as other cancers. It is downstream of PI3K, upstream from mTOR, and plays an important role in cell survival and proliferation.31 In vitro and in vivo data show that inhibiting AKT directly can lead to cell death; however, perifosine may also act by other means, including effects on the MAPK and JNK pathways.32 In vitro cytotoxicity is enhanced in the presence of other novel agents.
Use in lymphoma.
Perifosine has been studied in gastrointestinal, renal cancers, and hematopoietic malignancies, including multiple myeloma and Waldenström macroglobulinemia. A phase 2 clinical trial of single agent perifosine in 36 patients with rel/ref Waldenström macroglobulinemia showed responses in 35% (11% PR, 24% minimal response [MR]), and 54% had stable disease.33
Toxicity of perifosine.
Gastrointestinal symptoms of some degree occurred in more than 65% in the phase 2 trial. Although grade 3-4 hematologic toxicity was uncommon, grade 1-2 anemia and neutropenia were seen in 65% and 49%, respectively.
mTOR inhibitors
mTOR is a central component in signaling of normal and malignant cell processes such as growth and proliferation. Inhibition of mTOR leads to cell death. There are currently 4 mTOR inhibitors in the clinic: rapamycin (sirolimus) and the rapalogs temsirolimus (CC-779), everolimus (RAD001), and deforolimus. Temsirolimus and deforolimus are intravenous agents, whereas everolimus and sirolimus can be administered orally.
Mechanism of action.
The PI3K pathway is often dysregulated in human cancers as the result of mutation or loss of phosphatase and tensin homolog, mutation of PI3K, or amplification of AKT (protein kinase B).34 mTOR is a downstream target of the PI3K/AKT pathway and is represented by 2 components, mTORC1 and mTORC2. Only the mTORC1 component is inhibited by rapamycin and the rapalogs, and it appears that mTORC2 is activated by pathways distinct from PI3-K/AKT. mTOR activation by AKT leads to cell proliferation and survival by modulating protein synthesis of critical molecules such as cyclin D1. mTOR signaling also activates NF-κB–induced survival pathways.35 The rapalogs are specific inhibitors of mTOR and in vitro can induce cell death. Inhibition of mTOR can sometimes lead to up-regulation of AKT by negative feedback, which may be a possible mechanism of resistance to the rapalogs.
Use in lymphoma.
Temsirolimus (CCI-779) was studied in relapsed MCL as a single agent given once weekly at a dose of 250 mg/m2. The OR in 34 patients was 38%, with 1 CR and 12 PRs.36 At this dose level, 63% of patients experienced grade 3 thrombocytopenia, which often led to delays in therapy. A second cohort of MCL patients were treated at one-tenth the original dose (25 mg/m2) and again demonstrated activity with 41% OR despite the reduced dose.37 This reduced the thrombocytopenia to 39% and produced equal response (41% vs 38%) and DOR (6.0 months vs 6.9 month) as the greater dose. Temsirolimus also has activity in non-MCL as shown by Smith et al,38 with 40% OR, 15% CR, and 26% PR.
Everolimus (RAD001) has also shown preclinical activity in a variety of hematologic cancers. We presented the early results of this oral agent in aggressive lymphomas: 37 patients with a median age of 72 years and a median of 4 previous therapies received RAD001 at a starting dose of 10 mg daily, continuing until disease progression or undue toxicity.39 The OR was 32% (12/37) with 7 of 20 DLBCL and 4 of 14 MCL patients responding. The median duration of response was 5.5 months. Johnston et al40 used RAD001 in a group of heavily pretreated Hodgkin lymphoma patients and showed an OR of 47% (7/15). Ghobrial et al41 recently published results of RAD001 in relapsed Waldenström macroglobulinemia; The OR in 50 patients was 70% with 42% PR and 28% MR. These data confirm that mTOR inhibitors have significant activity in malignant lymphoma, giving proof to the concept that targeting mTOR is relevant in this disease.
mTOR inhibitor toxicity.
Temsirolimus and everolimus both produce reversible myelosuppression, particularly thrombocytopenia. In addition everolimus can cause hyperglycemia (gr2 16%, gr3 11%), hyperlipidemia (gr2 11%, gr4 2%), and a small number of patients develop aphthous stomatitis. An uncommon development has been the appearance of interstitial pneumonitis on routine follow-up computed tomography scans.
Ras pathway inhibitors
The Ras/Raf/mitogen-activated kinase ½ (MEK1/2)/MAPK pathway is one of the most frequently dysregulated signaling cascades in cancer. Activating mutations of Ras and Raf occur frequently in both solid tumors and hematologic malignancies, leading to activation of their downstream targets MEK1/2 and extracellular signal-regulated kinase 1/2.42
Mechanism of action.
The Ras pathway is involved in multiple cellular processes, including cell proliferation, differentiation, and transformation. However, for the Ras protein to function, prenylation (farnesylation) is required. Ras then activates Raf, MEK1/2, extracellular signal-regulated kinase 1/2, and MAPK. Furthermore, there is accumulating evidence that cross-talk between this pathway and various other signaling pathways exists.43 These findings have resulted in the clinical development of small molecule inhibitors targeting specific components of the Ras/MAPK pathway, including farnesyltransferase inhibitors (eg, tipifarnib), Raf-1 inhibitors (eg, sorafenib), and MEK1/2 inhibitors (eg, AZD6244, TAK-733).44
Use in lymphoma.
The oral farnesyltransferase inhibitor, tipifarnib, has been used to treat patients with relapsed DLBCL, LF3, or MCL.45 Of the 38 patients who were evaluated, 18% had PR, and 21% had stable disease. A second study in MCL showed 1 response of 11 patients treated (9%).46
The multikinase inhibitor sorafenib (BAY 43-9006) initially was developed as a Raf-1 inhibitor but has subsequently been shown to inhibit multiple other kinases, including FLT3, platelet-derived growth factor receptor, vascular endothelial growth factor receptor 1 (VEGFR1), and VEGFR2.47 Sorafenib has been approved for the treatment of advanced renal cell carcinoma and hepatocelluar carcinoma. It has in vitro activity in lymphoma and multiple myeloma cell lines but has not been studied as a single agent in vivo.
Toxicity of Ras inhibitors.
Tipifarnib causes myelosuppression. Sorafenib side effects include hand-foot syndrome, rash, fatigue, anorexia, and diarrhea.
PKC inhibition
PKC beta (PKCβ) plays a pivotal role in normal B-cell signaling and survival. Overexpression of PKCβ is implicated in the pathogenesis of B-cell lymphoma and has prognostic significance in diffuse large B-cell lymphoma.48 Enzastaurin, an oral serine/threonine kinase inhibitor, targets the PKCβ as well as the PI3K/AKT pathways to inhibit tumor cell proliferation, induce apoptosis, and suppress tumor-induced angiogenesis.
Mechanism of action.
PKC-α is the major PKC isoform expressed by normal and malignant B lymphocytes and its activity is pivotal for survival signals triggered by the B-cell receptor.49 In addition to the direct effects on tumor cells, PKC and PKC-α signaling pathways are also linked to VEGF-induced angiogenesis.50 The antiangiogenic activity of PKC inhibitors may therefore represent an important functional aspect of these compounds. In fact, the antiangiogenic effect of enzastaurin has been demonstrated in several preclinical B-cell malignancy models.51,52
Use in lymphoma.
Responses to single-agent enzastaurin in patients with aggressive lymphomas have been rare. However, enzastaurin has appeared to prolong the time to progression in these patients. Similarly, in 60 patients with MCL, no objective tumor responses occurred, but 22 patients (37%) were free from progression for > 3 months; 6 of 22 were free from progression for > 6 months; and 2 patients remain on treatment and free from progression at > 2 years.53 In contrast, patients with FL have had greater response rates when treated with enzastaurin. Of 64 patients with FL treated in a phase 2 trial, 1 (1.6%) had a CR and 15 (23.4%) had PR, for an overall RR of 25%.54 The median DOR had not been reached (59-687 days).
Toxicity of enzastaurin.
Grade 3-4 adverse events are uncommon and in general it is well tolerated.
NFκB modulation with the use of proteasome inhibitors
The proteasome has been identified as a novel target in cancer cells, given the role it plays in cell cycling, growth, and survival. The proteasome is responsible for the degradation of ubiquinated proteins, and there are more proteasomes in malignant compared with normal cells. The first proteasome inhibitor, bortezomib (Btz), has become an important treatment in the management of multiple myeloma and has more recently been shown to have activity in lymphoma. A second proteasome inhibitor, carfilzomib, is now being studied in phase 1 and 2 trials. Oral proteasome inhibitors are in early phases of development.
Mechanism of activity.
Although Btz clearly inhibits the proteasome, there is some controversy as to the mechanism of its antitumor activity. In multiple myeloma it has long been accepted that Btz acted via the inhibition of NF-κB, a transcriptional factor that has been associated with cancer cell survival not only in myeloma but in lymphoma as well. New information suggests that Btz may also activate NF-κB, suggesting that other mechanisms may be responsible, including inhibition of the aggresome and activation of the unfolded protein stress response.55,56
Use in lymphoma.
Btz as a single agent produced responses in a group of relapsed NHL patients as shown by Goy et al.57 Of 29 evaluable patients with MCL, the ORR was 41% with 20.5% CR and 20.5% PR. In the other B-cell NHL patient group, 4 of 21 patients responded (19%). In a second trial, O'Connor et al58 treated 24 NHL patients who had relapsed after a median of 3 previous therapies. The ORR was 58% with a 50% response in MCL and 77% response in follicular NHL. In a larger study (“Pinnacle”)59,60 overall responses were seen in 45 of 141 (32%) relapsed MCL patients, with 8% CR/CRu and 24% PR. The median TTP was 6.7 months and for responding patients 12.4 months. The results of this trial led to the Food and Drug Administration approval of Btz for treatment of relapsed MCL. Di Bella et al61 reported results of Btz in patients with indolent lymphoma who had relapsed after rituximab therapy. Six of 36 FL and 1 of 6 patients with marginal zone lymphoma had objective responses; however, many were noted to have stable disease. Recently, O'Connor et al62 reported responses in 9 of 18 with FL and pointed out that the time to response was longer (12 vs 4 weeks) than in MCL.
Toxicity of proteasome inhibitors.
The most common grade 3 + 4 adverse events with Btz are neuropathy (up to 50% of patients experience some symptoms), fatigue (12%), and thrombocytopenia (11%). Carfilzomib does not appear to cause neuropathy at such high frequency but has been associated with tumor lysis and elevations in creatinine.
New agents to promote apoptosis
Histone deacetylase inhibitors
Overexpression and underexpression of certain genes are hallmarks of malignant cells and are often caused by duplication or deletion of critical genes. Expression can also be affected by epigenetic factors such as histone proteins, which are regulated by an acetylation/deacetylation enzyme system. Cancer cells frequently show an over activity of deacetylases, and inhibiting this activity can restore a more normal expression profile.
Several histone deacetylase inhibitors (HDACi) have been studied and 2, vorinostat (SAHA, Zolinza) and romidepsin (depsipeptide), have been approved for treatment of CTCL. LBH589 (panobinostat) and MGCD0103 are in phase 2 clinical trials, and all show activity in treating lymphoma.
Mechanism of action.
HDACi have been shown to promote normal apoptotic pathways,63 and in vitro this can lead to death of malignant cells while sparing normal cells. HDAC inhibition has also been shown to inhibit angiogenesis. An interesting feature of HDACi is the induction of p21 that leads to cell cycle arrest. Not all the HDACi are the same, and some appear to not only inhibit histone deacetylases but other nonhistone proteins, such as heat shock protein 90, hypoxia-inducible factor-1α, and α-tubulin, all which play roles in promoting proliferation, migration, angiogenesis, and oncogenesis.
Use in lymphoma.
Panobinostat (LBH589) is an oral HDACi that has shown activity in a variety of cancers. In a phase 2 trial including many advanced hematologic cancers, a subgroup of 13 relapsed Hodgkin lymphoma patients received LBH589 in a dose of either 20 mg orally 3 days per week or every other week, and 5 of 13 had a PR.64 In a more recent study, Younes et al65 showed an OR of 18% (1 CR, 10 PR) in a group of heavily treated relapsed HL patients. LBH589 is now being studied in B-cell NHL and in combination with other novel agents and will be discussed in “The use of new drugs in rational combinations.”
MGCD0103 is a new oral HDACi. In a phase 2 trial for relapsed NHL, Crump et al66 showed an OR of 14% (8/59). Bociek et al67 used 2 different doses, 110 mg and 85 mg in a group of relapsed HL patients, and responses were 35% and 13%, respectively. The median duration of response was 6 months.
Suberoylanilide hydroxamic acid (Zolinza, vorinostat) was approved for treatment of stage ≥ 2B CTCL failing 2 or more previous therapies but has shown activity in non-cutaneous lymphoma too. Kirschbaum et al68 presented results of a phase 2 relapsed/refractory NHL trial that included follicular, mantle cell and mantle zone subtypes. The dose was 200 mg by mouth twice a day, the same as is used in therapy of CTCL. An overall response of 29% was seen and 37% of the follicular and MZL patients responded.
HDACi toxicity.
HDACi have similar toxicities, the most common being fatigue, nausea, thrombocytopenia, and neutropenia. Grade ≥ 3 toxicities occur in < 30% of patients.
Bcl-2 Inhibitors
The Bcl-2 family proteins (Bcl-2, Bcl-X, Bcl-w, Mcl-1, etc) are key regulators of cell survival through their effects on the mitochondrial-mediated pathway of apoptosis, or programmed cell death. Targeting Bcl-2 proteins has therefore been a logic goal for the treatment of cancer. Several compounds have been identified that have anticancer activity in xenograph models through their ability to block Bcl-2 antiapoptotic proteins.
Mechanism of activity.
The Bcl-2 family includes proapoptotic and antiapoptotic proteins, and the balance of these can control whether a cell lives or dies. Cancer cells are known to have altered expression of these proteins, and up-regulation of antiapoptotic Bcl-2 proteins is associated with tumorigenesis and resistance to chemotherapy. Inhibition of these antiapoptotic proteins can restore normal apoptosis mediated by caspase activation. These agents have single agent activity in a variety of cancer cell lines and in addition show augmentation in cell killing when used in conjunction with chemotherapy agents.
Use in lymphoma.
ABT-263 is an oral BH-3 mimetic that inhibits multiple Bcl-2 family proteins, including Bcl-2, Bcl-w, and Bcl-X, all of which are prosurvival molecules found in lymphoma. In a phase 1 study Wilson et al69 used 2 dosing schedules. Responses (3 PR, 7 MR) were seen in 42 patients with CLL/SLL, FL, and NK/T-cell lymphoma. Another phase 1 trial of ABT-263 in patients with relapsed CLL70 showed responses characterized by decreasing lymphocytosis in 7 patients and reduction in lymphadenopathy in 3.
Obatoclax showed some minimal clinical activity in phase 1 trials in patients with relapsed CLL. A phase 1 trial of obatoclax with escalating doses of bortezomib was performed in patients with relapsed MCL.71 Obatoclax was give by intravenous infusion over 3 hours on days 1, 4, 8, and 11 followed by Btz on the same day. Responses were seen in 3 of 9 patients, including one patient previously treated with Btz.
Toxicity of Bcl-2 inhibitors.
Myelosuppression, especially thrombocytopenia, has been seen with most of these agents. Obatoclax causes CNS symptoms of euphoria and somnolence during infusion but no lasting toxicity has been seen.
New agents targeting DNA synthesis
Bendamustine
A bifunctional alkylating agent first developed in East Germany more than 40 years ago, bendamustine has now been studied in North America and has received approval for treatment of CLL and more recently, relapsed indolent NHL not responding or progressing within 6 months after rituximab-based therapy. It is currently approved in Europe for treatment of NHL, Hodgkin lymphoma, CLL, and multiple myeloma.
Mechanism of action.
The chemical structure of bendamustine suggests the possibility of both alkylator-like activity as well as that of purine nucleosides. Furthermore, in vitro and in vivo data show noncross resistance to commonly used alkylators such as cyclophosphamide and chlorambucil. This agent activates p53-dependent stress pathways leading to apoptosis and inhibits mitotic checkpoints. DNA damage is more extensive and it occurs with slower and different DNA repair pathways than other alkylators.72,73
Use in lymphoma.
A phase 2 trial of bendamustine in patients with rituximab-refractory or -intolerant, -indolent, or -transformed lymphoma showed an overall response of 77%.74 Patients received a dose of 120 mg/m2 on days 1 and 2, every 3 weeks and received up to 6 cycles. Responses were observed in alkylator-refractory (61%) and fludarabine refractory (62%) patients, confirming the in vitro data that suggested non-cross resistance. Of the 74 evaluable patients, there were 34% CR/CRu and 43% PR, with a median duration of response 6.6 months. In a second study presented by Kahl et al75 100 patients with rituximab-refractory indolent lymphoma were treated with the same schedule and dose. The OR was 84% with 32% CR/CRu and 52% PR. Again, alkylator-refractory patients responded.
Toxicity of bendamustine.
Grade 3 or 4 neutropenia (54%) and thrombocytopenia (25%) are not unexpected. Nonhematologic toxicity, however, is mild with nausea, fatigue, diarrhea, and vomiting being the most common occurrences.
Pralatrexate
This agent is a new antifolate that more selectively targets the tumor cell than methotrexate. It has shown significant activity in T-cell lymphomas.
Mechanism of action.
Pralatrexate, like other antifolates, interferes with DNA synthesis and cell replication by reversibly inhibiting dihydrofolate reductase, which prevents formation of necessary purine nucleotides. It is cell cycle specific (S phase). The authors of an early study showed pralatrexate to be more effectively internalized in malignant cells than methotrexate as the result of the presence of the reduced folate carrier, which is expressed only in malignant and fetal tissue.76 Once internalized, it is polyglutamylated, resulting in intracellular accumulation. It is less effective as an inhibitor of dihydrofolate reductase than methotrexate, but because of its greater intracellular accumulation, it has more antitumor activity and, theoretically, less toxicity in normal tissue.
Use in lymphoma.
Pralatrexate was studied in a phase 1/2 trial by O'Connor et al77 where 20 patients with relapsed/refractory non-Hodgkin and Hodgkin lymphoma were treated. The MTD was determined to be 30 m/m2 every week for 6 of 7 weeks. Of 4 patients with T-cell disease, all achieved a CR. There was stable disease at best in patients with B-cell disease.
Toxicity of pralatrexate.
The results of a phase 1 study demonstrated mucositis to be the dose-limiting toxicity and occurs in 21% to 59% of patients, even in lower dose schedules. This seems to be more common in NHL than in lung cancer patients. Thrombocytopenia is seen in 33%, whereas anemia and neutropenia are less common (12% and 11%, respectively).
The use of new drugs in rational combinations
As outlined previously in this review, multiple new agents targeting various pathways important for malignant cell growth have shown clinical activity in lymphoma as single agents. Unfortunately, in these studies a minority of patients responded, and the duration of benefit was short lived. Clearly, combining these agents with other effective therapy may enhance the combination resulting in greater benefit for lymphoma patients.
Combining new agents with rituximab
The anti-CD20 monoclonal antibody rituximab has become a standard of care as a single agent in indolent B-cell lymphoma patients with a relatively low burden of disease. Rituximab in this setting is associated with overall response rates of 50%-80% and durations of response of 18-28 months. Although effective, rituximab therapy does not result in a high rate of CRs, and patients eventually relapse. Adding new agents with potential promise to rituximab is a reasonable approach, especially given its low toxicity as a single agent. Bendamustine has been combined with rituximab for relapsed indolent lymphoma, and the authors of 2 published trials show high overall response rates of 90%-92% with a high CR rate of 41%-60%.78,79 Bendamustine has also been used as upfront therapy in patients with indolent, follicular, and MCL. Rummel et al80 demonstrated that this combination provided results similar to R-CHOP (ie, CHOP therapy with rituximab) in this randomized study, with ORR 94%, CR 41%, and with less toxicity. Of note, the doses of bendamustine used in combination are 90 mg/m2 rather than the initial 120 mg/m2. Similarly, lenalidomide has been added to rituximab and in a study of 30 patients with low-burden indolent lymphoma, an ORR of 86% was seen with 79% CRs.81 This result supports the hypothesis that IMiDs enhance antibody-dependent cell-mediated cytotoxicity. Dd has been combined with rituximab82 in patients with relapsed B-cell NHL. Eighty percent of these 38 patients were refractory to rituximab, and still the OR was 32%. Combining mTOR inhibitors such as temsirolimus with rituximab also appears promising. Ansell et al83 showed that temsirolimus could be safely combined with rituximab and in a group of relapsed MCL patients, an ORR of 48% (CR 20%, PR 28%) was seen. Future studies of novel agents in combination with rituximab will need to show not only improvements in efficacy over rituximab alone but with minimal additional toxicity. Furthermore, assessing the true benefits of these combinations will be proven only in randomized studies.
The addition of new agents to established lymphoma regimens
In the past decade, the addition of rituximab to combination chemotherapy has improved response rates, time to progression, and OS in B-cell NHL. Despite this progress, many patients still relapse, and outcomes with this chemoimmunotherapy approach may be improved if new agents are added to these combinations. Proteasome inhibition can be safely added to alkylator-based therapies and several combinations have encouraging results. Combinations include Btz + R-CVP,84 Btz + R-CHOP,85 Btz + fludarabine and rituximab,86 Btz + cyclophosphamide, dexamethasone, and rituximab (CyBorD-R; see http://clinicaltrials.gov/ct2/show/NCT00711828), Btz + Hyper-CVAD,87 and Btz + rituximab and bendamustine.88,89 Given the single-agent activity of lenalidomide in both indolent and aggressive NHL, current trials are testing the benefit of adding it to established regimens. At our institution, we are completing trials with lenalidomide added to R-CHOP (R2-CHOP) for newly diagnosed aggressive lymphoma and to cyclophosphamide, rituximab, and dexamethasone (ie, LR-CD) for low-grade lymphoma, including Waldenström macroglobulinemia. Multi-institutional studies are testing maintenance lenalidomide after R-CHOP therapy.
The use of combinations of new agents
Because many of the new agents selectively inhibit specific cell signaling pathways, combining agents that inhibit different mechanisms of cell growth and survival is particularly attractive. Our institution is currently piloting a combination of lenalidomide and the mTOR inhibitor RAD001 in a phase 1 trial, postulating that the different mechanisms of activity will be complementary.
Another rational approach would be inhibiting Ras/Raf/MEK and mTOR pathways: sorafenib is being tested in combination with the mTOR inhibitor everolimus, and in an initial study of 26 patients, an ORR of 33% was seen with 2 CRs and 5 PRs (S. Kumar, L. F. Porrata, S. M. A., manuscript submitted, 2010). Because sorafenib is nonmyelosppressive, it could be combined with more standard, often myelosuppressive regimens, as well as lenalidomide, a Btk inhibitor, or Bcl-2inhibitor.
Patients who do not respond to mTOR inhibitors often have up-regulation of AKT; therefore, the combination of RAD001 or temsirolimus plus perifosine would be of interest. Because we are aware that the HDACi LBH589 also inhibits AKT, we are studying the combination of RAD001 plus panobinostat (LBH589) in a phase 1 trial for NHL. The addition of an HDACi to Btz is another rational combination as has been explored in patients with relapsed refractory multiple myeloma.
Summary
We are optimistic that many of these novel agents may play a role in the future management of B-cell lymphoma. Indeed with as many as 8-9 new agents showing hints or clear evidence of activity in lymphoma it is highly likely that modern paradigms will evolve rapidly in the next few years. The optimal combinations of these drugs with existing agents and the most efficacious timing of use may be best directed when an individual's lymphoma targets are identified by gene profiling and immunohistochemistry. As rational combinations are developed, we must keep in mind that many of the novel agents have “off-target” activity and may in fact act by multiple (and as yet unknown) mechanisms. We also need to be conscious of potential toxicities and be certain these combinations are safe.
In the current management of multiple myeloma, novel agents have extended the survival from a dismal 3 years to well over 5 years, and importantly, many of those agents appear to be active in malignant lymphoma. The novel agents hold great promise for improving the outcomes of treatment and perhaps achieving the ultimate goal of curing malignant lymphoma.
Acknowledgment
We thank Dr A. Keith Stewart for his encouragement to write this article.
Authorship
Contribution: C.B.R. and S.M.A. wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Craig B. Reeder, MD, Division of Hematology and Oncology, Mayo Clinic Arizona, 13400 E Shea Blvd, Scottsdale, AZ 85259; e-mail: reeder.craig@mayo.edu.