Abstract
Inhibition of Cdk4/Cdk6 by p18INK4c (p18) is pivotal for generation of noncycling immunoglobulin (Ig)-secreting plasma cells (PCs). In the absence of p18, CD138+ plasmacytoid cells continue to cycle and turnover rapidly, suggesting that p18 controls PC homeostasis. We now show that p18 selectively acts in a rare population of rapidly cycling CD138hi/B220hi intermediate PCs (iPCs). While retaining certain B-cell signatures, iPCs are poised to differentiate to end-stage PCs although the majority undergo apoptosis. p18 is dispensable for the development of the PC transcriptional circuitry, and Blimp-1 and Bcl-6 are expressed fully and mutually exclusively in individual iPCs. However, a minor proportion of iPCs express both, and they are preferentially protected by p18 or Bcl-xL overexpression, consistent with expansion of the iPC pool by Bcl-xL overexpression, or loss of proapoptotic Bim or Noxa. Expression of Noxa is induced during B-cell activation, peaks in iPCs, and selectively repressed by p18. It is required to promote apoptosis of cycling B cells, especially in the absence of p18. These findings define the first physiologic function for Noxa and suggest that by repressing Noxa, induction of G1 arrest by p18 bypasses a homeostatic cell-cycle checkpoint in iPCs for PC differentiation.
Introduction
Terminal differentiation of B cells to plasma cells (PCs) secreting antigen-specific antibodies requires exquisite coordination of cell-cycle control, differentiation, and apoptosis. PCs are permanently withdrawn from the cell cycle. Most are short-lived but some, particularly those residing in the bone marrow, can live for a long time.1 Gene targeting and in vitro studies have demonstrated that through inhibiting Cdk4 and Cdk6, induction of early G1 arrest by the Cdk inhibitor (CKI) p18INK4c2,3 is pivotal for the generation of end-stage PCs in the T-dependent (TD) antibody response.4 In the absence of p18, memory B cells and plasmacytoid cells expressing CD138 (syndecan-1), a proteoglycan present on PCs but not B cells, are formed, but they continue to cycle and are eliminated by cell death in situ.4 These findings provide the first direct evidence for cell-cycle control of PC differentiation in a physiologic setting. They suggest that p18 imposes a final homeostatic checkpoint in PC differentiation but that the mechanism is unknown. Because p18 is frequently deleted in lymphoma and myeloma,5,6 understanding the mechanism by which p18 controls homeostasis has important implications for the pathogenesis of hematologic malignancies as well.
Cell-cycle control of the PC transcriptional program represents one possible mechanism, because PC differentiation is a continuum marked by orderly transition of gene expression. It requires the activation of key transcription factors such as Blimp-1,7 IRF-4,8 and XBP-19 in concert with repression of other transcription factors, notably Bcl-6 required for germinal center (GC) formation10,11 and Pax-5.12 Blimp-1 and Bcl-6 repress each other.13 Pax-5 is another target of Blimp-1 repression,14 which, in turn represses XBP-1.9 IRF-4 has been shown to be necessary for both Ig class switch recombination (CSR) and the generation of IgG-secreting PCs.15,16 Although Blimp-1 protein expression is unabated in p18-deficient CD138+ plasmacytoid cells,4 it is unclear whether the transcriptional circuitry for PC differentiation is intact in the absence of p18.
At the cellular level, the increase in surface CD138 expression during B-cell terminal differentiation is accompanied by a gradual loss of B-cell surface markers, so that end-stage PCs express CD138 but not B220. However, a low level of B220 has been detected on CD138+ precursors of long-lived bone PCs.17 It is also known that cycling CD138+ plasmablasts emerge dynamically in TD and T-independent antibody responses and secrete Ig.18–20 However, the intermediate steps linking antigen-activated B cells to cycling plasmablasts and noncycling, Ig-secreting end-stage PCs are not fully understood.
To elucidate the mechanism for cell-cycle control of PC differentiation, we show that p18 selectively acts in a rare population of rapidly cycling and apoptotic PC precursors (referred to as intermediate plasma cell, or iPC), which express the signatures of both B cells and PCs. The PC transcription program appears intact in the absence of p18. Blimp-1 and Bcl-6 are expressed fully and mutually exclusively in individual iPCs, except for a small proportion, which expresses both, and they are protected by p18 and Bcl-xL. Through cell-cycle attenuation, p18 maintains the iPC pool for timely differentiation to end-stage PCs, in part by selective repression of the proapoptotic BH3-only Noxa, which is preferentially expressed in iPCs. Collectively, our data suggest that by attenuating cell-cycle progression though G1 and repressing Noxa, p18 controls homeostasis during PC differentiation in the transitional iPCs.
Methods
Isolation and culturing of primary B cells and PCs
Mice deficient in p18,21 Bim,22 or Noxa23 (kindly provided by Dr Andreas Strasser [Walter and Eliza Hall Institute of Medical Research, Parkville, Australia] via Dr Navdeep Chandel [Northwestern University, Chicago, IL]), Bcl-xL transgenic mice24 (kindly provided by Dr Tim Behrens [Genentech]), and C57BL/6 mice (The Jackson Laboratory), were immunized intraperitoneally at 7-10 weeks of age with 75 μg of alum-precipitated 4-hydroxy-3-nitrophenylacetyl (NP)-CGG (23:1; Biosearch Technologies Inc), as described.4
Splenic resting B cells present in the high density (60%-70%) fraction and activated B cells, iPCs, and PCs present in the low-density (50%-60%) fraction of a discontinuous Percoll gradient25 were isolated by sorting determined by CD138 and B220 expression after staining with phycoerythrin-conjugated anti–mouse CD138 and phycoerythrin-Cy5–conjugated anti–mouse CD45R/B220 rat antibodies (BD Biosciences) with the use of a BD FACS Vantage SE Cell Sorter and Diva software Version 4.1 (Becton Dickinson). They were cultured in RPMI supplemented with 10% heat-inactivated fetal bovine serum (B media; Hyclone), as described.25 In some experiments, resting B cells were cultured in B media in the presence of goat anti–mouse immunoglobulin M (IgM; 5 μg/mL; Jackson ImmunoResearch Laboratories) or recombinant soluble human BAFF (75 ng/mL; Peprotech), and polymyxin B (200 units/mL; Sigma-Aldrich), as described.26 They were also differentiated to PCs in vitro by coculturing with CD40L or MC3T3 cells (mouse embryo calvaria fibroblasts; German Collection of Microorganisms and Cell Cultures) in the presence of interleukin 6 (IL-6) and soluble IL-6 receptor (sIL-6R; both 40 U/mL). Recombinant mouse IL-4 (200 U/mL) and OX40-Ig (1:60 dilution) were added from day 4 onward, as described.4
Enzyme-linked immunosorbent assay and enzyme-linked immunosorbent spot
The relative NP-specific immunoglobulin G (IgG) titer after NP-CGG immunization was determined by enzyme-linked immunosorbent assay as described.4 The only modification was the coating of wells with 5 μg of NIP16 -BSA (Biosearch Technologies) or with 2.5 μg of a goat anti–mouse κ antibody (Southern Biotec). The frequency of cells secreting NP-specific IgG was determined by enzyme-linked immunosorbent spot (Elispot), also as described.4
Immunofluorescence staining
IgM and IgG expression was detected by simultaneously staining with fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgM and Texas Red–conjugated goat anti–mouse IgG (Southern Biotec), counterstained with DAPI (Roche), and visualized by immunofluorescence microscopy.4 The IgM- and IgG-positive cells from each sorted population were counted in triplicate, with at least 500 cells counted in each sampling.
Fluorescence-activated cell sorting
To detect surface marker expression, cells were incubated first with rat antimouse CD16/CD32 (FcγIII/II receptor; BD Pharmingen) to block nonspecific binding and then stained with FITC-conjugated rat antimouse IgG1, CD40, GL-7, or CXCR4 (BD Pharmingen) on ice and analyzed by fluorescence-activated cell sorting (FACS) by the use of FACSCalibur and CellQuest software Version 5 (Becton Dickinson). To detect intracellular IgG, sorted cells were fixed in 4% paraformaldehyde and then stained with FITC-conjugated anti–mouse IgG1 (Southern Biotec) on ice before FACS analysis, as described.26
Bromodeoxyuridine uptake
For bromodeoxyuridine (BrdU) uptake in vivo, mice were injected intraperitoneally with BrdU (2.5 mg in 200 μL of phosphate-buffered saline; Sigma-Aldrich) at 2 hours before isolation of activated splenic B cells. After staining with FITC-conjugated anti-BrdU (Roche), BrdU uptake was determined by FACS by the use of FACSCalibur (Becton Dickinson) and FlowJo software Version 8 (TreeStar), as described.26
Apoptosis
To assess DNA fragmentation, TO-PRO-3 (Invitrogen) was added to cells at a concentration of 1nM and analyzed immediately by FACS.
Quantitative polymerase chain reaction
Total RNA was isolated from cells by the use of TRIzol (Invitrogen), and cDNA was synthesized with SuperScript III and oligo (dT)20 primers (Invitrogen). Quantitative polymerase chain reaction (qPCR) analysis was performed with the use of Assays-on-Demand gene expression mixes specific for indicated genes (Applied Biosystems). Reactions were conducted in an ABI PRISM 7900 HT Sequence Detection System with TaqMan Universal PCR Master Mix (Applied Biosystems). The relative amount of product was determined by the comparative threshold cycle method, according to Applied Biosystems. Unless otherwise noted, resting splenic B cells isolated from unimmunized mice were the reference and actin served as a base line control within each sample.
Single-cell qPCR
Activated B cells, iPCs, and PCs present in the low-density fraction were sorted into 384-well PCR plates containing 1 μL of nuclease-free water in each well. The plates were frozen immediately and stored at −80°C before analysis of Bcl-6, Blimp-1, and β-actin mRNA by real-time PCR, as described.27 Primers and probes (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were added to the lysate together with QuantiTect Multiplex RT-PCR buffer (QIAGEN) in a total volume of 12 μL. Reactions were run for 40 cycles in a 7500 Real-Time PCR System (Applied Biosystems). The relative RNA level in single cells was quantified by setting thresholds within the logarithmic phase of the PCR and determining the cycle number at which the threshold was reached (Ct). Data were plotted as cycle number at which target gene was higher than threshold (Ct) in reverse order from wells that were positive for β-actin. This corresponds to a log2 scale.
Retroviral shRNA knockdown
293T cells in 6-well plates were transfected with 900 ng of ecotropic envelope vector, 100 ng of gag/pol vector (a kind gift from Dr Louis Staudt [National Cancer Institute]), and 1 μg of pSM2c shRNA vector containing either a Noxa-specific or a nonsense control shRNA (Open Biosystems), using TransIT-LT1 (Mirus) per the manufacturer's protocol. The virus-containing medium (viral supernatant) was harvested at 48 hours after transfection, with a media change at 24 hours, and mixed with 4 μg/mL of polybrene (Sigma-Aldrich). Freshly isolated primary resting B cells (1 × 106) were suspended in 1 mL of the viral supernatant in the presence or absence of 5 μg/mL anti-IgM (Jackson ImmunoResearch Laboratories) and 2 μg/mL anti-CD40 (eBioscience) in a 12-well plate at room temperature for 20 minutes, centrifuged at 750g at 30°C for 2 hours, and incubated in B media in the presence or absence of anti-CD40 and anti-IgM overnight. The virally infected cells were then rinsed, resuspended at 4 × 105 cells/mL in the same B media, and incubated for 72 hours. Live cells were determined by Trypan Blue exclusion in triplicate at 48 and 72 hours.
Statistical analysis
All statistical analyses were performed by the use of a 2-tailed Student t test, assuming unequal variances, where the significance level was set at P < .05. Unless otherwise noted, error bars in figures are displayed as ± SEM, and data presented represent at least 3 independent experiments.
Results
p18INK4c is critical for differentiation of transient precursors to end-stage PCs
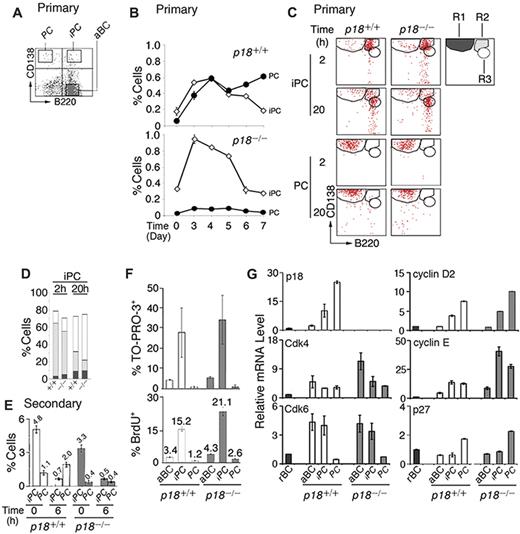
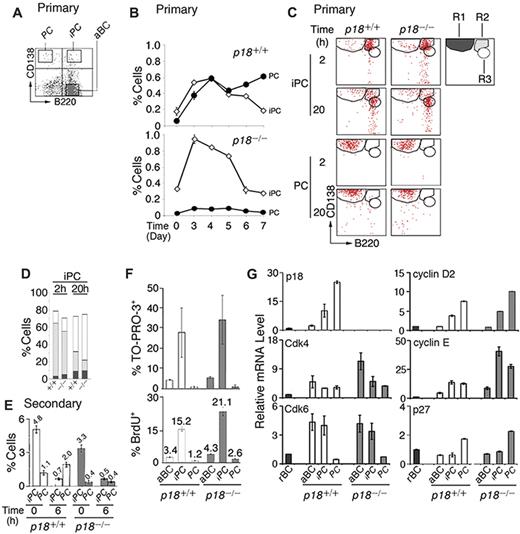
p18 is required for cell-cycle termination in proliferating extrafollicular CD138+ plasmaycytoid cells,4 suggesting that it targets PC precursors. Among CD138+ cells present in the low-density splenic B-cell fraction after immunization with the TD antigen NP-CGG, some coexpressed a high level of B220 (CD138hi/B220hi, iPCs; Figure 1A). iPCs increased in parallel with PCs (CD138hi/B220lo) until day 4, when they began to decrease, whereas PCs continued to increase (Figure 1B). In the absence of p18, the increase in PCs was negligible despite the initial amplification of iPCs (Figure 1B). These results demonstrate that p18 is required for the generation of PCs but not iPCs and suggest a dynamic precursor-product relationship between them in the TD response.
Generation of rapidly cycling and apoptotic iPCs in the TD response. (A) A representative FACS analysis of CD138 and B220 expression in the low-density splenic B cells isolated from p18+/+ mice. (B) Percentage of p18+/+ and p18−/− iPCs and PCs in the low-density fraction on indicated days after NP-CGG immunization. (C) FACS analysis of iPCs and PCs at indicated hours after sorting and incubation in B media; R1 (CD138hi/B220lo), R2 (CD138hi/B220hi), and R3 (CD138m/B220hi) are as marked. (D) The percentage of R1, R2, and R3 generated from iPCs at 2 and 20 hours as shown in panel C. (E) Percentage of iPCs and PCs in p18+/+ and p18−/− mice on day 4 of the secondary NP-CGG response, before or 6 hours after coculture with MC3T3 cells. (F) Percentage of TO-PRO-3+ and BrdU+ cells after a 2-hour-pulse in vivo in p18+/+ and p18−/− mice. (G) qPCR analysis of relative mRNA levels in sorted p18+/+ and p18−/− populations. rBC indicates resting B cell; aBC, activated B cell; iPC, intermediate plasma cell; PC, plasma cell.
Generation of rapidly cycling and apoptotic iPCs in the TD response. (A) A representative FACS analysis of CD138 and B220 expression in the low-density splenic B cells isolated from p18+/+ mice. (B) Percentage of p18+/+ and p18−/− iPCs and PCs in the low-density fraction on indicated days after NP-CGG immunization. (C) FACS analysis of iPCs and PCs at indicated hours after sorting and incubation in B media; R1 (CD138hi/B220lo), R2 (CD138hi/B220hi), and R3 (CD138m/B220hi) are as marked. (D) The percentage of R1, R2, and R3 generated from iPCs at 2 and 20 hours as shown in panel C. (E) Percentage of iPCs and PCs in p18+/+ and p18−/− mice on day 4 of the secondary NP-CGG response, before or 6 hours after coculture with MC3T3 cells. (F) Percentage of TO-PRO-3+ and BrdU+ cells after a 2-hour-pulse in vivo in p18+/+ and p18−/− mice. (G) qPCR analysis of relative mRNA levels in sorted p18+/+ and p18−/− populations. rBC indicates resting B cell; aBC, activated B cell; iPC, intermediate plasma cell; PC, plasma cell.
Confirming that iPCs are transient precursors of PCs, a small proportion of iPCs (R2) differentiated to PCs (R1) by 2 hours of sorting independent of p18 (Figure 1C and supplemental Figure 1A). However, most iPCs failed to maintain CD138 expression, leading to the emergence of a distinct population expressing reduced CD138 while maintaining B220 (CD138m/B220hi, R3) by 20 hours in vitro. This finding was more prominent in the absence of p18 (Figure 1C-D). By contrast, the sorted PCs remained unchanged throughout (Figure 1C and supplemental Figure 1B). These data suggest that iPCs can rapidly differentiate to the more stable PCs or the less PC-like CD138m/B220hi cells and that p18 promotes PC differentiation.
The proportion of iPCs was expanded in the secondary NP response (Figure 1E). When the low-density splenic B cells were cocultured with MC3T3 stromal cells in the presence of IL-6 and the sIL-6R and OX40-Ig to promote IgG+ PC differentiation, iPCs were reduced 7-fold within 6 hours independent of p18. This correlated with a ∼2-fold increase in PCs but only in the presence of p18. These data are consistent with iPCs being transient precursors of the more stable PCs and show that p18 promotes the longevity of iPCs or differentiation to end-stage PCs in the TD response.
p18 attenuates the cell cycle and apoptosis in rapidly cycling iPCs
iPCs were significantly more apoptotic than activated B cells or end-stage PCs, particularly in the absence of p18, as evidenced by TO-PRO-3 analysis of DNA fragmentation (Figure 1F). Importantly, 15% of iPCs took up BrdU in 2 hours in vivo, and this was markedly increased in the absence of p18 (Figure 1F). These findings validated the detection of extensive cell death in cycling CD138+ plasmacytoid cells in situ in the p18-null mice4 and suggest that unscheduled progression through G1 is coupled to enhanced cell death in rapidly cycling iPCs.
Little is known about cell-cycle control of PC differentiation, despite the knowledge that in mammalian cells progression through G1 is driven by Cdk4/6-cyclin D in opposition to INK4 CKIs and by Cdk2-cyclin E, which is inhibited by Cip/Kip CKIs.28 Cdk4 and Cdk6 mRNAs were coordinately elevated in activated B cells and iPCs. Although Cdk4 was maintained in PCs, Cdk6 was silenced. p18 mRNA was undetected in resting B cells, induced in activated B cells, and increased progressively in iPCs and PCs in parallel with cyclin D2 (Figure 1G). By contrast, p27 mRNA was selectively increased only in PCs (Figure 1G). The absence of p18 markedly augmented the elevation of Cdk4 mRNA in activated B cells, and cyclin E mRNA in both iPCs (40-fold) and PCs (30-fold) over resting B cells. These increases were substantially greater than those seen in their wild-type counterparts (Figure 1G).
Taken together, these data suggest that in iPCs, the levels of p18 and p27 were insufficient to fully oppose the increases in Cdk4, Cdk6, cyclin D2, and cyclin E; hence, the cell cycle progresses through G1. In PCs the concomitant gain of p18 and p27, together with loss of Cdk6, results in G1 arrest despite high cyclin D2 and cyclin E expression. By inhibiting both Cdk4/Cdk6 catalytic activity and cyclin E expression, p18 acts in tandem with p27 to ensure G1 arrest in PCs and is required to attenuate progression through G1 in iPCs.
iPCs are transitional in IgG synthesis and secretion while expressing B-cell signatures
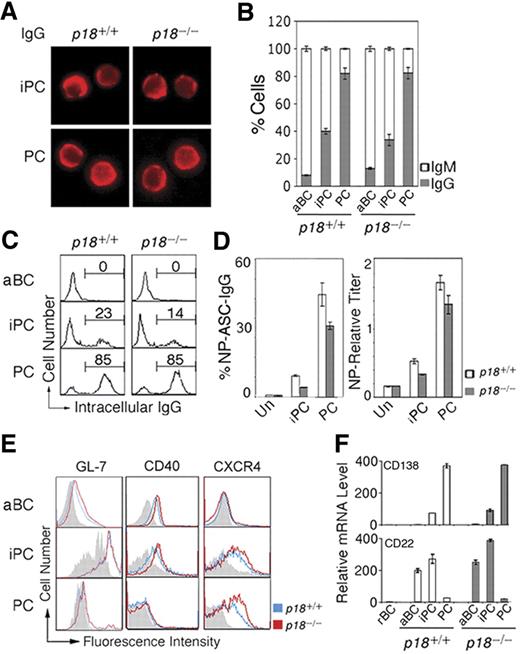
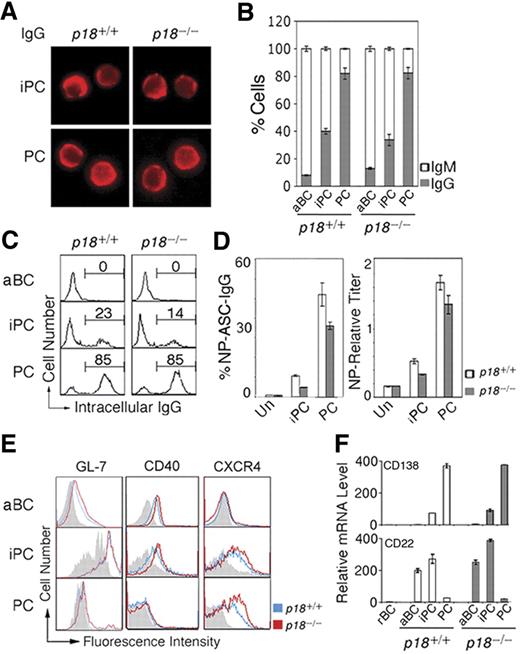
iPCs resemble PCs in morphology (Figure 2A). Independent of p18, ∼40% of iPCs had switched to IgG1 compared with ∼ 80% of PCs by day 7 after immunization (Figure 2B). Some iPCs (23%) express cytoplasmic IgG at the PC level, suggesting that iPCs may secrete IgG when Ig synthesis has reached a threshold level (Figure 2C). Indeed, Elispot showed that when protected by stromal cells, iPCs secreted NP-specific IgG1 at 20% of the frequency of PCs and this frequency was lower in the absence of p18 (Figure 2D). Thus, p18 is dispensable for CSR but required to promote Ig synthesis or survival of high IgG-expressing iPCs.
iPCs are transitional in IgG secretion and retain B-cell signatures. (A) IMF of intracellular IgG in sorted p18+/+ and p18−/− iPCs and PCs on day 7 after NP-CGG immunization. (B) Proportion of IgG- or IgM-expressing cells by IMF and (C) FACS analysis of cytoplasmic IgG, both in sorted p18+/+ and p18−/− populations. (D) Elispot analysis of cells secreting NP-specific IgG (ASC) in unsorted cells (Un) and sorted p18+/+ and p18−/− populations after coculturing with MC3T3 cells for 2 days; enzyme-linked immunosorbent assay of relative NP-specific IgG secreted into media. (E) FACS analysis of surface markers in p18+/+ and p18−/− populations; gray represents isotype control. (F) qPCR analysis of relative mRNA in sorted p18+/+ and p18−/− populations.
iPCs are transitional in IgG secretion and retain B-cell signatures. (A) IMF of intracellular IgG in sorted p18+/+ and p18−/− iPCs and PCs on day 7 after NP-CGG immunization. (B) Proportion of IgG- or IgM-expressing cells by IMF and (C) FACS analysis of cytoplasmic IgG, both in sorted p18+/+ and p18−/− populations. (D) Elispot analysis of cells secreting NP-specific IgG (ASC) in unsorted cells (Un) and sorted p18+/+ and p18−/− populations after coculturing with MC3T3 cells for 2 days; enzyme-linked immunosorbent assay of relative NP-specific IgG secreted into media. (E) FACS analysis of surface markers in p18+/+ and p18−/− populations; gray represents isotype control. (F) qPCR analysis of relative mRNA in sorted p18+/+ and p18−/− populations.
Further characterization of iPCs revealed that they express CXCR4 required for GC dark and light zone segregation29 and homing of PCs to the bone marrow30 at the level of PCs. In addition, iPCs express CD40, the GC marker GL7, and the BCR coreceptor CD22 before they are extinct on PCs (Figure 2E). CD138 mRNA was lower in iPCs compared with PCs despite comparable high surface expression, suggesting that CD138 is subject to transcriptional and posttranscriptional regulation during PC differentiation (Figure 2F). Thus, iPCs express CD138 and CXCR4 in common with PCs but retain characteristics of B cells (B220 and CD40), activated B cells (CD22), and GC B cells (GL-7 and CD22), and CXCR4 and CD22 expression is selectively attenuated by p18. On this basis, it seems likely that iPCs, some having switched to IgG, are proliferating PC precursors in transition from GCs to the extra follicular foci.
p18 is required within B cells to promote PC differentiation
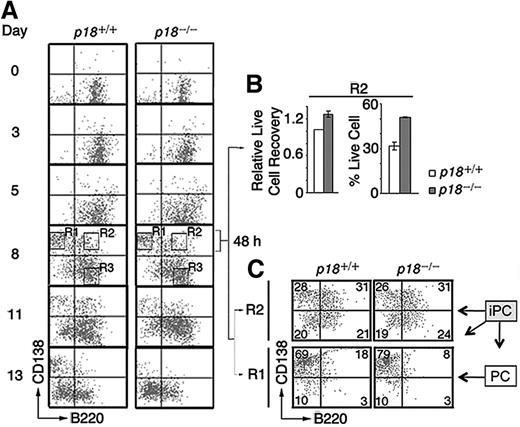
To verify that p18 is required within iPCs for differentiation to PCs, we reconstituted the differentiation of polyclonal IgG-secreting PCs in vitro by coculturing resting splenic B cells with mitomycin-C arrested mouse CD40L-expressing L (CD40L)-cells and orderly addition of IL-6, IL-6R, IL-4, and OX40.4 CD138+/B220+ iPCs were detected at day 3, peaked by day 8 along with abundant CD138+/B220− PCs, and then disappeared with time, more rapidly in the absence of p18. By day 13, the number of p18−/− PCs was < 30% of p18+/+ PCs (Figure 3A), which correlated with a ∼ 50% reduction of total live cells.4 These data suggest that p18 is required within iPCs to promote differentiation to PCs, most likely though attenuation of apoptosis.
p18 is required within B cells for differentiation of iPCs to PCs. (A) Differentiation of polyclonal PCs from resting B cells in vitro. p18+/+ and p18−/− iPCs and PCs were sorted on day 8 and cultured separately for 48 hours with MC3T3 stromal cells. (B) The recovery of live p18−/− iPCs relative to p18+/+ iPCs (left) and the percentage of live cells (right) (C) FACS analysis of sorted p18+/+ and p18−/− PCs and iPCs after 48 hours in coculture. Percentage of cells in each quadrant is indicated.
p18 is required within B cells for differentiation of iPCs to PCs. (A) Differentiation of polyclonal PCs from resting B cells in vitro. p18+/+ and p18−/− iPCs and PCs were sorted on day 8 and cultured separately for 48 hours with MC3T3 stromal cells. (B) The recovery of live p18−/− iPCs relative to p18+/+ iPCs (left) and the percentage of live cells (right) (C) FACS analysis of sorted p18+/+ and p18−/− PCs and iPCs after 48 hours in coculture. Percentage of cells in each quadrant is indicated.
If enhanced cell death in the absence of p18 limits the generation of PCs from iPCs, protection from death should compensate for the loss of p18 in iPCs. Indeed, when iPCs (R2) and PCs (R1) sorted on day 8 were cocultured separately with MC3T3T stromal cells, the recovery of p18+/+ and p18−/− iPCs was comparable (Figure 3B), as was the differentiation of iPCs (CD138hi/B220hi) to PCs (CD138hi/B220lo) at 48 hours (Figure 3C). Some iPCs lost the expression of CD138 (CD138lo/B220hi), as observed in iPCs generated in the TD response (Figure 1C), or both CD138 and B220 (CD138lo/B220lo), whereas PCs remained unchanged (Figure 3C). These results suggest that polyclonal iPCs generated in vitro are also transient precursors of the more stable PCs and that stromal protection compensates for the loss of p18 in the differentiation of iPC to PC.
iPCs express transcription factors required for PC differentiation independent of p18
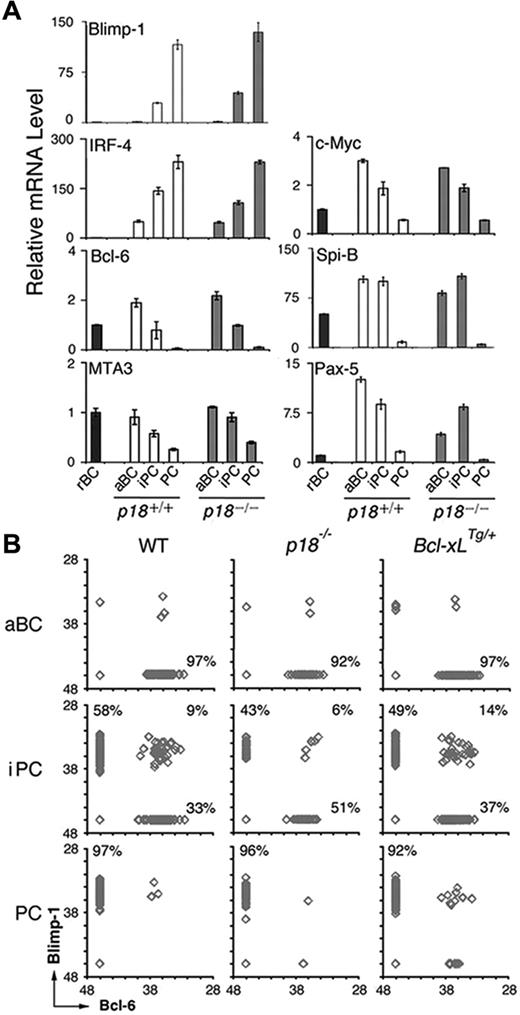
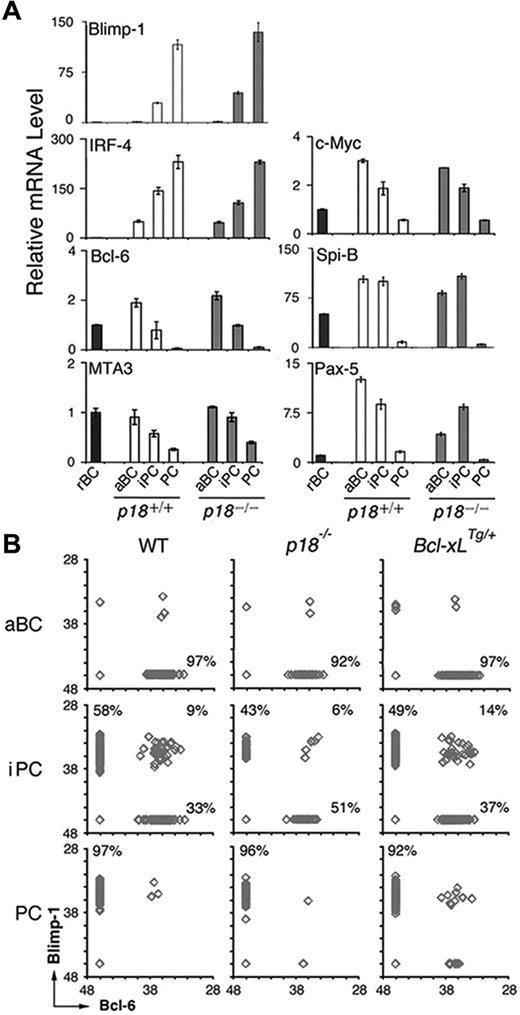
We then investigated whether cell-cycle attenuation by p18 could also promote PC differentiation through regulating the PC transcriptional program. Blimp-1 mRNA appeared in iPCs and was increased in PCs in the TD response, consistent with its requirement for PC differentiation. IRF-4 mRNA was induced in activated B cells and increased further in iPCs and PCs, in line with its dual roles in CSR and PC differentiation (Figure 4A). In keeping with mutual repression, the increase in Blimp-1 mRNA was accompanied by a reciprocal decrease in Bcl-6 mRNA along with its corepressor for Blimp-1 transcription, MTA3,13 and c-Myc, another target of Blimp-1 (Figure 4A). Of interest, after induction in activated B cells, the expression of Spi-B required for GC formation31 was maintained in iPCs but not PCs. The absence of p18 did not alter their temporal expression, apart from a 3-fold reduction in Pax-5 mRNA in activated B cells (Figure 4A). On this basis, it appears that iPCs express transcription factors essential for GC formation and PC differentiation at levels between activated B cells and PCs independent of p18.
iPCs express Blimp-1 and Bcl-6 mutually exclusively and the minority expressing both are maintained by p18 and Bcl-xL. (A) qPCR analysis of relative mRNA levels in sorted p18+/+ and p18−/− populations. (B) Single-cell qPCR analysis of Bcl-6 and Blimp-1 expression in aBCs, iPCs, and PCs from wild-type, p18−/−, and Bcl-xLTg/+ mice on day 7 after NP-CGG immunization. Percentages were calculated from the total number of cells expressing Bcl-6, Blimp-1, or both.
iPCs express Blimp-1 and Bcl-6 mutually exclusively and the minority expressing both are maintained by p18 and Bcl-xL. (A) qPCR analysis of relative mRNA levels in sorted p18+/+ and p18−/− populations. (B) Single-cell qPCR analysis of Bcl-6 and Blimp-1 expression in aBCs, iPCs, and PCs from wild-type, p18−/−, and Bcl-xLTg/+ mice on day 7 after NP-CGG immunization. Percentages were calculated from the total number of cells expressing Bcl-6, Blimp-1, or both.
iPCs express either Blimp-1 or Bcl-6, and the minority expressing both is protected by p18 and Bcl-xL
The transient nature of iPCs, however, suggests that they may be molecularly heterogeneous. To address this possibility, we determined whether Blimp-1 and Bcl-6 were expressed at graded levels in individual iPCs by single-cell qPCR. Activated B cells expressed Bcl-6 only and PCs expressed Blimp-1 exclusively, as expected (Figure 4B). Most iPCs expressed either Blimp-1 or Bcl-6 fully and mutually exclusively independent of p18. However a minor proportion (∼9%) expressed both. This proportion was diminished (to 6%) in the absence of p18, correlating with a reduction in iPCs expressing Blimp-1 only (58% to 43%) and an increase in those expressing Bcl-6 only (33% to 51%; Figure 4B). Thus, despite uniformly high expression of CD138 and B220, iPCs consist of cells expressing Bcl-6 or Blimp-1 fully and mutually exclusively and a minority expressing both.
The reduction in Blimp-1+/Bcl-6+ iPCs in the absence of p18 further suggests that they may be direct targets of p18 cell-cycle control. Because Bcl-xL prolongs lymphocyte survival32 and increases antibody production when overexpressed under the control of the thymidine kinase (TK) promoter and the Ig heavy chain (5′) enhancer (supplemental Figure 2),24 we asked whether the Blimp-1+/Bcl-6+ iPCs are preferentially protected by Bcl-xL. Indeed, expressing one copy of the TK-Bcl-xL transgene expanded iPCs markedly (9%-14%; Figure 4B). Given that the TK promoter is active only in S phase, the Blimp-1+/Bcl-6+ iPCs are likely the most rapidly cycling iPCs in dynamic transition from expressing Bcl-6 only to expressing Blimp-1 exclusively. They are protected by p18 and by Bcl-xL, further suggesting a role of Bcl-2 family genes in cell-cycle control of iPC homeostasis.
Bcl-xL overexpression or loss of Bim promotes iPC survival
Loss of p18 markedly enhanced iPC apoptosis (Figure 1F). Coordinated with increased p18 expression (Figure 1G), Bcl-xL and the proapoptotic BH3-only Bim, known to be required for homeostatic control of B cells and PCs,22 were increased progressively during B-cell activation and differentiation to iPCs and PCs (Figure 5A). By contrast, Mcl-1 was elevated significantly only in PCs, which is consistent with its role in promoting PC survival,33 and Bcl-2 was reduced progressively in iPCs and PCs (Figure 5A). These results demonstrate that Bcl-2 family genes are temporally regulated in B-cell activation and PC differentiation. The coordinated increase in Bim and Bcl-xL in iPCs and PCs further imply that they may mediate p18 cell-cycle control of apoptosis in PC differentiation.
Regulation and role of Bcl-xL, Bim, and Noxa in PC differentiation. (A) qPCR analysis of mRNA levels in sorted p18+/+ and p18−/− populations on day 7 after NP-CGG immunization. (B) Percentage of live aBCs, iPCs, and PCs in individual Bim+/+ and Bim+/− mice (P < .05, n = 4). (C) Percentage of live and TO-PRO-3+ aBCs, iPCs, and PCs in individual Bcl-xL+/+ and Bcl-xLTg/Tg mice (P < .001, n = 4); and (D) Total live aBCs, iPCs, and PCs in each spleen and percentage of live and TO-PRO-3+ cells in Noxa+/+ and Noxa−/− mice (P < .05, n = 4).
Regulation and role of Bcl-xL, Bim, and Noxa in PC differentiation. (A) qPCR analysis of mRNA levels in sorted p18+/+ and p18−/− populations on day 7 after NP-CGG immunization. (B) Percentage of live aBCs, iPCs, and PCs in individual Bim+/+ and Bim+/− mice (P < .05, n = 4). (C) Percentage of live and TO-PRO-3+ aBCs, iPCs, and PCs in individual Bcl-xL+/+ and Bcl-xLTg/Tg mice (P < .001, n = 4); and (D) Total live aBCs, iPCs, and PCs in each spleen and percentage of live and TO-PRO-3+ cells in Noxa+/+ and Noxa−/− mice (P < .05, n = 4).
Loss of one copy of Bim resulted in a 2-fold increase in iPCs and a greater (4-fold) increase in PCs by day 7 of the TD response (Figure 5B), suggesting that Bim promotes apoptosis in cycling iPCs but more effectively in the noncycling PCs. Overexpression of a Bcl-xL transgene in S phase24 led to a 7-fold increase in iPCs because of attenuation of apoptosis and increased PCs (2-fold) and secretion of NP-specific IgG (4-fold; Figure 5C and supplemental Figure 2). Apoptosis of iPCs is therefore is modulated in part by Bim and Bcl-xL.
Noxa is repressed by p18 for homeostatic control of cycling B cells and iPCs
Bim and Bcl-xL, however, were not regulated by p18 (Figure 5A). To understand the basis for enhanced apoptosis in p18−/− iPCs, we focused on Noxa, a proapoptotic BH3-only Bcl-2 family gene34 whose expression is specific to S phase in B-lineage tumor cells (X.H., M.D.L., and S.C.-K., unpublished data, October 1, 2009). Undetectable in resting B cells, Noxa expression was induced in activated B cells and further increased iPCs (4-fold) before it declined in PCs in the TD response. The absence of p18 augmented Noxa expression in iPCs (8-fold) and PCs (3-fold; Figure 5A). Noxa, therefore, is preferentially expressed in rapidly cycling iPCs and repressed by p18 in the TD response.
Loss of Noxa amplified the expansion of activated B cells, iPCs, and PCs, and the ∼3-fold increase in Noxa−/− iPCs occurred because of attenuation of apoptosis (Figure 5D). Thus, Noxa is required for apoptotic control of activated B cells and rapidly cycling iPCs in PC differentiation.
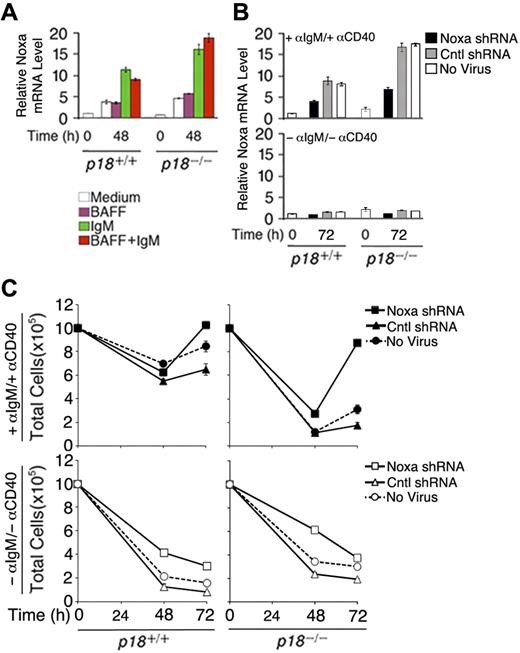
To verify that Noxa expression is induced by cell-cycle activation, resting B cells were stimulated with anti-IgM, which induces cell-cycle entry and progression to S phase by 48 hours, or BAFF (BLyS), which induces partial G1 progression but not S-phase entry26 while attenuating apoptosis independent of the cell cycle,25 or both. Compared with cells left in the media, Noxa mRNA was increased 4-fold by anti-IgM stimulation, alone or in combination with BAFF, but not by BAFF (Figure 6A). Correlating with accelerated S-phase entry and apoptosis,26 the absence of p18 augmented the induction of Noxa by anti-IgM (Figure 6A). These data provide the first direct evidence that Noxa is induced in S phase and repressed by p18 in primary B cells in vitro and in the TD response.
Noxa mediates cell-cycle control of apoptosis. (A) qPCR analysis of relative Noxa mRNA in p18+/+ and p18−/− rBCs (A) before and after stimulation with BAFF or anti-IgM for 48 hours and (B) before and after stimulation with anti-IgM and anti-CD40 for 72 hours and infection with a Noxa shRNA- or nonsense shRNA (Cntl)-expressing retrovirus or left uninfected. (C) Total live cells from cultures in panel B at 48 and 72 hours after shRNA-retrovirus infection.
Noxa mediates cell-cycle control of apoptosis. (A) qPCR analysis of relative Noxa mRNA in p18+/+ and p18−/− rBCs (A) before and after stimulation with BAFF or anti-IgM for 48 hours and (B) before and after stimulation with anti-IgM and anti-CD40 for 72 hours and infection with a Noxa shRNA- or nonsense shRNA (Cntl)-expressing retrovirus or left uninfected. (C) Total live cells from cultures in panel B at 48 and 72 hours after shRNA-retrovirus infection.
To further confirm that Noxa is required within B cells for cell-cycle control of apoptosis, we knocked down Noxa expression during activation of primary resting B cells with anti-IgM and anti-CD40 by using an shRNA-expressing retrovirus. Regardless of p18 expression, induction of Noxa expression by anti-IgM and anti-CD40 was reduced more than 2-fold by 72 hours of infection with the Noxa shRNA-expressing retrovirus but not with a nonsense shRNA-expressing virus (Cntl) or in the absence of viral infection (Figure 6B). This led to a modest increase in the number of activated p18+/+ cells but restored the number of activated p18−/− B cells, which was drastically reduced earlier (48 hours) before shRNA knockdown could antagonize the activation of Noxa by anti-IgM and anti-CD40 (Figure 6B) and apoptosis26 (Figure 6C). Thus, Noxa expression is required within B cells to mediate cell-cycle control of apoptosis, in particular during unscheduled cell-cycle progression in the absence of p18.
Discussion
In this study, we have identified iPCs as rapidly cycling and apoptotic, transient precursors of the more stable, noncycling end-stage PCs. We demonstrate that the Cdk4/Cdk6-specific inhibitor p18 selectively acts in iPCs for homeostatic cell-cycle control of PC differentiation by repressing Noxa expression through attenuation of G1-S progression.
iPCs are generated dynamically in the TD antibody response and during in vitro differentiation of polyclonal IgG PCs by CD40L and cytokines. They secrete antigen-specific IgG at a low frequency, contingent on achieving a threshold of IgG synthesis, but are poised to mature to high IgG-secreting, noncycling PCs. In addition to expressing uniformly high levels of surface CD138 and B220, iPCs express Blimp-1, IRF-4, and CXCR4, characteristic of PCs. Expression of CXCR4 suggest that iPCs may home to the bone, where they serve as self-renewing precursors of CD138+/B220lo cells for further differentiation to long-lived PCs.17 The absence of p18-augmented CXCR4 expression on iPCs, which helps to explain the homing of p18 null CD138+ plasmacytoid cells to the bone marrow4 and suggests that G1 arrest is not a prerequisite for bone homing of PC precursors. iPCs retain CD22, GL-7, and CD40, expression, thereby resembling the rapidly proliferating GC B cells,29 as well as Bcl-6 and Spi-B required for GC formation.10,11,31 On this basis, it is tempting to speculate that although iPCs may also be derived from the extrafollicular response early in the TD response, some of the iPCs generated in the TD response are PC precursors of GC origin.
We showed that iPCs cycle and turnover at an extraordinarily high rate, in striking contrast to PCs, which are G1-arrested and more stable. iPCs likely represent a molecularly defined subset of the broadly termed plasmablasts that express CD138, proliferate, and secrete Ig in TD and T-independent antibody responses.18–20 Plasmablasts are thought to turnover rapidly in situ.19,35 The relative turnover rate of PCs and plasmablasts has not been determined, but the instability of splenic plasmacytoid cells may largely be attributed to rapid turnover of iPCs, because end-stage PCs, having bypassed the iPC homeostatic checkpoint, are in fact quite stable.
We have previously shown that inhibition of Cdk4/Cdk6 and induction of G1 arrest by p18 is crucial for the generation of Ig-secreting PCs in vivo and in vitro.4,36 We now identify iPCs as a target for p18 cell-cycle control of PC differentiation. In theory, p18 could modulate PC differentiation by coupling the PC transcriptional circuitry to the cell cycle. At a first glance, the expression of transcription factors critical for PC differentiation seems to be graded from activated B cells to end-stage PCs, and at intermediate levels in iPCs independent of p18. However, single-cell qPCR analysis reveals that Blimp-1 and Bcl-6 mRNAs are expressed fully and mutually exclusively in the majority of iPCs, with a minority expressing both Blimp-1 and Bcl-6 mRNAs and these iPCs require p18 for maintenance.
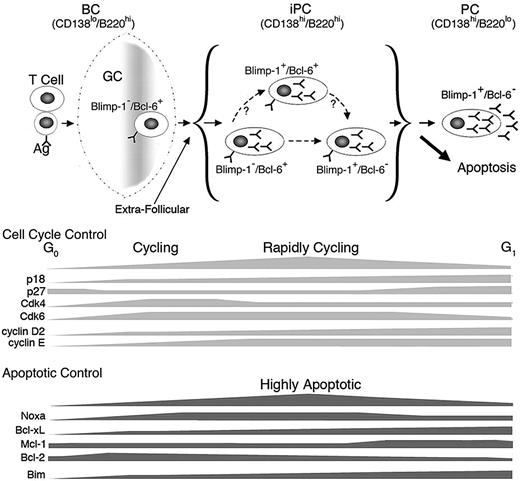
On this basis, we propose a working model for homeostatic cell cycle control of PC differentiation. Activated B cells differentiate to iPCs expressing high levels of both CD138 and B220 while retaining Bcl-6. They transiently gain Blimp-1 expression, which rapidly give rise to iPCs expressing Blimp-1 only by repressing Bcl-6 expression. Loss of B220 ensues during final differentiation to PCs expressing CD138. Induction of early G1 arrest by p18 and repression of Noxa promote the generation or maintenance of iPCs expressing both Blimp-1 and Bcl-6, thereby facilitating timely differentiation to PCs (Figure 7).
Model for homeostatic cell-cycle control of PC differentiation. PCs are generated via the GC reaction or the extrafollicular response in a TD response. iPCs are transient intermediates between aBCs and PCs and consist of 3 populations: Blimp-1−/Bcl-6+, Blimp-1+/Bcl-6+, and Blimp-1+/Bcl-6−. p18 and Noxa mediate cell-cycle control of iPC homeostasis during differentiation to end-stage PCs.
Model for homeostatic cell-cycle control of PC differentiation. PCs are generated via the GC reaction or the extrafollicular response in a TD response. iPCs are transient intermediates between aBCs and PCs and consist of 3 populations: Blimp-1−/Bcl-6+, Blimp-1+/Bcl-6+, and Blimp-1+/Bcl-6−. p18 and Noxa mediate cell-cycle control of iPC homeostasis during differentiation to end-stage PCs.
Apart from a requirement for p18,4 cell-cycle control of PC differentiation is not well understood. We present evidence that the decision to cycle rapidly in iPCs or not at all in PCs rests on the temporal expression of positive and negative G1 cell-cycle regulators and the balance among them during the TD response. Undetected in resting B cells, p18 mRNA is induced in activated B cells and increased progressively in iPCs and PCs. By inhibiting the Cdk4/Cdk6 catalytic activities, p18 attenuates progression through early G1 and the release of E2F from phosphorylated Rb, which promotes transcriptional activation of cyclin E.37 Although p27 and p21 both inhibit Cdk2/cyclin E in late G1 and are repressed by Bcl-6,38,39 only p27 expression is restored on extinction of Bcl-6 expression in PCs. In PCs, in conjunction with silencing of CDK6, elevation of p27 and p18 defies high cyclin D2 and cyclin E expression to induce G1 arrest. In iPCs, the p18 level is insufficient to oppose Cdk4, Cdk6, and cyclin D2, and p27 and p21 are insufficient to impede the dramatic increase in cyclin E. This leads to rapid cycling. However, to maintain homeostasis, p18 acts as a “break” for cell cycle acceleration in particular in iPCs expressing both Blimp-1 and Bcl-6.
Our data further suggest that selective Bcl-2 family genes mediate cell-cycle control of apoptosis in iPCs. By binding to and neutralizing all antiapoptotic Bcl-2 family proteins,40 Bim is known to control homeostasis in BCR-signaling and IgG PCs.22,41 The iPC pool is expanded modestly by loss of Bim and more significantly by S-phase specific overexpression of Bcl-xL. Noxa promotes cytochrome c release indirectly by neutralizing antiapoptotic Mcl-1 and A-142 and by inducing Mcl-1 degradation.43 Noxa expression is cell cycle dependent: undetected in resting B cells, induced in activated B cells, and markedly elevated in rapidly cycling iPCs in vivo and by anti-IgM activation in vitro. Because A-1 is only marginally detected in iPCs (data not shown), expression of Noxa at high levels in cycling iPCs could enhance Mcl-1 degradation and the apoptotic functions of other proapoptotic Bcl-2 family proteins, especially Bim. Importantly, Noxa expression is markedly repressed by p18 attenuation of G1-S progression in iPCs in vivo and in anti-IgM activation of B cells in vitro. Furthermore, iPCs are expanded in Noxa−/− mice in the TD response, and knocking down Noxa expression by shRNA interference compensates for the enhancement of apoptosis in cycling p18−/− B cells.
Noxa is dispensable for development and has no known physiologic function.23 This study demonstrates, for the first time, that Noxa is regulated by the cell cycle and required to mediate cell-cycle control of apoptosis during PC differentiation in vivo. Because Noxa is similarly activated in S-phase in malignant B cells (X.H., M. Di Liberto, and S.C.-K., unpublished data, October 1, 2009) and is thought to mediate proteasome inhibitor killing of lymphoma and multiple myeloma cells in vitro,44–46 Noxa may similarly mediate cell-cycle control of cytotoxic killing in lymphoma and myeloma. Elucidating cell-cycle control of Noxa expression should provide new insights into cell-cycle control of homeostasis in B-cell immunity and cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Ian C. M. MacLennan and Theresa Lu for insightful suggestions; Dr Andreas Strasser for providing the Bim and Noxa deficient mice, and Dr Timothy Behrens for providing the Bcl-xL transgenic mice; Drs Maurizio Di Liberto, Eric Meffre, and Virginia Pedicord for helpful comments; and Suzhen Chen for technical assistance.
This work was supported by a National Institutes of Health T-32 predoctoral training grant to J.B., a Leukemia & Lymphoma Society postdoctoral fellowship to J.G., and National Institutes of Health grants AR 49436 and CA120531 to S.C.-K.
National Institutes of Health
Authorship
Contribution: J.B., J.G., X.H., L.K., K.-M.T., and S.C.-K. designed the experiments; J.B., J.G., X.H., L.K., Y.Z., and K.-M.T. performed the experiments; J.B., J.G., K.-M.T., and S.C.-K. analyzed the data; and J.B. and S.C.-K. prepared the manuscript.
The current affiliation for J.G. is the US Naval Medical Research Center Detachment, Lima, Peru.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Selina Chen-Kiang, Department of Pathology, Rm C-338, Weill-Cornell Medical College, 1300 York Ave, New York, NY 10065; e-mail: sckiang@med.cornell.edu.
References
Author notes
J.B. and J.G. contributed equally to this work.