Abstract
Standards for clinical trial design, execution, and publication have increased in recent years. However, the current structure for interaction among the pharmaceutical sponsor funding a drug or device development program, the contract research organization (CRO) that typically assists in executing the trial, regulatory agencies, and academicians, provides inadequate leadership and oversight of the development process. Conventional academic steering committees are not provided with the independent infrastructure by which to verify statistical analyses and conclusions regarding safety and efficacy. We propose an alternative approach centered on partnerships between CROs and university-based academic research organizations (AROs). In this model, the ARO takes responsibility for processes that address journal requirements and regulatory expectations for independent academic oversight (including oversight of Steering Committee and Data and Safety Monitoring Board activities), whereas the CRO provides infrastructure for efficient trial execution, site monitoring, and data management. The ARO engages academic experts throughout the trial process and minimizes conflicts of interest in individual industry relationships via diversification of sponsors, agents, and therapeutic areas. Although numerous models can be entertained, the ARO-CRO model is uniquely structured to meet the demand for greater assurance of integrity in clinical trials and the needs of each stakeholder in the process.
Introduction
The current landscape of drug and device development brings both the opportunity for treatment advances and the challenge of choosing among agents to assure maximum benefit, minimum risk, and acceptable cost. The execution of large industry-sponsored clinical trials as part of this development process involves a dynamic interaction between the sponsor funding the development program, a contract research organization (CRO) typically hired to help the sponsor execute the trial, and academic experts who provide scientific oversight and interpret trial results. Although academicians play an important role in oversight and safety monitoring as part of steering committees and data and safety monitoring boards (DSMBs), principal control of trial design and data handling rests with 2 for-profit entities: the sponsoring company that owns the product and the CRO.
The present structure for the interaction among the sponsor, CRO, and academicians, however, provides inadequate leadership and oversight of the development process, including trial design, interpretation, and reporting of results. As such, the conventional model does not best serve the interests of patients, clinicians, academia, regulatory agencies, or industry. A current alternative is to engage a university-based academic research organization (ARO) in lieu of a CRO in the industry-sponsored trials; however, a challenge for the academic model is efficiency in completing large trials. Furthermore, although there are several examples of successful large AROs, few offer a complete range of CRO services, and most rely on academicians from a single institution or organization to provide expertise for all trials in a given disease area. The authors of this “Perspectives,” 2 of whom have leadership roles in a global for-profit CRO and 2 in a university-affiliated ARO, instead propose an approach based on partnerships between CROs and AROs. Although numerous models could be entertained, we think that the ARO-CRO model is uniquely structured to meet the demand for greater assurance of integrity in clinical trials and the needs of each stakeholder in the process, recognizing the pragmatic requirement that they all play important roles. The ARO-CRO model is novel in that it is not previously described in the literature and has infrequently been used in the conduct of industry-sponsored clinical trials.
Proposal
Standards for the design, monitoring, and analysis of data from clinical trials for publication require independent and unrestricted access to data and analyses by academicians.1,2 Although these policies are particularly pertinent to large, industry-sponsored trials that address prevalent conditions associated with considerable morbidity and mortality, their enforcement is lax. Recent examples of either inadequate or inappropriate interactions among industry sponsors, the U.S. Food and Drug Administration, and academic committees involved in trial oversight highlight concerns about the integrity of this process.2-4 The importance of constituting independent DSMBs by a nonprofit organization has also been emphasized.4 Furthermore, even assuming complete access to data, the current paradigm for Steering Committee involvement relies on the infrastructure of the CRO and sponsor, rather than an independent infrastructure that provides the academicians with the means to verify statistical analyses of original data and assure truly independent conclusions about safety and efficacy.
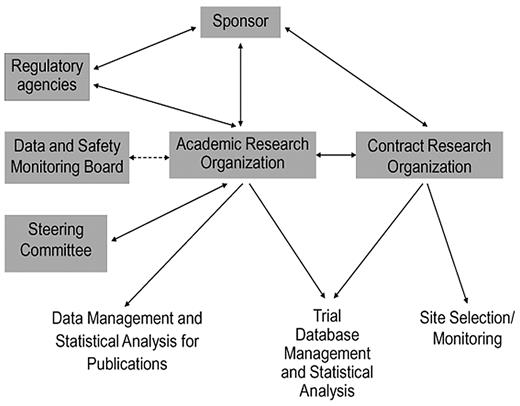
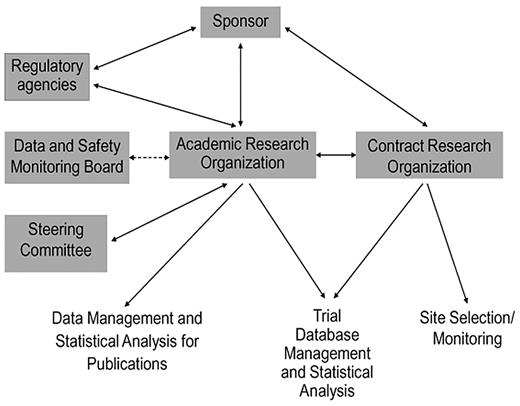
The mandates for transparency, integrity, and independent academic expertise in industry-sponsored trials can be realized through a thoughtfully structured partnership between an ARO and a CRO. In this model (Figure 1), the ARO takes responsibility for processes that address journal requirements and regulatory expectations for independent academic oversight, whereas the CRO provides the broad infrastructure for efficient trial execution, site monitoring, and data management (Table 1). Because selected, appropriate roles are transitioned from the for-profit CRO to the nonprofit university-based ARO, rather than creating new roles, the ARO-CRO model is not anticipated to appreciably increase cost relative to the conventional CRO model. A nonprofit, university-based ARO is well suited to engage a diverse team of academic experts from multiple institutions throughout the trial process, and its independence from the for-profit entities is assured by public contracts as well as by charters governing academic oversight (eg, steering, adjudication, publications committees, and DSMBs). Compared with conventional industry-academia relationships, the ARO's engagement of academic experts across a variety of agents and sponsors in a given therapeutic area reduces the impact of individual conflicts of interest. The ARO can provide continuity across a development program, with an additional charge to report follow-up data after the main objectives of the trial have been met, regardless of whether the primary outcome of the trial is negative or positive. The ARO also provides a unique venue for mentoring young clinical investigators for careers as academic trialists.
Schematic representation of the ARO-CRO model, showing the interactions among principal organizations, committees, and agencies involved in industry-sponsored clinical trials, and the division of responsibilities between the ARO and CRO.
Schematic representation of the ARO-CRO model, showing the interactions among principal organizations, committees, and agencies involved in industry-sponsored clinical trials, and the division of responsibilities between the ARO and CRO.
Other models to develop and manage clinical trials should continue to be considered. Historically, large trials were supported by government agencies and performed by academic institutions. For example, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial was entirely funded by the National Institutes of Health and addressed the management of hypertension across classes of existing drugs.5 Yet a purely federally funded model is challenged both economically and logistically. Federally sponsored trials are often underfunded and frequently fail to achieve accrual goals. Because of increased stringency of regulatory requirements relative to historical standards, many academic institutions have come to rely on sponsors and CROs to assure compliance with trial management and monitoring responsibilities.
Alternatively, new product development could use the model of an academic-industry partnership, abandoning the CRO. However, in such a model, smaller pharmaceutical and biotechnology companies would not be able to compete successfully in the conduct of large trials, leading to less competition and perhaps less innovation in the drug development process. The success of CROs in providing national or global management of large trials and the challenges faced by government or academic institutions in executing such trials in a timely fashion also suggest that the efficiencies provided by the CRO should not be overlooked in an optimal model. The for-profit orientation of the pharmaceutical industry must, likewise, be acknowledged as a key driver to the rapid advancement of new agents from bench to bedside, wherein patients can benefit. Hence, to shift toward a drug development model in which the pharmaceutical industry is no longer a key player would not only be impractical, but inefficient.
As a last option, the conventional CRO-based model could simply be maintained. Yet, the CRO and sponsor may lack substantial content expertise in the disease state or endpoints monitored during a trial. In addition, the conventional CRO-based model, which prioritizes brisk study initiation and efficient execution, does not emphasize active involvement of academic trialists during trial execution, and rarely engages these persons in interactions with regulatory agencies until late in the development process. In the absence of appropriately structured academic engagement, and given a lack of enforcement of the requirements established by major medical journals for detailed disclosure of design and analytic plans during clinical trial registration, it is not surprising that problems of delayed and selective publication of findings persist.1,6
For these reasons, we propose the ARO-CRO model as a preferred paradigm, and we have established the Antithrombotic Trials Leadership and Steering (ATLAS) group within the framework of an ARO-CRO collaboration, to provide independent oversight of clinical trials in our fields of expertise. In an era when multiple new drugs are under development for specific indications, investigators and practitioners must look beyond individual development programs to assessments of comparative effectiveness and safety that inform future therapeutic choices. Equally important is the identification of target populations that stand to benefit most from new products, rather than the largest potential market to accelerate return on the sponsor's investment. An ARO's involvement in the clinical trials process can retarget development programs to focus on these issues. For example, emerging therapies in the antithrombotic arena primarily target common conditions, including ischemic heart disease, atrial fibrillation, and venous thromboembolism, but other indications, such as malignancies, mechanical heart valves, and periprocedural management, are in need of study, as are underrepresented populations, such as pediatrics, geriatrics, and patients with renal insufficiency. In addition, an ARO can provide much-needed guidance for crafting development programs for novel therapeutics with complex safety and efficacy profiles. Conventional anticoagulants have high risk-to-benefit ratios, narrow therapeutic ranges, requirements for frequent monitoring of anticoagulation intensity, and (in the case of warfarin) a substantial propensity for drug and food interactions. New oral agents inhibiting specific components of the coagulation pathway (eg, direct thrombin inhibitors and factor Xa inhibitors) offer potential advantages, including predictable pharmacokinetics, fixed dosing, and perhaps improved risk-benefit profiles. With several new anticoagulants in phase 2 and 3 development programs, there is a critical need for ethical, informed scientific leadership of upcoming trials. Similar concerns apply to many other therapeutic areas.
Challenges
Challenges to implementing the ARO-CRO model include corporate reluctance to provide necessary resources and relinquish authority over trial design and publication, hesitation by academicians to assume greater responsibility, and the perception among sponsors and CROs that academicians cannot function with sufficient business sophistication or at the pace necessary to meet competitive timelines. Overcoming these concerns requires that AROs have nimble structures and decision-making processes and that academic trialists are highly experienced and continuously engaged. Academic experts must take greater responsibility for trial oversight than the industry-defined roles of “key opinion leader,” “consultant,” or “advisory board member” permit. At the same time, sponsors must adapt to an evolving regulatory climate in which academic leadership and independent oversight are critical to the approval and commercialization of new products for clinical use.
A sustainable ARO-CRO collaboration must assure efficiency to realize the potential for optimized trial designs and endpoints to reduce development costs. Academic trialists should facilitate communication between government regulators and industry sponsors, and must in turn defend key elements of trial design. Academic leadership is critical to provide impetus for, and oversight of, extensions of trials to vulnerable and underrepresented populations, investigation of foreseeable and clinically important off-label applications, and evaluation of comparative effectiveness and safety of new and existing therapeutics. Ultimately, the success of the ARO-CRO model depends on the relationships among sponsors, CROs, and academic experts who must firmly commit to establishing the infrastructure necessary for independent academic oversight of industry-sponsored clinical trials.
Conclusion
The ARO-CRO model offers a mechanism for improved academic leadership and oversight in industry-sponsored clinical trials. The ARO provides academic committies (eg, DSMBs, steering and leadership committees such as the ATLAS group) with the independent infrastructure to verify statistical analyses and conclusions regarding safety and efficacy, whereas the CRO provides the infrastructure for efficient trial execution, site monitoring, and data management. In this manner, the needs of each stakeholder in the process can be met, while also meeting the critical demand for greater assurance of integrity in clinical trials.
Presented in part at the 8th Annual American Society of Hematology Clinical Research Training Institute symposium on industry-academia relationships, La Jolla, CA, August 3, 2010.
Authorship
Contribution: N.A.G., A.C.S., J.L.H., C.M.K., S.S., A.G.G.T., A.M.S., N.R.C., and W.R.H. conceptualized the work, revised the manuscript, approved its submission for publication, were fully responsible and autonomous in the conceptualization and writing of the manuscript, and have confirmed that no pharmaceutical industry writers were involved in any phase of its development; and N.A.G. and W.R.H. codrafted the initial manuscript.
Conflict-of-interest disclosure: N.A.G. receives salary and research support from the National Institutes of Health, National Heart, Lung, and Blood Institute in the form of a Career Development Award and salary support in pharmaceutical industry-sponsored research from CPC Clinical Research, a nonprofit ARO affiliated with the University of Colorado. A.C.S. receives fees from sanofi-aventis, Boehringer Ingelheim, and Bristol Myers-Squibb for consulting activities, from Astellas Pharma for Data and Safety Monitoring Committee activities, and from Eisai Inc, Johnson & Johnson, and Bayer Healthcare for consulting, Data and Safety Monitoring Committee, and Steering Committee activities. He also receives consultant fees from the American Society of Hematology and received honoraria from CPC Clinical Research. J.L.H. receives consulting fees for advisory and/or Steering Committee activities from Astellas Pharma, Bayer Healthcare, Biotronik Inc, Boehringer Ingelheim, Bristol Myers-Squibb, Dai-ichi Sankyo, Johnson & Johnson, Pfizer Inc, and sanofi-aventis. He also receives honoraria from Portola Pharmaceuticals Inc for Data and Safety Monitoring Board activities, receives research support from the National Institutes of Health, National Heart, Lung, and Blood Institute and received honoraria from CPC Clinical Research. He is a member of the Cardiovascular and Renal Drugs Advisory Committee of the US Food and Drug Administration. C.M.K. receives consulting fees for advisory, Data and Safety Monitoring Board, and/or Steering Committee activities from Baxter Immuno, Bayer Healthcare, Boehringer Ingelheim Canyon Pharmaceuticals, CSL Behring, Eisai Inc, Incyte, King Pharmaceutical, NovoNordisk, Octapharma, Pfizer Inc, and sanofi-aventis and received honoraria from CPC Clinical Research. Georgetown University receives research support on his behalf from the National Institutes of Health, the Maternal and Child Health Bureau, and the Centers for Disease Control and Prevention, as well as from Amgen, Baxter Immuno, Eisai Inc, Genentec, GlaxoSmithKline, Griffols, NovoNordisk, Octapharma, and sanofi-aventis. S.S. receives fees from Bayer Healthcare and Boehringer Ingelheim for Data and Safety Monitoring Committee and Steering Committee activities and received honoraria from CPC Clinical Research. A.G.G.T. receives fees from Bayer Schering Pharma, Astellas, Portola, Takeda, Eisai, and Pfizer for consulting, CSL Behring for Data and Safety Monitoring Committee, and Bayer Schering Pharma, Takeda, and Astellas for Steering Committee activities and received honoraria from CPC Clinical Research. A.M.S. is employed by Worldwide Clinical Trials Inc, a for-profit international CRO. N.R.C. is President and CEO of Worldwide Clinical Trials Inc. W.R.H. is President of CPC Clinical Research. He receives research support from grants provided by the National Institutes of Health and from the pharmaceutical industry for sponsored research initiatives. He also receives partial salary support through research grants provided to CPC Clinical Research and the University of Colorado, as well as fees from the US Food and Drug Administration as a Special Government Employee for several advisory committees. He provides consulting services to the pharmaceutical industry via a grant-funded mechanism though CPC Clinical Research; he does not receive fees from industry directly. Current relationships include GlaxoSmithKline, sanofi-aventis, Arca Biopharma, Cytokinetics, Diffusion Pharma, Kowa, Portola, ReNeuron, Sigma Tau, TheraVasc, Vermillion, and Zona.
Correspondence: William R. Hiatt, University of Colorado School of Medicine, Division of Cardiology, and CPC Clinical Research, 13199 E Montview Blvd, Ste 200, Aurora, CO 80045; e-mail: Will.Hiatt@ucdenver.edu.