Abstract
B-cell migration into and within lymphoid tissues is not only central to the humoral immune response but also for the development of malignancies and autoimmunity. We previously demonstrated that SWAP-70, an F-actin-binding, Rho GTPase-interacting protein strongly expressed in activated B cells, is necessary for normal B-cell migration in vivo. SWAP-70 regulates integrin-mediated adhesion and cell attachment. Here we show that upon B-cell activation, SWAP-70 is extensively posttranslationally modified and becomes tyrosine phosphorylated by SYK at position 517. This phosphorylation inhibits binding of SWAP-70 to F-actin. Phospho-site mutants of SWAP-70 disrupt B-cell polarization in a dominant-negative fashion in vitro and impair migration in vivo. After CXCL12 stimulation of B cells SYK becomes activated and SWAP-70 is phosphorylated in a SYK-dependent manner. Use of the highly specific SYK inhibitor BAY61-3606 showed SYK activity is necessary for normal chemotaxis and B-cell polarization in vitro and for entry of B cells into lymph nodes in vivo. These findings demonstrate a novel requirement for SYK in migration and polarization of naive recirculating B cells and show that SWAP-70 is an important target of SYK in this pathway.
Introduction
Migration of B cells into secondary lymphoid organs is required for an antigen-specific humoral immune response. B cells migrate from the bloodstream to lymph nodes by extravasation from high endothelial venules. The response to chemokines and integrin-mediated adhesion to endothelial cells is a key step in this process.1 B cells in secondary lymphoid organs are critical to homeostatic and inflammatory regulation of these tissues. If the lymph node becomes inflamed, B-cell migration is a prerequisite for normal hypertrophy and the further recruitment of dendritic and T cells.2 B-cell trafficking within lymph nodes is also important during the generation of the germinal center reaction and humoral immune responses.3-6 Abnormal chemoattractant-mediated B-cell migration is central to infiltration of malignant B cells into tissues and formation of ectopic germinal centers in autoimmune diseases.7-9
The transduction of signals from cell surface receptors to the actin cytoskeleton to regulate cell adhesion and migration requires small G proteins of the Ras superfamily.10-12 These include members of the Ras family, including Ras and Rap1, and members of the Rho family, such as Rac, Cdc42, and Rho.13 Upstream activators of Rho proteins such as the Dbl homology domain proteins of the Vav family are required for β-integrin and chemokine-mediated Rac, Cdc42, and Rho activation and stable adhesions of cells.14
Although B-cell migration is vital for their development, the pathways underlying integrin and chemokine signals in B cells are poorly defined. We identified the signaling protein SWAP-7015 as required for B-cell migration and adhesion in vitro and in vivo.16 Swap70−/− B cells show aberrant integrin-mediated adhesion, defective polarization, and do not form uropods and stabilized lamellipodia in vitro. This leads to a defect in migration and homing of B cells to lymph nodes in vivo. In correlation with these defects in migration and adhesion, Swap70−/− transitional B cells hyperadhere to the splenic red pulp and are impaired in differentiation into marginal zone B-cells.17 In addition, Swap70−/− B cells do not generate a normal germinal center response,18 a process requiring high B-cell motility and a dynamic morphology,6 which are most likely disturbed by the absence of SWAP-70.
SWAP-70 interacts with and regulates proteins of the Rho family, in particular Rac and RhoA.19,20 Unusually, SWAP-70 has a Dbl homology domain to the C-terminus of its pleckstrin homology (PH) domain.20 The PH domain binds PIP3 and SWAP-70 also binds F- but not G-actin.20,21 The binding of SWAP-70 to F-actin depends on cell stimulation and is required for regulation of cytoskeletal rearrangements such as membrane ruffles.21
Although its regulation of Rac and actin polymerization are likely to be important for SWAP-70's function in regulating B-cell migration, little is known about how SWAP-70 itself is regulated, other than its interaction with PIP3, which is required for its localization.22 Therefore, we asked whether and how SWAP-70 is posttranslationally modified, and, if phosphorylated, which kinase might be responsible. Upon identifying SYK as a kinase that phosphorylates SWAP-70, the requirement for SYK in regulating B-cell migration was determined.
Methods
For further description of materials and methods, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All experiments were ethically approved by the State of Saxony. Mice were bred in the animal facility of the Medical School, Dresden University of Technology, according to state regulations.
Purification of SYK
The kinase that phosphorylates SWAP-70 was purified from cytoplasmic extracts of lipopolysaccharide (LPS)-activated splenic B cells. The extracts were prepared by Dounce homogenization of 108 to 109 cells in a hypotonic buffer (20mM Tris-HCl, pH 7.4 at 4°C, 1mM dithiothreitol [DTT], 1mM ethylene diamine tetraacetic acid [EDTA], proteinase inhibitor cocktail); the yield was 5-40 mg of protein in 10-80 mL (Fraction I). Extracts were precleared from nucleic acids by running through a 10-mL diethylaminoethyl cellulose-Sepharose column at 500mM NaCl, where nucleic acids but few proteins bind. The flow-through (Fraction II, 95% of input protein) was diluted 1:10 with buffer A (10mM 3-[4-{2-hydroxyethyl}-1-piperazinyl] propanesulfonic acid, pH 7.8, 1mM DTT, 5% glycerol, 0.1mM EDTA, 0.02mM Na-vanadate, proteinase inhibitors) and fractionated by chromatography on 2 ion-exchange columns (10 mL Macro S, a macroporous strongly acidic cation exchange resin, yielding Fraction III, and 2 mL Macro Q, a macroporous strongly basic anion exchange resin, yielding Fraction IV, both resins from BioRad Inc.) and a 1-mL heparin Sepharose column (HiTrap; Amersham/Pharmacia), which served as a mixed affinity/weak cation exchange resin (yielding Fraction V).
Protein fractions were assayed for kinase activity by incubation for 20 minutes at room temperature with 0.5 μg of SWAP-70 in the presence of 10mM MgCl2, 2mM MnCl2, 0.1 μCi γ-32P-ATP (3000 Ci/mmol), 10mM ATP, 20mM EPPS (pH 7.0), 1mM DTT, and 20mM NaCl. Conditions were optimized at each step of purification. After incubation, the reaction mixture was loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide protein gels; after electrophoresis, the gels were stained with Coomassie blue dried to visualize the SWAP-70 protein besides marker proteins and exposed to autoradiography films or in a phospho imager. Protein fractions that transferred 32P to SWAP-70 were thus identified for further purification. Fraction V contained approximately 10 polypeptides, as seen on silver-stained gels. Mass spectrometric identification of these peptides revealed SYK as the only kinase in this preparation.
2D and 1D Western blotting
For 2D Western blots cytoplasmic extracts were prepared from stimulated and unstimulated B cells by Dounce homogenization. The buffer was then exchanged by methanol/chloroform precipitation and resuspension in urea lysis buffer (7M urea, 2M thiourea, 4% CHAPS, 30mM Tris HCl, pH 9.1-9.3). The protein sample was then loaded onto an IPG strip (pH 4-7; GE Healthcare) then rehydrated for 30 minutes before isoelectric focusing on an Ettan IPGphor 3 isoelectric focusing system (GE Healthcare). Focused strips were then run on a polyacrylamide gel and underwent Western blotting with rabbit anti–SWAP-70. For anti-SYK Phospho Y348 (BD Biosciences) analysis RIPA, extracts of stimulated and unstimulated cells were prepared, and then they underwent Western blotting before we stripped the membrane and reprobed with anti SYK (N19; Santa Cruz Biotechnology). For Western blotting of tyrosine phosphorylation, we used a mouse monoclonal antiphospho tyrosine (4G10; ATCC).
In vitro chemotaxis assays
Chemotaxis of B cells to CXCL12 (R&D Systems) was performed in Transwell plates with a 5-μm pore size (Corning) as described23 in the presence of increasing concentrations of the SYK inhibitor BAY61-3606 or mock treated with carrier (dimethyl sulfoxide; by use of the carrier dilution corresponding to that found at the highest concentration of BAY61-3606). Haptotactic migration assays were carried out by coating the Transwell membranes with VCAM-1 via incubation overnight in a 5 μg/mL solution of recombinant vascular cell adhesion molecule 1 (VCAM-1; R&D Systems) at 4°C. Membranes were then rinsed and used as for Transwell assays.23
Fractionation of cytoskeletal and soluble proteins
Cell extracts were made by lysis of cells in a buffer containing 1% Triton ×-100, 1mM ATP, 1M ethylene glycol tetraacetic acid, 2.5mM MgCl2, 250mM NaCl, and 250mM Tris, pH 7.4. Lysates were then cleared by low-speed centrifugation in a benchtop Eppendorf centrifuge at 16 100g for 30 minutes. A sample of the total extract was kept for later analysis. The resulting supernatant was then placed in a bench top ultracentrifuge (Beckman TL-100) and spun at 40 000g for 2 hours. The resulting pellets and supernatants were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
F-actin binding assay
Human platelet actin (Cytoskeleton) was polymerized in 5mM Tris (pH 8), 50mM KCl, 2mM MgCl2, 1mM ATP, and 0.2mM DTT at room temperature for 30 minutes and incubated with His-SWAP-70 C-terminus for 30 minutes at room temperature. After ultracentrifugation at 109 000g for 30 minutes at 25°C, the proteins in the pellet were analyzed by SDS-PAGE and Coomassie blue staining. Proteins in the supernatant were precipitated with the use of ice-cold acetone before analysis.
Competitive migration assays
Purified wild-type (Wt) B cells were labeled with cell tracker green or cell tracker red (Invitrogen). Either green or red cells were treated with BAY61-3606 and the other color mock treated with dimethyl sulfoxide alone. Equal numbers of each color lymphocytes (1 × 107 of each) were injected into the tail veins of Wt 129Sv mice. After 30 minutes or 1 hour, lymph nodes, spleen, and blood were collected, homogenized, and the number of fluorescent cells present was ascertained by fluorescence-activated cell sorting (FACS). The fluorescent dyes used on the cells were reversed with the same results. T-cell analysis was performed by taking the cells remaining after negative selection of splenic B cells and treating them as previously described. The migrated Wt CD4+ T cells were identified by staining samples with anti-CD4 (BD Biosciences) before FACS analysis of the CD4+ cells in the samples.
To determine whether infection with a SWAP-70Y517F expressing retrovirus impairs migration in vivo, Wt splenocytes were infected with retrovirus expressing either SWAP-70, SWAP-70Y517F, or green fluorescent protein (GFP). Seventy-two hours after infection the cells infected with either SWAP-70 expression vector were labeled with cell tracker red, and GFP-infected cells were labeled with cell tracker green. Infection efficiency was determined by FACS analysis. The SWAP-70– or SWAP-70Y517F–expressing cells were then mixed with GFP-expressing cells and coinjected via the tail vein into Wt mice. Lymph nodes and blood were then collected after 2 hours, cell suspensions were prepared, and B220+ cells were stained with anti-B220. The input cell mixtures were also stained with anti-B220. The numbers of red and green B220+ cells in blood, lymph nodes, and input were determined by FACS analysis. The ratio of red to green B220+ cells in the input was then determined and adjusted to 1, and the same factor was used to adjust the ratio in blood and lymph nodes.
Results
SWAP-70 is posttranslationally modified by tyrosine phosphorylation
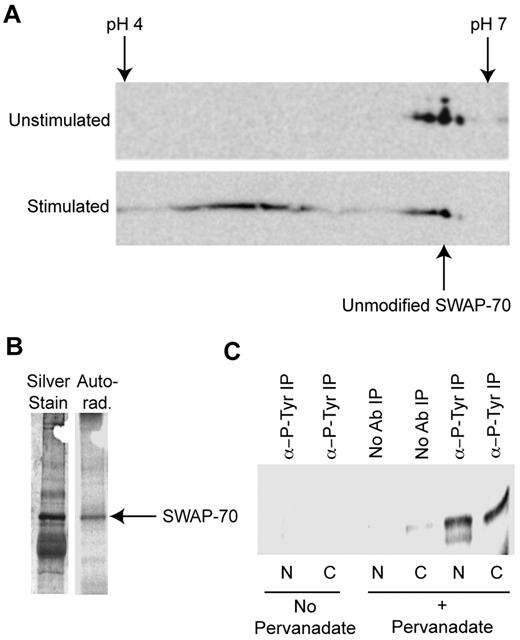
To investigate whether SWAP-70 is posttranslationally modified in B cells, cytoplasmic extracts from LPS-stimulated and -unstimulated cells were analyzed by 2D SDS-PAGE. Western blotting of the resulting gel with anti–SWAP-70 revealed only 3 modified forms in unstimulated cells, with the majority of SWAP-70 having a pI corresponding to the unmodified protein (as indicated in Figure 1A). After stimulation little SWAP-70 remains in the unmodified form, whereas multiple newly modified forms appear with a more acidic pI.
SWAP-70 is modified by tyrosine phosphorylation. (A) SWAP-70 is posttranslationally modified after LPS stimulation. Cytoplasmic extracts from stimulated and unstimulated B cells were analyzed by 2D Western blotting with anti–SWAP-70. Isoelectric focusing was performed between pH 4 and 7. The unmodified form of SWAP-70 has an approximate pI of 5.85 and is indicated, after activation several modified forms of SWAP-70 appear. (B) SWAP-70 is phosphorylated. LPS-stimulated B cells were grown in the presence of γ-32P-ATP and then SWAP-70 underwent IP from cytoplasmic extracts. The presence of SWAP-70 in precipitates was confirmed by SDS-PAGE of the precipitate and subsequent silver staining. On the silver stain, a band at 70 kDa corresponding to SWAP-70 can be seen, a corresponding band is found in the autoradiograph. (C) Stimulation of B cells leads to tyrosine phosphorylation of SWAP-70. IPs were made from nuclear (N) and cytoplasmic (C) extracts of stimulated and unstimulated cells with antiphospho-Tyr (α-P-Tyr). The resulting precipitates underwent IB with anti–SWAP-70 to determine in which samples SWAP-70 is Tyr phosphorylated. SWAP-70 was precipitated with antiphospho-Tyr from stimulated cell nuclear and cytoplasmic extracts but not unstimulated cells. Shown are representative images from experiments performed at least 3 times.
SWAP-70 is modified by tyrosine phosphorylation. (A) SWAP-70 is posttranslationally modified after LPS stimulation. Cytoplasmic extracts from stimulated and unstimulated B cells were analyzed by 2D Western blotting with anti–SWAP-70. Isoelectric focusing was performed between pH 4 and 7. The unmodified form of SWAP-70 has an approximate pI of 5.85 and is indicated, after activation several modified forms of SWAP-70 appear. (B) SWAP-70 is phosphorylated. LPS-stimulated B cells were grown in the presence of γ-32P-ATP and then SWAP-70 underwent IP from cytoplasmic extracts. The presence of SWAP-70 in precipitates was confirmed by SDS-PAGE of the precipitate and subsequent silver staining. On the silver stain, a band at 70 kDa corresponding to SWAP-70 can be seen, a corresponding band is found in the autoradiograph. (C) Stimulation of B cells leads to tyrosine phosphorylation of SWAP-70. IPs were made from nuclear (N) and cytoplasmic (C) extracts of stimulated and unstimulated cells with antiphospho-Tyr (α-P-Tyr). The resulting precipitates underwent IB with anti–SWAP-70 to determine in which samples SWAP-70 is Tyr phosphorylated. SWAP-70 was precipitated with antiphospho-Tyr from stimulated cell nuclear and cytoplasmic extracts but not unstimulated cells. Shown are representative images from experiments performed at least 3 times.
A change to an acidic pI might represent phosphorylation. To discover whether SWAP-70 is phosphorylated, B cells were cultured in the presence of 32P and stimulated with LPS. SWAP-70 then underwent immunoprecipitation (IP) from cytoplasmic extracts. SDS-PAGE, followed by silver staining, revealed a distinct band at 70 kDa that corresponded to SWAP-70, and autoradiography revealed SWAP-70 was phosphorylated (Figure 1B).
To determine whether SWAP-70 was tyrosine phosphorylated, phospho-tyrosine–containing proteins underwent IP from cytoplasmic or nuclear extracts of stimulated B cells with antiphospho-tyrosine. Tyrosine-phosphorylated SWAP-70 was found in B cells stimulated and treated with the phospho-tyrosine phosphatase inhibitor sodium pervanadate (Figure 1C). IP with anti–SWAP-70 followed by immunoblotting (IB) with antiphospho-tyrosine (as in Figure 6B) confirmed these results.
SYK phosphorylates SWAP-70
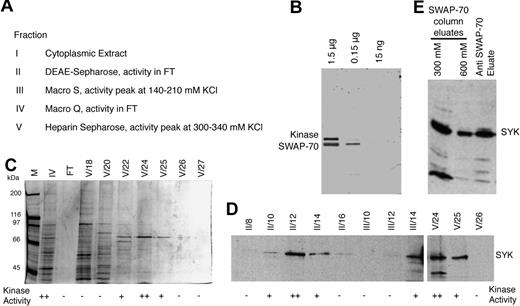
To identify a kinase that phosphorylates SWAP-70, we performed kinase assays by using cytoplasmic extracts from LPS-stimulated splenic B cells, incubated with purified recombinant SWAP-70 and γ-32P-ATP as described in “Purification of SYK.” Phosphorylation of SWAP-70 was assessed by autoradiography of SDS-PAGE protein gels. The cytoplasmic extract (Fraction I; depending on the starting number of cells, between 108 to 109 cells, the extract contained from 5 to 40 mg of protein) was fractionated by chromatography, and the peak kinase activity at each step was further purified as indicated in Figure 2A. Nucleic acids were removed by passing the extract through DEAE-Sepharose at 500mM NaCl, yielding Fraction II (ca. 95% of starting amount of protein, ie, 4.5 mg from 108 cells). After a 10-fold dilution with buffer A, Fraction II was loaded at 50mM NaCl onto a 10-mL Macro S column and proteins were eluted with a 50-600mM KCl gradient. Kinase activity eluted between 140 and 210 mM KCl. The fractions containing the peak activity were found from 150 to 200 mM KCl, they were pooled (Fraction III; 0.3 mg of protein from 108 cells), and further purified. Upon incubation with SWAP-70, Fraction III yielded 2 32P-labeled signals in autoradiography of SDS protein gels, representing SWAP-70 and the auto-phosphorylated kinase (Figure 2B). This kinase migrates at approximately 72 kDa, which consistent with it being SYK. Fraction III was diluted 1:4 with buffer A to ca. 45mM KCl and loaded onto a 2-mL Macro Q column. The kinase activity was found in the flow through; this was collected (Fraction IV, 0.07 mg of protein from 108 cells) and loaded onto a 1-mL heparin-Sepharose column. Proteins were eluted by a linear gradient from 50 to 600mM KCl, and kinase activity eluted between 270 and 360mM KCl, with a peak at 300-340mM KCl. This peak fraction was pooled (Fraction V, ca. 5-μg protein from 108 cells). Silver staining of gels containing Fractions IV and V showed approximately 10 polypeptides in the active fractions of Fraction V (fractions no. V/22-V/25), including the most prominent protein of approximately 72 kDa (Figure 2C).
SWAP-70 is phosphorylated by Syk. (A) SWAP-70 kinase purification scheme with the resulting fractions and the respective active peak fractions indicated; FT indicates flow through. (B) Kinase reactions performed with 3 different amounts of recombinant SWAP-70 as indicated, and Fraction V of the kinase preparation. Autoradiograph of the gel showing phosphorylated SWAP-70 (at ca. 70 kDa) and autophosphorylated kinase (at ca. 75 kDa). (C) Silver-stained SDS-polyacryl amide gel showing the pooled activity peak fraction IV (IV), and subsequent fractions as eluted from the heparin Sepharose column (Fraction V, No. 18 to 27), and the heparin Sepharose flow-through (FT), besides a mass marker (M). Kinase activity of the individual fractions is indicated below. (D) IB of consecutive fractions from steps II, III, and V from the kinase purification. Kinase activity of the individual fractions is indicated below. The blot was probed with anti-SYK antibody, and the SYK protein signal is indicated. (E) Anti-SYK IB of an eluate from anti–SWAP-70 IP (right lane) and of 2 high-salt eluates (300 and 600mM ammonium sulfate) obtained from a SWAP-70 affinity column, onto which cytoplasmic extract from activated B cells was loaded. The specific SYK protein signal is indicated.
SWAP-70 is phosphorylated by Syk. (A) SWAP-70 kinase purification scheme with the resulting fractions and the respective active peak fractions indicated; FT indicates flow through. (B) Kinase reactions performed with 3 different amounts of recombinant SWAP-70 as indicated, and Fraction V of the kinase preparation. Autoradiograph of the gel showing phosphorylated SWAP-70 (at ca. 70 kDa) and autophosphorylated kinase (at ca. 75 kDa). (C) Silver-stained SDS-polyacryl amide gel showing the pooled activity peak fraction IV (IV), and subsequent fractions as eluted from the heparin Sepharose column (Fraction V, No. 18 to 27), and the heparin Sepharose flow-through (FT), besides a mass marker (M). Kinase activity of the individual fractions is indicated below. (D) IB of consecutive fractions from steps II, III, and V from the kinase purification. Kinase activity of the individual fractions is indicated below. The blot was probed with anti-SYK antibody, and the SYK protein signal is indicated. (E) Anti-SYK IB of an eluate from anti–SWAP-70 IP (right lane) and of 2 high-salt eluates (300 and 600mM ammonium sulfate) obtained from a SWAP-70 affinity column, onto which cytoplasmic extract from activated B cells was loaded. The specific SYK protein signal is indicated.
Fractions from different stages of purification underwent IB, and we found that only the fractions with kinase activity contained SYK (Figure 2D); neighboring kinase-inactive fractions lacked this protein. By the use of mass spectrometric analysis of the active fraction V we identified SYK as the only kinase present in that fraction. Thus, we conclude SYK is a kinase that phosphorylates SWAP-70 in vitro.
Anti–SWAP-70 IPs from cytoplasmic extracts of activated B cells were found by IB to contain SYK, which is indicative of interaction between the 2 proteins. In a separate approach, SWAP-70 protein affinity columns were loaded with cytoplasmic extracts from activated B cells and eluted with increasing salt. SYK was found to elute at the 2 greatest salt concentrations (Figure 2E). These data suggest association of SWAP-70 with SYK.
SYK phosphorylates the C-terminus of SWAP-70
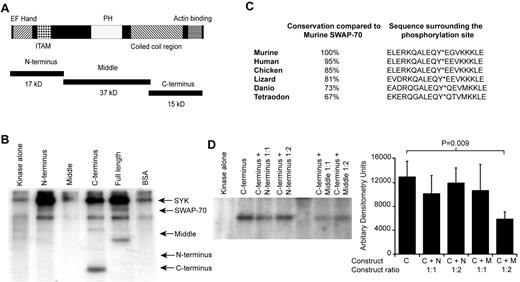
To identify the SWAP-70 residue phosphorylated by SYK recombinant SWAP-70 fragments were generated corresponding to the N-terminus, middle, and C-terminus (Figure 3A). These fragments were incubated with a recombinant SYK-Ig heavy chain fusion protein in the presence of γ-32P-ATP. After autoradiography only the C-terminus of SWAP-70 was found to be phosphorylated (Figure 3B), the only tyrosine in this region is at position 517 (of the full-length protein). Thus, SWAP-70 is phosphorylated by SYK at residue 517.
Phosphorylation by SYK occurs on tyrosine 517 of SWAP-70. (A) Schematic representation of SWAP-70 showing its known domains and the bacterial expression constructs used to identify the phosphorylation site. (B) SWAP-70 is phosphorylated at its C-terminal. Different domains of SWAP-70, the full-length protein, or BSA were incubated with a recombinant SYK-IgH fusion protein in the presence of γ-32P-ATP. After SDS-PAGE the phosphorylation was detected by autoradiography. Arrows indicate the position of the different constructs, the full-length protein, and BSA. Only the C-terminal of SWAP-70 and the full-length protein are phosphorylated. Figure is representative of 5 independent experiments. (C) Comparison of the murine Swap-70 with other orthologues showing the overall conservation of the phosphorylation site and surrounding sequence. (D) Phoshorylation of the C-terminal is inhibited by the presence of the middle construct. Kinase reactions involving the C-terminus of SWAP-70 in the presence and absence of the N-terminal or middle-region proteins were analyzed by SDS-PAGE followed by autoradiography. The image shows the phosphorylated C-terminus. The accompanying bar graph shows the densitometric analysis of the C-terminus band with SD from 4 experiments. Only the 1:2 mixture of C-terminus with the middle of SWAP-70 showed significant inhibition of phosphorylation as indicated.
Phosphorylation by SYK occurs on tyrosine 517 of SWAP-70. (A) Schematic representation of SWAP-70 showing its known domains and the bacterial expression constructs used to identify the phosphorylation site. (B) SWAP-70 is phosphorylated at its C-terminal. Different domains of SWAP-70, the full-length protein, or BSA were incubated with a recombinant SYK-IgH fusion protein in the presence of γ-32P-ATP. After SDS-PAGE the phosphorylation was detected by autoradiography. Arrows indicate the position of the different constructs, the full-length protein, and BSA. Only the C-terminal of SWAP-70 and the full-length protein are phosphorylated. Figure is representative of 5 independent experiments. (C) Comparison of the murine Swap-70 with other orthologues showing the overall conservation of the phosphorylation site and surrounding sequence. (D) Phoshorylation of the C-terminal is inhibited by the presence of the middle construct. Kinase reactions involving the C-terminus of SWAP-70 in the presence and absence of the N-terminal or middle-region proteins were analyzed by SDS-PAGE followed by autoradiography. The image shows the phosphorylated C-terminus. The accompanying bar graph shows the densitometric analysis of the C-terminus band with SD from 4 experiments. Only the 1:2 mixture of C-terminus with the middle of SWAP-70 showed significant inhibition of phosphorylation as indicated.
The Mus musculus Swap-70 gene product was used for all biochemical analysis. If this sequence is compared with other Swap70 orthologues from both closely related species (eg, Homo sapiens, 95% protein sequence identity) or more distantly related genes (eg, Tetraodon nigroviridis, 67% protein sequence identity), the tyrosine residue is 100% conserved (Figure 3C), the surrounding protein sequence is also highly conserved. In addition, SYK orthologues are found in all species were SWAP-70 is seen.
It was noted that although SYK does not phosphorylate the N-terminus of SWAP-70, the N-terminus causes a strong increase in SYK autophosphorylation (Figure 3B). In addition, the full-length protein caused increased autophosphorylation of recombinant SYK (Figure 3B) and SYK from the purified kinase fraction (Figure 2B). The middle of SWAP-70 had no, or a slightly inhibitory, effect and the C-terminus caused an increase compared with a nonspecific protein such as bovine serum albumin (BSA; Figure 3B).
The phosphorylation of full-length SWAP-70 is less efficient than that of the isolated C-terminus (Figure 3B). This could be attributable to the tertiary structure of the protein hindering the interaction between SWAP-70 and SYK. Some other proteins phosphorylated by SYK such as Vav have their phosphorylation inhibited by their PH domain.24 To determine whether the SWAP-70 PH domain might impair phosphorylation of the C-terminus, the C-terminus was incubated with SYK in presence or absence of the PH containing middle region protein (Figure 3D). A 2-fold excess of the middle region significantly decreased C-terminus phosphorylation by 45% ± 8.1%, and the N-terminus had no significant effect. Hence, in full-length SWAP-70 phosphorylation of the C-terminus may be reduced by intra-molecular interactions with the central region of SWAP-70.
SWAP-70 phosphorylation regulates its binding to F-actin
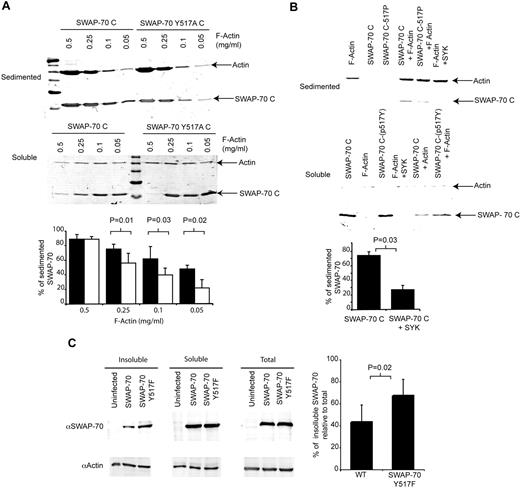
The conservation of tyrosine 517 and its surrounding sequence suggests its phosphorylation has an important function. The C-terminus of SWAP-70 is important for F-actin binding, as removal of the 12 C-terminal amino acids (from 573 to 585) destroys the F-actin binding domain.21 It is unknown how far toward the N-terminus the F-actin binding domain extends. The tyrosine at position 517 might be sufficiently close to influence F-actin binding. To test this, the residue was mutated from tyrosine to alanine and the resulting F-actin binding activity of the mutant C-terminus (SWAP-70C Y517A) tested. After centrifugation F-actin forms a pellet and any bound SWAP-70 C-terminus cosediments. At high F-actin (0.5 mg/mL) concentrations, the majority (> 85% of input) of SWAP-70C is in the pellet for both the wt and mutant SWAP-70C. As the amount of F-actin decreases less SWAP-70C sediments, yet a significant proportion (48% ± 5.1%) still sediments at concentrations of F-actin as low as 0.05 mg/mL (Figure 4A). However, the mutant SWAP-70C Y517A cosedimented in lower amounts, at 0.1 mg/mL F-actin, only 40% ± 9.4% sedimented; by 0.05 mg/mL F-actin, 22% ± 11.3% sedimented (Figure 4A). Conversely, more SWAP-70C Y517A than SWAP-70C remains in the supernatant.
SWAP-70 phosphorylation regulates its binding to F-actin. (A) Tyrosine 517 is important for F-actin binding. Either the Wt C-terminus or Y517A mutant C-terminus were incubated with decreasing amounts of F-actin, then centrifuged such that the F-actin sediments and F-actin binding proteins bound cosediment. The remaining soluble proteins were also collected. The figure shows the Coomassie-stained SDS-PAGE gel of the sedimented and soluble proteins. The bar chart represents average values (± SD of 5 independent experiments) of the percentage of SWAP-70 found in the insoluble fraction as determined by densitometery. (B) Phosphorylation of SWAP-70C reduces its binding to F-actin. To ascertain the effect of SWAP-70 phosphorylation on F-actin binding, the F-actin binding assay was repeated with the use of phosphorylated or nonmodified SWAP-70C. The bar graph shows the percentage of phosphorylated and nonphosphorylated SWAP-70C found in the insoluble fraction as determined by densitometery (n = 4). (C) SWAP-70 Y517F is enriched in an insoluble cytoskeletal fraction in vivo in comparison to Wt SWAP-70. The potential effect of phosphorylation on F-actin binding in vivo was measured by infecting Swap70−/− cells with retroviruses expressing either wt or a SWAP-70 Y517F mutant. After 72 hours, cell extracts were made and the insoluble fraction containing the cytoskeleton was collected by centrifugation. Proteins present in the soluble, insoluble, and total extracts were determined by Western blotting with the antibodies indicated. The ratio of each form of SWAP-70 relative to actin in the insoluble fraction was used to calculate the percentage of insoluble SWAP-70 compared with the same ratio in the unfractionated total preparation and plotted as shown (n = 5).
SWAP-70 phosphorylation regulates its binding to F-actin. (A) Tyrosine 517 is important for F-actin binding. Either the Wt C-terminus or Y517A mutant C-terminus were incubated with decreasing amounts of F-actin, then centrifuged such that the F-actin sediments and F-actin binding proteins bound cosediment. The remaining soluble proteins were also collected. The figure shows the Coomassie-stained SDS-PAGE gel of the sedimented and soluble proteins. The bar chart represents average values (± SD of 5 independent experiments) of the percentage of SWAP-70 found in the insoluble fraction as determined by densitometery. (B) Phosphorylation of SWAP-70C reduces its binding to F-actin. To ascertain the effect of SWAP-70 phosphorylation on F-actin binding, the F-actin binding assay was repeated with the use of phosphorylated or nonmodified SWAP-70C. The bar graph shows the percentage of phosphorylated and nonphosphorylated SWAP-70C found in the insoluble fraction as determined by densitometery (n = 4). (C) SWAP-70 Y517F is enriched in an insoluble cytoskeletal fraction in vivo in comparison to Wt SWAP-70. The potential effect of phosphorylation on F-actin binding in vivo was measured by infecting Swap70−/− cells with retroviruses expressing either wt or a SWAP-70 Y517F mutant. After 72 hours, cell extracts were made and the insoluble fraction containing the cytoskeleton was collected by centrifugation. Proteins present in the soluble, insoluble, and total extracts were determined by Western blotting with the antibodies indicated. The ratio of each form of SWAP-70 relative to actin in the insoluble fraction was used to calculate the percentage of insoluble SWAP-70 compared with the same ratio in the unfractionated total preparation and plotted as shown (n = 5).
Because the mutation of position 517 alters F-actin binding, its phosphorylation might affect the interaction between SWAP-70 and F-actin. To address this, we incubated SWAP-70C protein with recombinant SYK in kinase reaction buffer (to generate phosphorylated SWAP-70 517P) or left it in reaction buffer without SYK. The cosedimentation of both constructs with F-actin (0.25 mg/mL) was analyzed. The phosphorylated SWAP-70C was found at lower levels in the insoluble sedimented fraction (76% with SYK omitted vs 27% with SYK included) and at greater levels in the supernatant in comparison with nonphosphorylated protein (Figure 4B). Thus, phosphorylation of SWAP-70 by SYK reduces its binding to F-actin.
To investigate the effect of SWAP-70 phosphorylation, in vivo splenic cell cultures stimulated with LPS from Swap70−/− mice were infected with retroviruses expressing either GFP alone, GFP and SWAP-70, or GFP and a SWAP-70 Y517F mutant. Proteins associated with the cytoskeleton can be isolated by differential fractionation of the cells. Similar total levels of SWAP-70 and SWAP-70 Y517F proteins were found in infected cells (Figure 4C, right). However, more mutant SWAP-70 Y517F associates with the insoluble cytoskeleton fraction relative to SWAP-70 (Figure 4C), demonstrating that in cells the phosphorylation site at Y517 regulates F-actin binding, presumably through SYK phosphorylation.
Abolition of the phosphorylation site at position 517 impedes the ability of SWAP-70 to regulate B-cell polarization
The interaction of F-actin and SWAP-70 is important for regulation of cytoskeletal rearrangements in vivo21 and consequently can influence cell migration and adhesion. It has been demonstrated that Swap70−/− B cells do not polarize after adhesion on anti-CD44–coated surfaces. This phenotype can be rescued by restoration of SWAP-70 expression.16 On the basis of these observations, it seemed reasonable to assess the polarization of Wt or Swap70−/− B cells expressing SWAP-70 or SWAP-70 Y517F.
Wt B cells infected with an empty GFP-expressing vector or a SWAP-70-IRES-GFP vector polarize normally. Many infected B cells are found with a polarized morphology having a distinct uropod to one side of the cell body and lamellipodia to the other, as highlighted by phallodin staining of the F-actin cytoskeleton (Figure 5A-B). These data indicate infection of Wt cells with a SWAP-70–expressing retrovirus has no effect on cell polarization. In contrast, Swap70−/− B cells infected with empty GFP vector rarely form a polarized morphology (22% ± 3.0% for Wt vs 11% ± 1.5% for Swap70−/−), and very few cells have a uropod. If SWAP-70 is reintroduced into Swap70−/− B cells, they polarize in similar numbers to the Wt cells (26% ± 3.8% for Wt vs 25% ± 3.3% for Swap70−/− +SWAP-70) and can form uropods (Figure 5A-B).
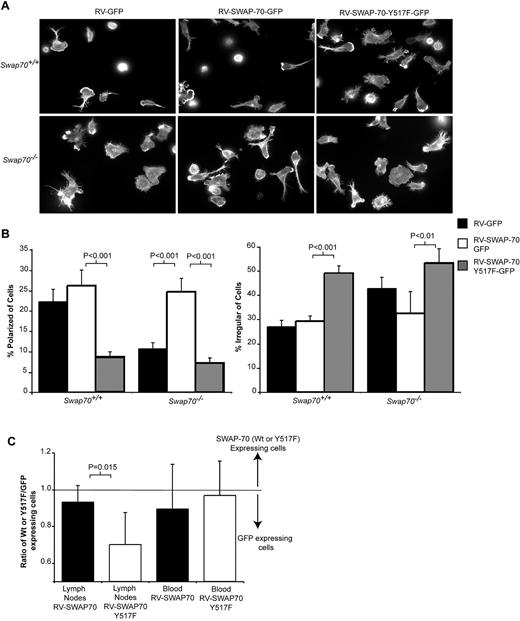
Mutant SWAP-70 Y517F inhibits B-cell polarization in vivo. (A) Wt or Swap-70−/− B cells were infected with retroviruses expressing GFP, SWAP-70, and GFP or SWAP-70, Y517F, and GFP. After 72 hours, the infected GFP-positive cells were sorted by FACS and then allowed to attach to anti-CD44 coated surfaces. The attached cells were stained with rhodamine-phallodin and images collected by fluorescence microscopy as shown. Representative images from one of 3 independent experiments performed in triplicate. (B) The number of cells with a particular morphology was quantified for each of the experiments. Polarized cells were defined as having one dendrite equal in length to the width of the cell body. Irregular cells were those cells that had several protrusions and or dendrites and whose shape was no longer largely round. Bars show mean ± SEM, and significance was calculated by the use of an unpaired t test. (C) Cells infected with either SWAP-70 or SWAP-70 Y517F expressing retrovirus were labeled with cell tracker red and combined with cells infected with GFP-expressing retrovirus labeled with cell tracker green. Two hours after the cells were injected into the tail vein of Wt mice, lymph nodes and blood were collected and the percentage of B cells determined by FACS analysis of anti-B220 stained samples. The ratio of SWAP-70 infected or SWAP-70Y517F infected to control cells infected with GFP retrovirus was calculated and plotted (data from 5 independent experiments). The value 1 represents the input ratio, and numbers lower than 1 show there are more control cells. The P value represents the comparison between the SWAP-70/GFP ratio to the SWAP-70 Y517F/GFP ratio.
Mutant SWAP-70 Y517F inhibits B-cell polarization in vivo. (A) Wt or Swap-70−/− B cells were infected with retroviruses expressing GFP, SWAP-70, and GFP or SWAP-70, Y517F, and GFP. After 72 hours, the infected GFP-positive cells were sorted by FACS and then allowed to attach to anti-CD44 coated surfaces. The attached cells were stained with rhodamine-phallodin and images collected by fluorescence microscopy as shown. Representative images from one of 3 independent experiments performed in triplicate. (B) The number of cells with a particular morphology was quantified for each of the experiments. Polarized cells were defined as having one dendrite equal in length to the width of the cell body. Irregular cells were those cells that had several protrusions and or dendrites and whose shape was no longer largely round. Bars show mean ± SEM, and significance was calculated by the use of an unpaired t test. (C) Cells infected with either SWAP-70 or SWAP-70 Y517F expressing retrovirus were labeled with cell tracker red and combined with cells infected with GFP-expressing retrovirus labeled with cell tracker green. Two hours after the cells were injected into the tail vein of Wt mice, lymph nodes and blood were collected and the percentage of B cells determined by FACS analysis of anti-B220 stained samples. The ratio of SWAP-70 infected or SWAP-70Y517F infected to control cells infected with GFP retrovirus was calculated and plotted (data from 5 independent experiments). The value 1 represents the input ratio, and numbers lower than 1 show there are more control cells. The P value represents the comparison between the SWAP-70/GFP ratio to the SWAP-70 Y517F/GFP ratio.
However, if SWAP-70 Y517F-IRES-GFP is expressed in place of SWAP-70 in Swap70−/− cells, polarization is not rescued (7% ± 1.2% of polarized cells with SWAP-70 Y517F vs 26% ± 3.3% with Wt SWAP-70). Mutant Y517F and WT SWAP-70 proteins are expressed at similar levels in infected Swap70−/− B cells (Figure 4C). In addition, expression of SWAP-70 Y517F in Wt B cells impairs their polarization (9% ± 1.2% of polarized cells with Y517F vs 25% ± 3.8% with Wt SWAP-70; Figure 5A-B). Polarization data were reanalyzed by blinded counting of the samples, and this pattern was reconfirmed (Swap70−/− B cells expressing SWAP-70 Y517F polarized 14% less than those expressing Wt SWAP-70, P = .01: Wt B-cell polarization was inhibited by 20% by SWAP-70 Y517F expression, P = .004). Thus, SWAP-70-Y517F has a dominant-negative effect on Wt-cell polarization and is unable to rescue Swap70−/− B-cell polarization. A similar effect is seen for a SWAP-70 Y517A mutant (supplemental Figure 1).
These data suggest SWAP-70 phosphorylation at Y517 by SYK is critical during B-cell polarization and might be important for regulation of B-cell migration in vivo. To analyze this assertion, we infected Wt splenocytes with retrovirus expressing SWAP-70, SWAP-70Y517F, or GFP only (SWAP-70 and SWAP-70Y517F vectors infected cells equally well [see supplemental Figure 2] and expressed protein to similar levels Figure 4C). The migration of SWAP-70– or SWAP-70 Y517F–expressing cells was measured relative to cells expressing just GFP by coinjecting either differently labeled SWAP-70 and GFP or differently labeled SWAP-70 Y517F and GFP cells. B cells were specifically identified in all samples by staining with anti-B220. The proportions of SWAP-70 or SWAP-70 Y517F relative to control GFP retrovirus-infected cells were similar in blood (Figure 5C). However, although SWAP-70 retrovirus-infected cells migrated into lymph nodes in similar numbers to control cells, significantly fewer SWAP-70 Y517F cells entered relative to control (Figure 5C). Thus, SWAP-70 Y517F has a dominant-negative effect on polarization in vitro and this correlates with significant impairment of migration in vivo.
SYK kinase activity is needed for normal B-cell migration in vitro and in vivo
SYK is an important regulator of integrin-mediated adhesion of neutrophils and is required to establish their leading edge during migration.25,26 In addition, it may play a role in the response of the chicken DT40 pre-B cell line to CXCL12.27 The interaction of SWAP-70 with SYK suggests SYK may regulate migration of mature primary B cells.
To investigate whether SYK is involved in signaling in response to CXCL12, extracts from CXCL12 stimulated B cells were analyzed by Western blotting, with antibodies specific for SYK phosphorylated at residue Y348. Phosphorylation of Y348 is required and diagnostic for SYK activation.28 Figure 6A shows SYK is phosphorylated rapidly after CXCL12 stimulation, reaching significantly greater levels of phosphorylation relative to unstimulated cells by 10 minutes. To confirm that SWAP-70 is phosphorylated in response to CXCL12 in a SYK-dependent manner, SWAP-70 underwent IP from extracts of cells stimulated with CXCL12 in the presence or absence of the specific SYK inhibitor Bay61-3606.29 IB of the resulting precipitates with antiphosphotyrosine demonstrated that SWAP-70 was rapidly tyrosine-phosphorylated (Figure 6B). Phosphorylation was largely dependent on SYK activity, as shown by its reduction through treatment of cells with Bay61-3606 (Figure 6B). Densitometry of the blots confirmed tyrosine phophorylation of SWAP-70 was elevated 2 minutes after stimulation, and this was reduced by treatment with Bay61-3606 (Figure 6B).
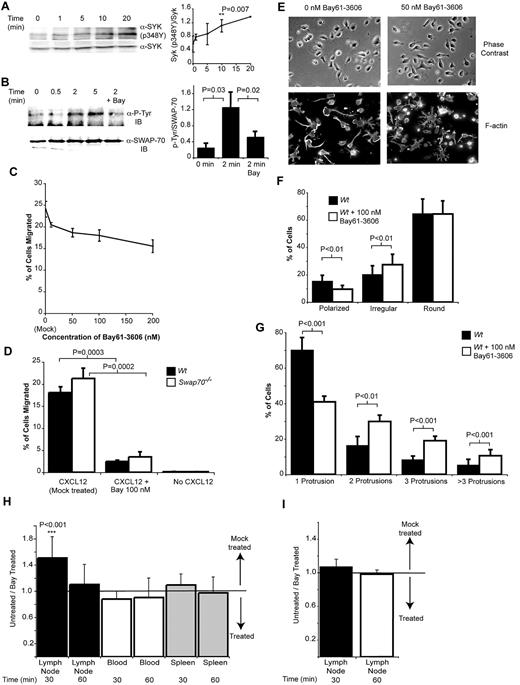
SYK is required for normal chemokine signaling and adhesion in vitro and for B-cell migration in vivo. (A) SYK is activated by CXCL12 stimulation of B cells. B cells were stimulated with CXCL12 for the times indicated, and total cell extracts were analyzed by Western blotting with anti-SYK (pY348P). Equal loading of the different samples was confirmed by reprobing the membranes with an antibody recognizing total SYK. The line graph represents densitometry data from 4 experiments showing the ratio of SYK-pY348 to total SYK. The time point at which the SYK-pY348P ratio is significantly elevated above the ratio seen at the 0 time point is marked. (B) SWAP-70 is phosphorylated after CXCL12 stimulation of B cells in a SYK-dependent manner. SWAP-70 underwent IP from B-cell extracts of cells stimulated with CXCL12, and then tyrosine phosphorylation was analyzed by Western blotting. The dependence of this upon SYK activity was ascertained by treating cells with 100nM of the SYK inhibitor Bay61-3606, which inhibited SWAP-70 phosphorylation. Reprobing the blot with anti–SWAP-70 checked equal loading of SWAP-70. (C) SYK is needed for maximal chemotaxis of B cells toward CXCL12. Transwell migration of B cells toward CXCL12 was measured in the presence of increasing doses of the SYK inhibitor Bay61-3606. The percentage of migrated cells was then analyzed. The chart shows mean data from 3 independent experiments performed in triplicate ± SEM (D) Haptotactic migration of B cells through a VCAM-1–coated membrane requires SYK activity. Migration toward CXCL12 was measured in the presence of Bay61-3606. To determine whether there might be any additive effects between SYK inhibition and SWAP70 deficiency both Wt and SWAP70−/− cells were analyzed. The chart shows mean data from 3 independent experiments performed in triplicate ± SEM (E) SYK is required for normal B-cell polarization on anti-CD44. B cells were allowed to attach to anti-CD44–coated surfaces in the presence or absence of Bay61-3606. Images of cells were then taken under polarized light before the F-actin cytoskeleton was stained by the use of rhodamine-phallodin. (F) Less polarized cells are found in the presence of Bay61-3606. The number of cells with a particular morphology was counted (as before in Figure 5). The percentage of polarized, irregular, and round cells with and without SYK inhibition is shown. The chart shows mean data from 3 independent experiments performed in triplicate ± SEM and significance analyzed by t test. A minimum of 100 cells per replicate was counted. (G) SYK inhibition leads to the development of many long dendritic protrusions from the surface of cells. The number of protrusions in each cell counted in panel F was determined. Then, the percentage of cells with 1, 2, 3, or more than 3 protrusions was calculated and plotted, and significance was analyzed by t test. (H) SYK is needed for normal B-cell homing. Purified B cells were labeled with either Cell Tracker Red or Green, and one set of cells was then treated with 100nM Bay61-3606 and the other mock treated and then injected via the tail vein into mice. After 30 or 60 minutes lymph nodes, spleen, and blood were collected and the number and hence ratio of migrated cells (untreated/treated) present was calculated and plotted. 1 represents the input ratio, and the arrows indicate the direction of relative increase of untreated or treated cell numbers. The data shown are from 6 independent experiments with the colors of treated and untreated cells being reversed 3 times. The significance of the difference from the input ratio of untreated/treated cells (1) is shown. (I) T-cell migration is not affected by treatment with Bay61-3606; this panel is the same as panel H except rather than B cells, the migration of T cells was analyzed.
SYK is required for normal chemokine signaling and adhesion in vitro and for B-cell migration in vivo. (A) SYK is activated by CXCL12 stimulation of B cells. B cells were stimulated with CXCL12 for the times indicated, and total cell extracts were analyzed by Western blotting with anti-SYK (pY348P). Equal loading of the different samples was confirmed by reprobing the membranes with an antibody recognizing total SYK. The line graph represents densitometry data from 4 experiments showing the ratio of SYK-pY348 to total SYK. The time point at which the SYK-pY348P ratio is significantly elevated above the ratio seen at the 0 time point is marked. (B) SWAP-70 is phosphorylated after CXCL12 stimulation of B cells in a SYK-dependent manner. SWAP-70 underwent IP from B-cell extracts of cells stimulated with CXCL12, and then tyrosine phosphorylation was analyzed by Western blotting. The dependence of this upon SYK activity was ascertained by treating cells with 100nM of the SYK inhibitor Bay61-3606, which inhibited SWAP-70 phosphorylation. Reprobing the blot with anti–SWAP-70 checked equal loading of SWAP-70. (C) SYK is needed for maximal chemotaxis of B cells toward CXCL12. Transwell migration of B cells toward CXCL12 was measured in the presence of increasing doses of the SYK inhibitor Bay61-3606. The percentage of migrated cells was then analyzed. The chart shows mean data from 3 independent experiments performed in triplicate ± SEM (D) Haptotactic migration of B cells through a VCAM-1–coated membrane requires SYK activity. Migration toward CXCL12 was measured in the presence of Bay61-3606. To determine whether there might be any additive effects between SYK inhibition and SWAP70 deficiency both Wt and SWAP70−/− cells were analyzed. The chart shows mean data from 3 independent experiments performed in triplicate ± SEM (E) SYK is required for normal B-cell polarization on anti-CD44. B cells were allowed to attach to anti-CD44–coated surfaces in the presence or absence of Bay61-3606. Images of cells were then taken under polarized light before the F-actin cytoskeleton was stained by the use of rhodamine-phallodin. (F) Less polarized cells are found in the presence of Bay61-3606. The number of cells with a particular morphology was counted (as before in Figure 5). The percentage of polarized, irregular, and round cells with and without SYK inhibition is shown. The chart shows mean data from 3 independent experiments performed in triplicate ± SEM and significance analyzed by t test. A minimum of 100 cells per replicate was counted. (G) SYK inhibition leads to the development of many long dendritic protrusions from the surface of cells. The number of protrusions in each cell counted in panel F was determined. Then, the percentage of cells with 1, 2, 3, or more than 3 protrusions was calculated and plotted, and significance was analyzed by t test. (H) SYK is needed for normal B-cell homing. Purified B cells were labeled with either Cell Tracker Red or Green, and one set of cells was then treated with 100nM Bay61-3606 and the other mock treated and then injected via the tail vein into mice. After 30 or 60 minutes lymph nodes, spleen, and blood were collected and the number and hence ratio of migrated cells (untreated/treated) present was calculated and plotted. 1 represents the input ratio, and the arrows indicate the direction of relative increase of untreated or treated cell numbers. The data shown are from 6 independent experiments with the colors of treated and untreated cells being reversed 3 times. The significance of the difference from the input ratio of untreated/treated cells (1) is shown. (I) T-cell migration is not affected by treatment with Bay61-3606; this panel is the same as panel H except rather than B cells, the migration of T cells was analyzed.
To determine whether SYK inhibition of CXCL12 signaling has a biologic effect on B-cell migration, Transwell chemotaxis toward CXCL12 was analyzed in the presence of increasing doses of Bay61-3606. At doses of Bay61-3606 as low as 10nM, some inhibition (20%) of cell migration occurred, and a dose-dependent increase in inhibition was observed, reaching 36% at 200nM in comparison with mock-treated cells (Figure 6C). Inhibition of B-cell receptor signaling, where SYK is essential, by Bay31-3606 has an IC50 of 58nM.29 There was no difference in apoptosis at any of the doses of Bay61-3696 used after 4 hours of treatment (supplemental Figure 3). Thus, SYK is involved in regulating chemotaxis to CXCL12.
Because SYK regulates neutrophil integrin mediated adhesion,30 its inhibition might have a greater effect on haptotactic migration. Thus, Transwell migration to CXCL12 was performed with the use of Transwells precoated with VCAM-1 in the presence or absence of 100nM Bay61-3606. Under these conditions cell migration was inhibited by 86% by Bay61-3606 (Figure 6D). In comparison, when uncoated Transwells were used, migration was inhibited by only 11% by 100nM BAY61-3606, relative to mock-treated cells. This finding suggests SYK activity is required in the response to both integrin signaling and CXCL12 signaling. In addition, no additive effect was seen between the inhibition of SYK and the absence of SWAP-70 (Figure 6D).
Because SWAP-70 phosphorylation by SYK is important for regulating cell polarization after adhesion on anti-CD44–coated surfaces (Figure 5) the effect of SYK inhibition on cell polarization was determined. Inhibition of SYK activity caused a slight but significant decrease in the number of polarized cells (15%-9%); conversely, the number of irregular cells was increased (20%-27%; Figure 6E-F). Blinded reanalysis confirmed this pattern (polarization of B cells is inhibited 3.9% by Bay61-3606 P = .06, and the number of irregular cells increases by 9% P = .03). When the morphology of the irregular cells is examined closely, it can be seen that Bay61-3606–treated cells frequently exhibit multiple protrusions (59% of cells have more than 1 protrusion), whereas untreated cells predominantly have just one obvious protrusion (70% of cells; Figure 6G). This was confirmed by blinded counting, 69.8% of untreated cells have one dendrite in comparison to 38.6% of treated cells P = .005; only 30.2% of cells have more than one dendrite compared with 61.4% of treated cells, P = .005. The irregular nature of the B cells is highlighted when rhodamine-phallodin–stained cells are examined (Figure 6E). Hence, SYK activity is needed for regulation of normal B-cell attachment and morphology in vitro.
Because SYK activity controls in vitro processes involved in many stages of cell migration in vivo, its in vivo role in cell migration was determined. Purified B cells labeled with either cell tracker green or red, green cells were treated with 100nM Bay61-3606, red cells were mock treated, washed, mixed 1:1 with the red cells, and injected intravenously into mice. After 30 or 60 minutes, lymph nodes (inguinal and axial), spleen, and blood were collected and the number and hence ratio of green (treated) and red cells (untreated) was determined by FACS. The experiment was repeated reversing the color of the treated cells. At 30 minutes significantly fewer Bay61-3606–treated B cells entered lymph nodes whereas more remained in the blood. By 1 hour, the treated B cells had begun to reach similar levels in lymph nodes in comparison to untreated cells (Figure 6H). No difference could be seen in the spleen at any time point.
To determine whether the SYK inhibitor might have a nonspecific effect on cell migration, the non-B cells remaining in the spleen cell suspension after purification of B cells were treated in the same manner as the B cells. CD4+ T-cell migration into lymph nodes was analyzed specifically by staining the cells collected from the tissues with anti-CD4. No effect of SYK inhibition was seen on migration of T cells, which although closely related to B cells do not express SYK (Figure 6I). This finding suggests the effect of SYK inhibition on B-cell migration in vivo is specific.
Discussion
SYK is essential for B-cell development31 and required for immunoreceptor tyrosine-based activation-like motif (ITAM)–dependent F-actin assembly,32 but little is known of the requirement for SYK during recirculating B-cell migration, although it is required during chicken pre-B cell haptotactic migration in vitro.27 Furthermore, SYK is involved in the chemotaxis and adhesion of B-cell chronic lymphocytic leukemia (CLL) cells and in polarization of a pro-B cell line.33-35 The data presented here establish a role for SYK in regulating B-cell chemotaxis and adhesion, in part through phosphorylation of SWAP-70. SYK phosphorylation of SWAP-70 occurs at residue 517, in a protein sequence conserved in even distantly related orthologues, such as Mus musculus and Danio rerio. Phosphorylation of SWAP-70 by SYK inhibits its interaction with F-actin in vitro. As a consequence of a Y517F mutation SWAP-70 was no longer able to rescue polarization of Swap-70−/− B cells in vitro and impairs Wt B-cell migration in vivo showing an important role for SWAP-70 phosphorylation and hence regulation of its F-actin binding by SYK.
SYK plays an important role in regulating migration of neutrophils and macrophages, both in chemokine signaling and integrin mediated adhesion.26,30,36 SYK is activated after integrin ligation on neutrophils, monocytic cell lines and platelets. The activation of SYK in migrating neutrophils is required for the establishment of stable lamellipodia and subsequent directed migration of the cells.25,26 In the absence of SYK activity the lamellipodia become unstable, in a manner similar to that seen for Swap70−/− B cells. Stabilization of the leading edge in migrating cells is associated with the accumulation of PIP3 at the lamellipodium in a positive feedback loop requiring phosphatidyl-inositol-3-kinase, Rac, and actin polymerization. SWAP-70 binds to PIP3, Rac, and F-actin and might regulate this positive feedback loop. This mechanism is also controlled by SYK, first through regulation of phosphatidyl-inositol-3-kinase, as seen previously25,26 and secondly through modulation of SWAP-70 binding to F-actin. A potential pathway is shown in supplemental Figure 4.
We determined that SYK is activated after CXCL12 stimulation of B cells. To investigate the role of SYK in regulating B-cell migration in vitro and in vivo, we used the highly specific B-cell inhibitor Bay61-3606.29 When we used this compound, we noted that SWAP-70 tyrosine phosphorylation in response to CXCL12 depends on SYK activity. In addition SYK is needed for normal B-cell migration toward CXCL12 in both Transwell and haptotactic (with VCAM1 as a ligand) Transwell migration assays in vitro. In addition, like SWAP-70, SYK activity is required for normal B-cell polarization after attachment on αCD44–coated plates. These in vitro observations correlate with a failure of BAY61-3606–treated B cells to migrate into lymph nodes in vivo, this is a specific effect of the inhibitor as T cells are unaffected by BAY61-3606.
Although SYK is activated by chemokine signaling and recruited to the leading edge of migrating cells,26 it is unclear how this is regulated. During immunoreceptor signaling SYK is recruited by binding ITAM motifs.37 We show here both by IP and affinity chromatography experiments that SWAP-70 associates with SYK. Additional evidence for a direct interaction between SWAP-70 and SYK comes from the stimulation of SYK autophosphorylation, particularly by the addition of the SWAP-70 N-terminal protein region to kinase reactions in vitro. The N-terminal domain of SWAP-70 contains an ITAM like sequence. Hence it is possible that in addition to being phosphorylated by SYK, SWAP-70 may in turn facilitate recruitment of SYK to the leading edge of the cell through its ITAM, forming a positive feedback mechanism, as seen for other ITAM sequences.37
This interaction between SWAP-70 and SYK may also be of relevance for fine-tuning cell migration during a germinal center reaction. B-cell migration during the germinal center response is modulated by cross-talk between the B-cell receptor (BCR) and chemokine receptors.38 BCR signaling down-regulates the responsiveness of B cells to CXCL12 and CCL3, highlighting another potential role for SYK in regulating B-cell migration. In addition, we have previously observed that SWAP-70 is able to associate with the BCR22 ; hence, it might be that SWAP-70 is in a unique position to modulate the intersection between the BCR and chemokine signaling pathways through its ITAM and its phosphorylation by SYK. This link might also explain, in part, the failure of Swap70−/− B cells to generate a normal germinal center response.
Similar crosstalk between BCR signals and chemokines is important for the maintenance of B-cell CLL cells.35 In addition, SYK activity is necessary for the migration of CLL cells toward CXCL12 and their adhesion to VCAM-1 in a BCR-independent manner.34 Because SWAP-70 is required for normal B-cell migration, a process regulated by its phosphorylation on tyrosine 517 by SYK, it is also likely to be important in migration of CLL cells. Moreover, because SYK activity is required for F-actin polymerization in CLL cells34 and SWAP-70 can regulate F-actin,21 SWAP-70 phosphorylation by SYK might modulate CLL cell migration and subsequent survival. Hence, the role SWAP-70 and its phosphorylation by SYK plays in the development of CLL is under investigation.
In addition, the interaction between SWAP-70 and SYK might play an important role in the migration of developing B cells. It has been noted that immature Syk−/− B cells (which can develop in Bcl-xl trangenic mice) fail to form mature follicular B cells and instead accumulate in the red pulp and outer T-cell zone of the spleen arrested at a transitional IgD− stage that has been termed T0.39 Rac signaling is also essential for the migration of transitional T0 B cells into the white pulp and their subsequent development.39 It has been suggested this migratory step is a key stage during which positive selection of B cells is regulated.39 Swap70−/− B cells also show a defect in development to MZB cells, which correlates with their hyper-adhesion, and accumulation in the splenic red pulp.17 SWAP-70 interacts with Rac and is phosphorylated by SYK. This raises the possibility that immature Syk−/− B cells fail to migrate into follicles and mature as the result of a failure to appropriately regulate SWAP-70 and, hence, Rac.
In conclusion, we show that SWAP-70 is phosphorylated by SYK and this modulates SWAP-70 F-actin binding in vitro and is required for normal B-cell polarization in vitro and migration in vivo. A novel mechanism is identified through which SYK regulates B-cell migration in vitro and homing to lymph nodes in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Annette Garbe and Carlos Ocana-Morgner for their critical reading of the manuscript, Florian Bilger for blinded analysis, and Dr Udo Maier (Boehringer-Ingelheim Inc) for BAY61-3606.
This study was funded by the DFG through the SFB655 (B4).
Authorship
Contribution: G.P. planned and performed experiments and cowrote the paper; T.A. performed experiments; and R.J. planned and performed experiments and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Glen Pearce or Rolf Jessberger, Institute of Physiological Chemistry, Faculty of Medicine Carl Gustav Carus, Dresden University of Technology, Fiedlerstr 42, MTZ, D-01307 Dresden, Germany; e-mail: 6305glen.pearce@mailbox.tu-dresden.de or rolf.jessberger@mailbox.tu-dresden.de.