Abstract
HIV infection can result in depletion of total CD4+ T cells and naive CD8+ T cells, and in the generation of dysfunctional effector CD8+ T cells. In this study, we show that naive CD8+ T cells in subjects with progressive HIV disease express low levels of CD8α and CD8β chains. Such naive CD8low T cells display broad signaling defects across the T-cell receptor complex, and their appearance correlates with generalized up-regulation of major histocompatibility complex class I (MHC-I) antigens on peripheral blood mononuclear cells (PBMCs). To explore a causal link between increased MHC-I up-regulation and the generation of naive CD8low T cells, we used the humanized SCID-hu Thy/Liv mouse model to show that HIV infection of the thymus and interferon α (IFNα) treatment alone result in MHC-I up-regulation and in the generation of dysfunctional CD3highCD8+CD4− single-positive 8 (SP8) thymocytes with low expression of CD8. We suggest that dysfunctional naive CD8low T cells are generated as a result of IFNα-mediated up-regulation of MHC-I on stromal cells in the thymus and antigen-presenting cells in the periphery, and that dysfunction in this naive compartment contributes to the immunodeficiency of HIV disease. This study is registered at www.clinicaltrials.gov as NCT00187512.
Introduction
Effector CD8+ T cells play a critical role in the prevention and control of viral infections. In HIV disease, however, their function appears to be inadequate: even in the presence of a high number of such effectors, virus spread usually proceeds unchecked, leading to progressive loss of CD4+ T cells and, ultimately, to immunodeficiency and death.1 For reasons that have not been fully elucidated, untreated HIV infection is associated with a progressive loss of both CD4+ and CD8+ naive and resting memory T-cell numbers over time2,3 as well as qualitative and quantitative changes in the CD8+ T-cell functions.4
HIV disease progression can be predicted by viral load as well as by the level of “chronic immune activation,” measured by levels of CD8+ T-cell activation5,6 and the increased production of proinflammatory cytokines.7 Among various proinflammatory cytokines that are associated with chronic immune activation and lentiviral disease progression, interferon α (IFNα) is perhaps best studied, although its effect in vivo is likely complex. Although this cytokine can block HIV replication when added to culture before infection, sustained high levels of IFNα and of IFN-inducible genes are associated with more rapid disease progression in simian immunodeficiency virus (SIV)–infected macaques,8 but not in nonpathogenic SIV infections.9,10
We have previously reported that HIV infection of the human thymus results in the production of IFNα that, in turn, leads to up-regulation of major histocompatibility complex class I (MHC-I) on thymocytes and on thymic epithelial cells (TECs)11 and to the generation of single-positive CD8 thymocytes (SP8) with low cell-surface expression of CD8 (CD8low SP8 thymocytes).12 In HIV-infected children, CD8low peripheral blood T cells were associated with poor responses to antigenic stimulation.12 Many studies using transgenic murine models13-15 have shown that CD8 expression is involved in fine-tuning of CD8+ T-cell responses in vivo and that CD8low T cells, when generated, are associated with defective T-cell responses in vitro and in vivo.15,16 Because T-cell receptor (TCR) activation exhibits exquisite functional sensitivity to CD8 cell-surface density,16-18 we hypothesized that CD8low SP8 and CD8low naive T cells may also display reduced functionality in the setting of HIV infection.
Here, we extend prior observations in humanized mice to HIV-infected human adults in various stages of HIV disease progression and treatment and find that progressive HIV disease is associated with MHC-I up-regulation in the peripheral immune system and the generation of CD8low T cells in the naive compartment. We show that naive CD8low T cells are functionally impaired in their early and late responses after TCR stimulation and that their prevalence is closely associated with high levels of chronic immune activation and generalized up-regulation of MHC-I cell-surface expression. Given data in the SCID-hu Thy/Liv mouse that such changes can also occur on direct administration of IFNα, we posit that these are IFN-mediated events that occur in the setting of chronic immune activation and that may play a role in crippling the host immune response against HIV.
Methods
Subjects
Cryopreserved peripheral blood mononuclear cells (PBMCs) were obtained from HIV-infected adults enrolled in the University of California, San Francisco (UCSF) SCOPE cohort or the UCSF Options cohort.6,19 Four separate studies of HIV-infected subjects from SCOPE and Options cohorts (study A to study D) contributed to this analysis, as described in Table 1 and in more detail in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All participants gave written, informed consent in compliance with the Declaration of Helsinki, using protocols approved by the Committee on Human Research, University of California, San Francisco.
Phenotypic and functional analysis in HIV-infected subject PBMCs
Immunophenotyping and calcium flux analyses in study A were performed simultaneously on cryopreserved PBMCs samples using 7-color multiparameter flow cytometry and the fluorescent calcium indicator Indo-1 AM (Molecular Probes) as described in Emu et al.19 Phosphokinase assays (study A) were performed in parallel on the remaining thawed PBMCs. The assay was carried out as described in Schweneker et al,20 with some modifications (see supplemental Methods). Immunophenotyping analyses in studies B and C were performed on cryopreserved PBMCs using 11-color multiparameter flow cytometry, as described in Loke et al21 and Favre et al.22 Finally, intracellular cytokine detection was performed on study D subjects, as described previously.19 Of note, samples from cohorts A and B were run on different instruments for technical reasons: a FACSDiVa cytometer (BD Biosciences) with a UV laser was required in cohort A for calcium flux analysis using the dye Indo-1, whereas an LSR-II cytometer (BD Biosciences) was used in cohorts B and C to increase antibody panels to 11 colors as well as sample processing using a high-throughput plate loader system. With the caveat that the superantigen Staphylococcus enterotoxin B (SEB) only stimulates T-cell clones expressing Vβ3, 12, 14, 15, 17, and 20, all of these stimuli are polyclonal in nature. Furthermore, in the case of SEB, we assumed that TCR signaling of subset Vβ naive CD8+ T cells was representative of a polyclonal stimulation of the naive CD8+ T-cell population in all HIV groups, because HIV disease progression appears to have limited influence on the naive TCR repertoire (see supplemental Methods for more details on each assay).
Viruses, implantation, and inoculation of SCID-hu Thy/Liv mice, and implant collection and viral load quantification
Virus strains, SCID-hu Thy/Liv mice, inoculation of the Thy/Liv implants with HIV and collection and viral load quantification were carried out as previously described12 (see supplemental Methods for more details). A total of 12 cohorts were included in this study with 15-40 SCID-hu Thy/Liv mice per cohort and for a total of 255 individual mice. Results were presented as an aggregate of 11 cohorts (224 animals) after inoculation with NL4-3, Ba-L, or JD HIV strains. A last cohort (31 animals) was inoculated or not at different time points to be analyzed on the same day after inoculation with NL4-3 (21 days), Ba-L (35 days), or JD (14 days) strains or treatment with Intron A (7 days; 1 × 106 units/day Schering recombinant human interferon “alfa-2b” once daily [200 μL] intraperitoneally for 6 days). Animal protocols were approved by the UCSF Institutional Animal Care and Use Committee.
Phenotypic and functional analysis in SCID-hu Thy/Liv mice
Phenotypic analysis of thymocytes.
Fresh thymocytes were stained with antibodies against CD3, CD4, CD8α, and MHC-I and, in some cohorts, with additional markers (see supplemental Methods), to measure the mean fluorescence intensity (MFI) of MHC-I in “double-positive” thymocytes (DP or CD3neg/dimCD4+CD8+) and median fluorescence intensity (MdFI) of CD8 in “single-positive” CD8 thymocytes (SP or CD3highCD4−CD8+) in each cohort. To compare the change of MHC-I and CD8 cell-surface expression from one cohort to another, the MFI (MHC-I) or MdFI (CD8) of each individual was converted into a fold-change over controls, corresponding to the ratio of MFI/MdFI over the average MFI/MdFI in mock-infected controls (RPMI 1640 medium). When presented by fold-change, mock-infected animals were not included in the linear regression analysis. Of note, groups of HIV-infected mice treated with 3TC showed low or no detectable viral loads, and presented the same phenotype as mock-infected control mice, as described.12 These groups were included in the linear regression analysis.
Tetramer/pentamer staining.
Fresh total thymocytes from a HLA-A*02+ SCID-hu Thy/Liv mouse cohort were stained with antibodies against CD3, CD4, CD8, and CD69, with the live/dead marker 7-aminoactinomycin D (7-AAD), and with phycoerythrin (PE)–conjugated HLA-A*0201/ELAGIGILTV (MelanA/MART-1 26-35) class I pentamers (ProImmune) or, as a negative control, iTAg HLA-A*0201/NLVPMVATV (HCMV/pp65 495-504) class I tetramer (Beckman Coulter).
Calcium flux analysis.
Calcium flux was assessed with the fluorescent calcium indicator Fluo-3 AM or Indo-1 AM (Molecular Probes). Calcium release was measured during fluorescence-activated cell sorting (FACS) analysis over time by the change of fluorescence intensity (Fluo-3 AM) or emission spectrum (Indo-1 AM). TCR activation was induced by adding streptavidin during FACS acquisition to cross-link biotinylated anti-CD3 antibodies, as well as anti-CD4 and anti-CD8 antibodies, as described in Schweneker et al20 (see supplemental Methods for more details).
Proliferation.
Proliferative responses in vitro were tracked with the intracellular CFSE dye (Molecular Probes) as described in Favre et al9 and Loke et al21 (see supplemental Methods for more details). The proliferation platform from FlowJo software (TreeStar) was used to refine the analysis with the following calculations: the % divided is the percentage of the cells of the original sample which divided; the proliferation index is the average number of divisions that those cells which divided underwent; the division index is the average number of divisions that a cell (that was present in the starting population) has undergone. These statistics are related in the following way: division index = (proliferation index)(% divided).
Statistical analysis
Nonparametric tests were used for all group analyses. Differences in variables between any 2 groups were analyzed using the Mann-Whitney U test. Differences between any 3 or more groups were analyzed by analysis of variance (ANOVA). For longitudinal analysis with 2 time points, a paired t test was used. Spearman rank correlation was used to determine correlations between variables.
Results
CD8low naive T cells are prevalent in the setting of HIV disease progression
Although previous studies have documented decreased levels of CD8α or CD8β cell-surface expression in peripheral CD8+ T cells of HIV-infected subjects,12,23,24 it has not been clear whether such levels were decreased equivalently in all CD8+ T-cell subpopulations. Because the relative proportion of naive and differentiated CD8+ T-cell subpopulations changes as a function of HIV disease progression,2,25 we examined cell-surface expression of CD8α and CD8β chains in peripheral blood CD8+ T-cell subpopulations of recently and chronically HIV-infected subjects in various stages of disease progression and treatment. Four studies (A to D) were performed from 2 cohorts of subjects, including a cohort of chronically HIV-infected subjects (SCOPE cohort) and a cohort of recently infected subjects (Options cohort; Table 1). Study A included 5 HIV-seronegative healthy donors (“Neg”), 10 immunologic “progressors” with high viral loads (“Prog. CD4 < 350”), and 10 virologically suppressed subjects on highly active antiretroviral therapy (“HAART”). Study B included 14 high-risk HIV-seronegative subjects, 14 subjects on HAART, and 48 untreated subjects at different stages of disease progression, including 10 long-term nonprogressors (“LTNP”), 10 “elite” viral controllers (subsequently referred to as “Controllers”), and 28 subjects with high viral loads (> 10 000 copies/mL) who had either early- (“Prog. CD4 > 350,” n = 14) or late-stage disease progression (“Prog. CD4 < 350,” n = 14). The expression of CD45RA and CD27 was used to discriminate distinct CD8+ T-cell subpopulations, including naive CD8+ T cells (CD45RAhighCD27high), central-memory (CM; CD45RA−CD27+), effector-memory (EM; CD45RA−CD27−), and terminally differentiated RA+ effector (TEMRA; CD45RA+CD27−) CD8+ T cells (Figure 1A).
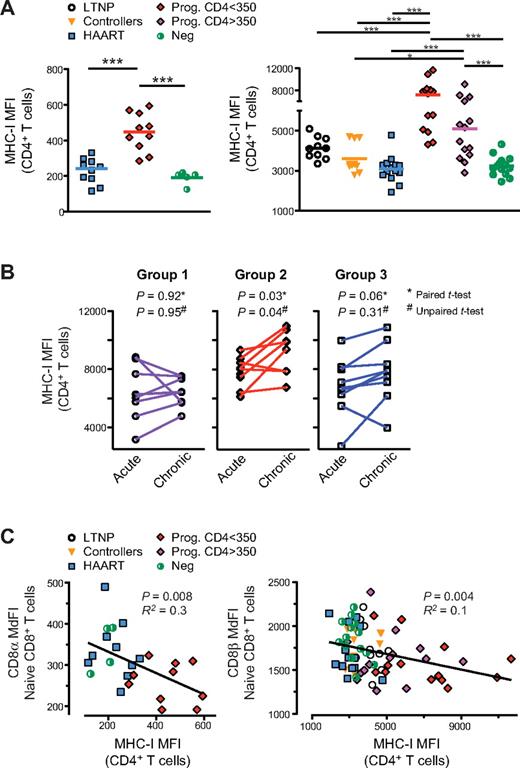
Naive CD8low T cells are prevalent in untreated HIV-infected human progressors. Cryopreserved PBMCs of HIV-negative and HIV-infected subjects in various stages of disease progression, viral control, and treatment were analyzed for cell-surface expression of the CD8α and CD8β chains in the naive pool of CD8+ T cells. 3 separate studies (study A, B, and C) were performed with samples from subjects of SCOPE and Options cohorts (see Table 1 and “Subjects” and supplemental Methods for more details and definitions of these groups). (A) FACS plot representing gating strategy, CD4 and CD8 expression in CD3+ T cells, and CD45RA and CD27 expression in CD8+ T cells (left to right panels). Naive (CD45RAhighCD27high) CD8+ T cells are shown from a subject with progressive disease (“Prog”), from a virologically suppressed subject (“HAART”) and from a HIV-negative subject (Neg) from study A (as indicated by the blue rectangle) with measurement of CD8α expression (MdFI). (B) CD8α expression (MdFI) in naive CD8+ T cells in all subjects from study A. (C) CD8α (top) and CD8β (bottom) expression in naive CD8+ T cell in all subjects from study B. (D) Grouping strategy of subjects in study C, based on viral load (“VL”) and immune activation (“IA” measured by CD38 expression) at approximately 12 months after infection. (E) Expression of CD8α (higher) and CD8β (lower) in naive CD8+ T cell over time, as measured in subjects at 3 and 12 months after infection (“acute” and “chronic,” respectively) from group 1 to 3 in study C (left to right). P values were calculated using the Mann-Whitney test for group analysis as indicated by the horizontal bar (B) or compared with HIV-Neg group when not indicated (C). The paired t test was used for longitudinal follow-up of the same subject (E). When the P value is not indicated on the graph, the symbols * and ** indicate that P < .05 or P < .005 for each test, respectively.
Naive CD8low T cells are prevalent in untreated HIV-infected human progressors. Cryopreserved PBMCs of HIV-negative and HIV-infected subjects in various stages of disease progression, viral control, and treatment were analyzed for cell-surface expression of the CD8α and CD8β chains in the naive pool of CD8+ T cells. 3 separate studies (study A, B, and C) were performed with samples from subjects of SCOPE and Options cohorts (see Table 1 and “Subjects” and supplemental Methods for more details and definitions of these groups). (A) FACS plot representing gating strategy, CD4 and CD8 expression in CD3+ T cells, and CD45RA and CD27 expression in CD8+ T cells (left to right panels). Naive (CD45RAhighCD27high) CD8+ T cells are shown from a subject with progressive disease (“Prog”), from a virologically suppressed subject (“HAART”) and from a HIV-negative subject (Neg) from study A (as indicated by the blue rectangle) with measurement of CD8α expression (MdFI). (B) CD8α expression (MdFI) in naive CD8+ T cells in all subjects from study A. (C) CD8α (top) and CD8β (bottom) expression in naive CD8+ T cell in all subjects from study B. (D) Grouping strategy of subjects in study C, based on viral load (“VL”) and immune activation (“IA” measured by CD38 expression) at approximately 12 months after infection. (E) Expression of CD8α (higher) and CD8β (lower) in naive CD8+ T cell over time, as measured in subjects at 3 and 12 months after infection (“acute” and “chronic,” respectively) from group 1 to 3 in study C (left to right). P values were calculated using the Mann-Whitney test for group analysis as indicated by the horizontal bar (B) or compared with HIV-Neg group when not indicated (C). The paired t test was used for longitudinal follow-up of the same subject (E). When the P value is not indicated on the graph, the symbols * and ** indicate that P < .05 or P < .005 for each test, respectively.
We quantified CD8α and CD8β cell-surface expression on subset naive and differentiated CD8+ T cells by flow cytometry using anti-CD8α and anti-CD8αβ anti–human antibodies and by measuring the MdFI on live CD3+ T cells (see “Phenotypic and functional analysis in HIV-infected subject PBMCs” and supplemental Methods). As in the case of HIV infection of the human thymus,12 CD8α and CD8β cell-surface expression (as measured by median fluorescence intensity [MdFI]) was decreased in total CD8+ T cells from HIV-infected subjects with progressive disease compared with HIV-negative donors (data not shown and supplemental Figure 1A for CD8β in study B).23,24 As expected,2,25 the composition of the CD8+ T-cell compartment was also altered, with an increase in the proportion of EM T cells and a corresponding decrease in the proportion of naive CD8+ T cells (P < .05, supplemental Figure 1B). When CD8α and CD8β expression was measured in each of these subpopulations, lower levels were observed in differentiated effector cells (eg, EM and TEMRA) compared with naive and CM pools (data not shown and supplemental Figure 1C, P < .05 for CD8β in study B), in the presence or absence of HIV infection. These results suggest that, in the setting of progressive HIV disease, low expression of CD8 on the total CD8+ T-cell population could simply be due to an increased fraction of EM cells with low levels of CD8 expression. However, when the expression of CD8α and CD8β was examined on CD8+ T-cell subpopulations between groups of subjects, it was evident that disease progression was associated with a uniquely low level of CD8 expression on naive CD8+ T cells, but not memory cells. HAART partially reversed this process because, in study A, low levels of CD8 expression were observed only on naive CD8+ T cells of progressors (Figure 1B, P = .03 and P = 0.009 for progressors vs HIV negatives and for progressors vs HAART, respectively). Analysis of CD8α and CD8β expression levels in study B confirmed that naive CD8low T cells were prevalent in early- and late-stage HIV disease progressors (with CD4 > and < 350, respectively) compared with the matched group of high-risk HIV-negative donors (Figure 1C, P < .05). As observed in study A, naive CD8low T cells were not found in treated subjects (HAART). We also failed to find consistent evidence of CD8low T cells in the untreated HIV-positive controllers and long-term nonprogressors (LTNPs; Figure 1C, P > .05 comparing the controllers or LTNPs to HIV seronegatives).
To determine whether the phenotype of low CD8 expression on naive CD8+ T cells might accompany other known predictors of disease progression, PBMCs from 27 subjects were studied at 2 time points after acute infection: one at an early time estimated to be approximately 3 months after infection (“acute”) and another estimated to be at 12 months after infection, that is, at a time when viral and immunologic set-points are established (“chronic”; study C from Options cohort). Given our previous results that viral load (VL) and levels of T-cell immune activation (IA; measured by cell-surface expression of CD38 in CD8+ T cells) are independent predictors of disease progression at this “chronic” stage,6 these subjects were subdivided into 3 groups (Table 1, Figure 1D, and supplemental Figure 2A): group 1 had low VL and low IA; group 2, high VL and high IA; and group 3, high VL but low IA (see “Subjects” and supplemental Methods for more detailed description). Predictably6 those in group 2 showed more rapid increases in VL and decreases in CD4+ T-cell counts in the absence of antiretroviral treatment than did those in groups 1 and 3 (supplemental Figure 2B-C, respectively). Based on this grouping strategy and prior results from cross-sectional studies A and B (above), we hypothesized that the more rapid disease progression evident in group 2 (compared with group 1 and group 3) subjects would be associated with more rapid down-regulation of CD8 expression in naive CD8+ T cells after acute infection. Indeed, comparing 3 and 12 months after infection, both CD8α and CD8β expression were significantly decreased in group 2 (P = .05 and P = .01, respectively, by paired t test) but not in groups 1 and 3 (P > .05; Figure 1E). Altogether, these results suggest that naive CD8low T cells are generated in the setting of high VL and high IA and that the presence of such cells is a consistent correlate of HIV disease progression.
CD8low naive T cells are dysfunctional with respect to Ca2+ flux, phosphorylation of p38MAPK, and IL-2 cytokine secretion
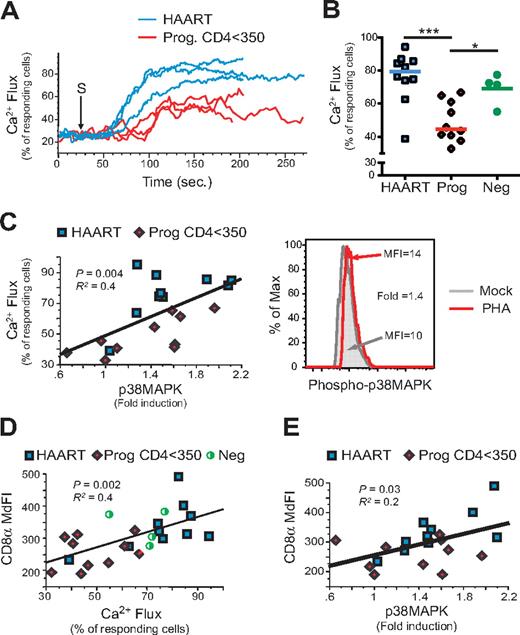
Given previous reports that the surface density of CD8 can contribute to the binding affinity between the TCR and peptide-MHC complexes16,18,26 and that down-regulation of CD8 is associated with impaired T-cell function,27 the ability of naive CD8low T cells to signal across the TCR was assessed. First, calcium flux was measured in each of the 4 different CD8+ T-cell subpopulations after cross-linking with anti-CD3 and anti-CD8 antibodies. As shown in Figure 2A, naive CD8+ T cells from 3 untreated progressors (Prog. CD4 < 350) showed a lower percentage of responding cells compared with naive CD8+ T cells from 3 virologically suppressed (HAART) subjects. When all subjects were analyzed in aggregate (Figure 2B), the difference in the response of naive CD8+ T cells between progressors and HAART subjects was highly significant (P = .0002).
Naive CD8low T cells from untreated HIV-infected subjects are impaired with respect to calcium flux response and phosphorylation of p38MAPK after TCR stimulation. Calcium flux and the level of phosphorylation of p38MAPK were measured by flow cytometry in naive (CD45RAhighCD27high) CD8+ T cells from PBMCs of study A (described in Figure 1B). Calcium flux was measured after TCR/CD8 cross-linking, and phosphorylation of p38MAPK was measured by the fold induction of p38MAPK phosphorylation from PHA-stimulated compared with mock-stimulated samples (see “Phenotypic and functional analysis in HIV-infected subject PBMCs” and supplemental Methods and Schweneker et al20 ). (A-C) Calcium flux responses over time were determined by the percentage of responding naive CD8+ T cells over 75% of the baseline level (prior TCR stimulation). Calcium flux responses are shown in 3 HAART and 3 Prog. CD4 < 350 (A) and for all subjects (B), and were correlated with the fold-induction of phopho-p38MAPK (C left) and shown in a representative example (C right). (D-E) Finally, both calcium flux (D) and phospho-p38MAPK (E) responses were correlated with the CD8α expression in naive (CD45RAhighCD27high) CD8+ T cells. P values are indicated for Spearman rank correlation test. R2 is also indicated (from the Pearson coefficient of correlation).
Naive CD8low T cells from untreated HIV-infected subjects are impaired with respect to calcium flux response and phosphorylation of p38MAPK after TCR stimulation. Calcium flux and the level of phosphorylation of p38MAPK were measured by flow cytometry in naive (CD45RAhighCD27high) CD8+ T cells from PBMCs of study A (described in Figure 1B). Calcium flux was measured after TCR/CD8 cross-linking, and phosphorylation of p38MAPK was measured by the fold induction of p38MAPK phosphorylation from PHA-stimulated compared with mock-stimulated samples (see “Phenotypic and functional analysis in HIV-infected subject PBMCs” and supplemental Methods and Schweneker et al20 ). (A-C) Calcium flux responses over time were determined by the percentage of responding naive CD8+ T cells over 75% of the baseline level (prior TCR stimulation). Calcium flux responses are shown in 3 HAART and 3 Prog. CD4 < 350 (A) and for all subjects (B), and were correlated with the fold-induction of phopho-p38MAPK (C left) and shown in a representative example (C right). (D-E) Finally, both calcium flux (D) and phospho-p38MAPK (E) responses were correlated with the CD8α expression in naive (CD45RAhighCD27high) CD8+ T cells. P values are indicated for Spearman rank correlation test. R2 is also indicated (from the Pearson coefficient of correlation).
To further investigate the mechanisms underlying dysfunction of the CD8low T-cell compartment, FACS analysis was coupled with antiphosphotyrosine antibodies to delineate the phosphorylation of key kinases involved in the Ras–mitogen-activated protein kinase (MAPK) pathway of TCR signaling (eg, Erk1/2 and p38MAPK) after cells were stimulated with phytohemagglutinin A (PHA). As detailed previously,20 phosphorylation responses were calculated as a fold-induction of MFI of phospho-p38MAPK or phospho-Erk1/2 in naive CD8+ T cells, comparing PHA-stimulated and unstimulated PBMCs. Phosphokinase and calcium flux experiments were conducted in parallel on the same samples, enabling direct comparison of CD8 expression levels, calcium flux responses, and the fold-induction of phosphorylation in CD8+ naive T cells. Decreased phosphorylation of p38MAPK in naive CD8+ T cells was associated with poor calcium flux (Figure 2C, P = 0.004) and normal levels of Erk1/2 phosphorylation (data not shown). Furthermore, CD8α expression in naive CD8+ T cells was directly correlated with impaired function as measured by lower levels of calcium flux (Figure 2D, P = .002) and of phosphorylation of p38 MAPK (Figure 2E, P = .03; but normal levels of Erk1/2 phosphorylation [data not shown]).
Finally, we addressed whether naive CD8low T cells were dysfunctional with respect to their ability to produce IL-2 (the principal cytokine expressed by naive CD8+ T cells). IL-2 and IFNγ production were assessed (using 8-color FACS analysis of naive CD45RA+CD27+CCR7+CD28+CD8+ T cell; Figure 3A) after superantigen (SEB) stimulation of cells from 10 virologically suppressed HAART subjects and 16 progressors with high VL (> 10 000 copies/mL) and CD4 counts < 350 cells/μL (Figure 3A; study D and also described previously).19 As expected, naive CD8+ T cells from both groups of subjects demonstrated very low IFNγ responses (Figure 3B) and low IL-2 responses (1%- to 3%-positive events) compared with memory CD8+ T cells (15%-20% for CM; data not shown). Notably, however, naive CD8+ T cells from progressors displayed significantly lower (P = .002) IL-2 responses compared with those of virologically suppressed HAART subjects (Figure 3B). In aggregate, these data indicate that HIV-mediated changes in CD8 expression correlate with the presence of dysfunctional naive CD8+ T cells.
CD8low CD8 naive T cells from untreated HIV-infected subjects are functionally impaired with respect to IL-2 secretion after TCR stimulation. PBMCs from subjects in study D, including 16 Prog. CD < 350 and 10 HAART subjects, were stimulated or not by the superantigen SEB to determine IFNγ and IL-2 intracellular cytokine response. Cells were stimulated overnight and analyzed for cytokine secretion in combination with surface staining for CD3, CD4, CD8, CD45RA, CD27, CD28, and CCR7. (A) Ethidium monoazide (EMA) labeling was also used to discriminate live from dead cells. (B) The frequency of IL-2 but not IFNγ-responding cells was determined in naive CD8+ T cells (EMA−CD3+CD8+CD45RA+CD27+CCR7+CD28+). P values were calculated using Mann-Whitney test for group analysis as indicated by the horizontal bar.
CD8low CD8 naive T cells from untreated HIV-infected subjects are functionally impaired with respect to IL-2 secretion after TCR stimulation. PBMCs from subjects in study D, including 16 Prog. CD < 350 and 10 HAART subjects, were stimulated or not by the superantigen SEB to determine IFNγ and IL-2 intracellular cytokine response. Cells were stimulated overnight and analyzed for cytokine secretion in combination with surface staining for CD3, CD4, CD8, CD45RA, CD27, CD28, and CCR7. (A) Ethidium monoazide (EMA) labeling was also used to discriminate live from dead cells. (B) The frequency of IL-2 but not IFNγ-responding cells was determined in naive CD8+ T cells (EMA−CD3+CD8+CD45RA+CD27+CCR7+CD28+). P values were calculated using Mann-Whitney test for group analysis as indicated by the horizontal bar.
Naive CD8low T cells are associated with MHC-I up-regulation in HIV-infected subjects
Many prior reports indicate that HIV infection triggers chronic inflammation characterized by increased circulating levels of proinflammatory cytokines.7 We have previously shown that HIV infection of the human thymus induces IFNα expression by plasmacytoid dendritic cells (pDCs) which, in turn, results in MHC-I up-regulation and the generation of CD8low SP8 thymocytes.11 To determine whether the generation of naive CD8low T cells in HIV-infected subjects also occurs in association with generalized MHC-I up-regulation, peripheral blood cells were evaluated from HIV-seropositive and -seronegative subjects in studies A and B. Data from these individuals showed that MHC-I cell-surface expression (measured by MHC-I MFI) was substantially increased in CD4+ T cells (Figure 4A) as well as all other populations of PBMCs, including CD8+ T cells, CD3-negative lymphocytes (including B and NK cells), and monocytes (supplemental Figures 3A and data not shown) from untreated progressors with early- (CD4 > 350 cells/μL) and late- (CD4 < 350 cells/μL) stage disease compared with other groups (eg, LTNP, controllers, HAART-suppressed and HIV-negative healthy controls). Such MHC-I up-regulation was directly correlated with increased immune activation (measured by CD38 expression on CD8+ T cells; supplemental Figures 3B-C, and data not shown) as well as decreased peripheral blood CD4+ and naive CD8+ T-cell counts (supplemental Figure 3D and data not shown; P < .005). To determine whether MHC-I expression correlated with more rapid disease progression during early HIV infection, we measured MHC-I MFI in CD4+ T cells at 3 and 12 months after infection in subjects within study C. Results showed that MHC-I MFI was significantly increased between these time points in subjects with high IA and high VL set-points, both on paired as well as per-group analyses, (ie, group 2, P = .03 by paired t test and P = .04 by unpaired t test) but not in subjects with low IA and low (ie, group 1) or high (ie, group 3) VL set-points (P > .05 by paired t test and by unpaired t test; Figure 4B). These results confirm prior observations that HIV disease progression is associated with increased levels of CD8+ T-cell activation (as defined by CD38 and other markers)6 and with a decline in the CD8+ naive T-cell count.23,24 They also demonstrate that there is generalized increased expression of MHC-I on PBMCs, despite the documented Nef-mediated down-regulation of MHC-I that occurs in HIV-infected cells in vitro.11 Finally, by linear regression analysis, we found that the degree of CD8α and CD8β down-regulation on naive CD8+ T cells was directly correlated with the degree of MHC-I up-regulation on CD4+ T cells (Figure 4C, P ≤ .008; and other PBMC populations [P < .05 for each pairwise comparison; data not shown]).
In HIV-infected subjects, generalized up-regulation of cell-surface MHC-I in PBMCs is associated with disease progression, chronic immune activation, and naive CD8low T cells. MHC-I cell-surface expression was measured by the MFI of MHC-I in PBMCs from subjects in studies A, B, and C, as shown here in CD4+ T cells. (A) MHC-I cell-surface expression in study A (left) and study B (right); of note, differences in MHC-I MFI measurements between study A and B are because of the use of different flow cytometers with different instrument settings. (B) MHC-I cell-surface expression in CD4+ T cells from study C at 3 and 12 months infection (“acute” and “chronic,” respectively). (C) Correlation between MHC-I cell-surface expression and CD8α expression in naive CD8+ T cells in study A (left) and study B (right). P values were calculated using the Mann-Whitney test for group analysis as indicated by the horizontal bar (A). Two-tailed paired and unpaired t tests were used for longitudinal follow-up of the same subject and for group analysis, respectively (B). When the P value is not indicated on the graph, the symbols * and *** indicate that P < .05 or P < .0005 for each test, respectively. P values are indicated for Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
In HIV-infected subjects, generalized up-regulation of cell-surface MHC-I in PBMCs is associated with disease progression, chronic immune activation, and naive CD8low T cells. MHC-I cell-surface expression was measured by the MFI of MHC-I in PBMCs from subjects in studies A, B, and C, as shown here in CD4+ T cells. (A) MHC-I cell-surface expression in study A (left) and study B (right); of note, differences in MHC-I MFI measurements between study A and B are because of the use of different flow cytometers with different instrument settings. (B) MHC-I cell-surface expression in CD4+ T cells from study C at 3 and 12 months infection (“acute” and “chronic,” respectively). (C) Correlation between MHC-I cell-surface expression and CD8α expression in naive CD8+ T cells in study A (left) and study B (right). P values were calculated using the Mann-Whitney test for group analysis as indicated by the horizontal bar (A). Two-tailed paired and unpaired t tests were used for longitudinal follow-up of the same subject and for group analysis, respectively (B). When the P value is not indicated on the graph, the symbols * and *** indicate that P < .05 or P < .0005 for each test, respectively. P values are indicated for Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
HIV infection of the human thymus results in up-regulation of MHC-I and to the selection of dysfunctional CD8low SP8 thymocytes
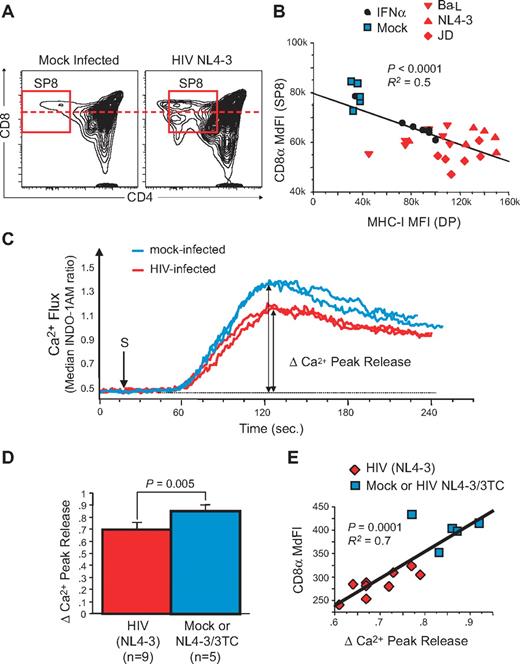
To further delineate the relationship between MHC-I up-regulation and the generation of dysfunctional naive CD8low T cells during HIV infection in vivo, we turned our attention to experimental infection of the SCID-hu Thy/Liv mouse. In previous experiments, HIV infection of the human thymic implant of such animals was found to lead to IFNα-induced up-regulation of MHC-I on thymocytes and thymic epithelial cells11 and to the selection of SP8 thymocytes that express low levels of CD8 (CD8low SP8 thymocytes) and that were less responsive to antigenic stimulation in HIV-infected pediatric patients.11,12 We have now extended these observations to an analysis of HIV infection in 12 separate cohorts of (a total of 255) SCID-hu Thy/Liv mice. In each experiment (data not shown) as well as in 11 experiments combined (supplemental Figure 4A), there was a highly significant (P < .0001) correlation between VL in the implants (as measured by HIV RNA content per million total thymocytes) and the fold-increase (relative to mock-infected controls) of MHC-I expression on CD3neg/dimCD4+CD8+ (double-positive [DP]) thymocytes. MHC-I up-regulation, in turn, was associated with a decrease in the MdFI of CD8 on SP8 thymocytes. As shown in Figure 5A, the decrease in CD8 MdFI was in some cases associated with the appearance of a discrete subpopulation of CD8low cells; in other cases, the fluorescence intensity of the entire CD8 SP8 population shifted downward in a unimodal manner. By linear regression analysis, the prevalence of CD8low SP8 thymocytes in infected Thy/Liv implants was inversely related to the fold-change of MHC-I (supplemental Figure 4B, P < .0001). This was the case whether the implants were infected with R5-tropic (eg, Ba-L), X4-tropic (eg, NL4-3), or dual-tropic (eg, JD) HIV, as shown as an example in one additional single cohort (Figure 5B). To confirm in this model that IFNα alone could solely cause such increases in MHC-I expression, SCID-hu Thy/Liv mice were treated for 6 days with IFNα (Intron A at 1 × 106 units/day). At day 7, CD8low SP8 thymocytes were also observed only when MHC-I up-regulation was evident on thymocytes (Figure 5B black circles).
HIV infection generates dysfunctional CD8low SP CD8 thymocytes in the SCID-hu Thy/Liv mouse model. Saline, or 1000 50% tissue culture infective dose (TCID50) of X4-tropic (NL4-3), R5-tropic (Ba-L), or dual-tropic X4-R5 (primary isolate JD) HIV were inoculated into individual cohorts of 30-40 SCID-hu Thy/Liv mice each. In most cohorts, several groups were also treated with antiretroviral drugs (eg, 3TC). Viral load (HIV RNA content per million total cells) as well as phenotypic and functional parameters were measured by flow cytometry in human thymocytes from each implant 3 to 7 weeks after inoculation. (A) FACS plot of SP8 thymocytes in Mock- and HIV-infected human thymus. (B) Correlation between CD8α expression in SP8 thymocytes and MHC-I expression in double-positive (DP) thymocytes from individuals within the same cohort of HLA-A2+ SCID-hu Thy/Liv mice, including animals inoculated with medium (Mock), HIV NL4-3, Ba-L, or JD, and compared with IFNα treatment alone. (C-E) Calcium flux response after TCR/CD8 cross-linking in SP8 thymocytes from a cohort of SCID-hu Thy/Liv mice, 21 days postinoculation with NL4-3: representative calcium flux kinetic from 2 mock-infected (blue line) or 2 NL4-3-infected mice (red line). (C) Calcium flux peak responses in all animals (D) and correlation with CD8α expression in SP8 thymocytes (E). P values were calculated using the Mann-Whitney test for group analysis and by Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
HIV infection generates dysfunctional CD8low SP CD8 thymocytes in the SCID-hu Thy/Liv mouse model. Saline, or 1000 50% tissue culture infective dose (TCID50) of X4-tropic (NL4-3), R5-tropic (Ba-L), or dual-tropic X4-R5 (primary isolate JD) HIV were inoculated into individual cohorts of 30-40 SCID-hu Thy/Liv mice each. In most cohorts, several groups were also treated with antiretroviral drugs (eg, 3TC). Viral load (HIV RNA content per million total cells) as well as phenotypic and functional parameters were measured by flow cytometry in human thymocytes from each implant 3 to 7 weeks after inoculation. (A) FACS plot of SP8 thymocytes in Mock- and HIV-infected human thymus. (B) Correlation between CD8α expression in SP8 thymocytes and MHC-I expression in double-positive (DP) thymocytes from individuals within the same cohort of HLA-A2+ SCID-hu Thy/Liv mice, including animals inoculated with medium (Mock), HIV NL4-3, Ba-L, or JD, and compared with IFNα treatment alone. (C-E) Calcium flux response after TCR/CD8 cross-linking in SP8 thymocytes from a cohort of SCID-hu Thy/Liv mice, 21 days postinoculation with NL4-3: representative calcium flux kinetic from 2 mock-infected (blue line) or 2 NL4-3-infected mice (red line). (C) Calcium flux peak responses in all animals (D) and correlation with CD8α expression in SP8 thymocytes (E). P values were calculated using the Mann-Whitney test for group analysis and by Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
The function of CD8low SP8 thymocytes in HIV-infected Thy/Liv implants was next assessed by calcium flux after TCR cross-linking with anti-CD3 and anti-CD8 antibodies. As shown in the representative examples of Figure 5C, peak calcium flux in cells from HIV-infected Thy/Liv implants was found to be lower than that observed in mock-infected implants. This change was statistically significant when analyzed across all samples (Figure 5D, P = .005), comparing cells from HIV-infected implants with those obtained from implants that had either been mock-infected or, alternatively, infected and treated with the antiretroviral agent, 3TC. Moreover, SP8 thymocytes that had the lowest levels of calcium flux also had the lowest levels of CD8 (Figure 5E, P = .0001).
The proliferation status of anti-CD3/anti-CD28-stimulated cells was assessed in parallel using carboxyfluorescein succinimidyl ester (CFSE) dilution (Figure 6). In preliminary experiments (data not shown), CD8low SP8 thymocytes from HIV-infected Thy/Liv implants were found to proliferate less well than those from mock-infected implants. To exclude the possibility that such a change in proliferation might be because of a deficiency of IL-2 in the culture (eg, secondary to the loss of thymocyte subpopulations that secrete IL-2), SP8 thymocytes (enriched after bead depletion of CD4+ thymocytes) were stimulated with plate-bound anti-CD3 antibodies and soluble anti-CD28 antibodies in the presence of IL-2. Even under these conditions, the proliferative capacity of CD8low SP8 thymocytes from an HIV-infected Thy/Liv implant was lower than that observed in a mock-infected implant, in terms of percentage of divided cells (43.7% vs 65.9%), proliferative index (1.8 vs 2.8), and division index (0.79 vs 1.44; Figure 6A). This observation was borne out in larger numbers of animals: as shown in Figure 6B, the percentage of divided cells was lower in SP8 thymocytes obtained from HIV-infected Thy/Liv implants compared with that obtained with cells from mock-infected of infected/treated implants (P = .0001). As in the case of calcium flux, cell populations with the lowest level of expression of CD8α also showed the lowest levels of proliferation (Figure 6C, P = .0001).
CD8low SP8 thymocytes have impaired proliferative capacities after TCR stimulation. Thymi from SCID-hu Thy/Liv mice were processed 21 days after direct intrathymic inoculation of saline (mock) or HIV JD, as described in Figure 5. MHC-I MFI and CD8 MdFI were determined at day 0 on total fresh thymocytes. In parallel, CD3 and CD28 proliferative responses were assessed on purified SP8 thymocytes after 5 days by CFSE dilution. (A) CFSE proliferative responses (flow plots, higher), histogram representation (red curve, lower), and modeling (blue curve, lower) in SP8 thymocytes from representative examples of mock- or HIV-infected SCID-hu Thy/Liv mice, indicating the percentage of divided, proliferative index, and division index after proliferation, as well as CD8 expression at the starting day (Day 0). (B) Percent of divided SP8 thymocytes (modeling) in HIV-infected SCID-hu Thy/Liv mice (n = 8) compared with mock- and virally suppressed 3TC/HIV-infected animals (n = 9, P = .001) and (C) correlation to CD8 MdFI at day 0 (P = .0001). P values were calculated using the Mann-Whitney test for group analysis and by Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
CD8low SP8 thymocytes have impaired proliferative capacities after TCR stimulation. Thymi from SCID-hu Thy/Liv mice were processed 21 days after direct intrathymic inoculation of saline (mock) or HIV JD, as described in Figure 5. MHC-I MFI and CD8 MdFI were determined at day 0 on total fresh thymocytes. In parallel, CD3 and CD28 proliferative responses were assessed on purified SP8 thymocytes after 5 days by CFSE dilution. (A) CFSE proliferative responses (flow plots, higher), histogram representation (red curve, lower), and modeling (blue curve, lower) in SP8 thymocytes from representative examples of mock- or HIV-infected SCID-hu Thy/Liv mice, indicating the percentage of divided, proliferative index, and division index after proliferation, as well as CD8 expression at the starting day (Day 0). (B) Percent of divided SP8 thymocytes (modeling) in HIV-infected SCID-hu Thy/Liv mice (n = 8) compared with mock- and virally suppressed 3TC/HIV-infected animals (n = 9, P = .001) and (C) correlation to CD8 MdFI at day 0 (P = .0001). P values were calculated using the Mann-Whitney test for group analysis and by Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
Finally, to delineate the effects of MHC-I up-regulation on antigen-specific SP8 thymocytes, we studied the frequency and phenotype of SP8 thymocytes reactive against the HLA-A2–restricted self-antigen, Melan-A in HLA-A2+ Thy/Liv implants infected with various HIV isolates or treated with IFNα (Figure 7A). SP8 thymocytes and naive CD8+ T cells with TCRs specific for the self-peptide MelanA have been previously shown to be detectable in HLA-A*02+ individuals with frequencies of 0.07% and 0.04%, respectively.28 As shown in the representative flow cytograms of Figure 7A and the aggregate data of Figure 7B, a discrete (0.04%) subpopulation of HLA-A2+ SP8 thymocytes was observed to bind to HLA-A2-MelanA tetramers in mock-infected Thy/Liv implants. After infection with R5 HIV (Ba-L) or with X4 HIV (NL4-3), or after treatment of Thy/Liv mice with IFNα, this population was found to be diminished in size relative to mock-treated animals (Figure 7B). Interestingly, both the frequency of MelanA+ SP8 thymocytes (Figure 7C, P = .002) and the expression of CD8 on the remaining MelanA+ SP8 thymocytes (Figure 7D, P = .01) fell as MHC-I expression was up-regulated in HIV-infected or IFNα-treated implants. These data suggest that up-regulation of MHC-I in the thymus leads both to increased negative selection against antigen-specific SP8 thymocytes and to the selection of antigen-specific cells that have lower levels of CD8 expression.
Generation of CD8low SP8 thymocytes correlates with reduced frequency of Melan-A–specific thymocytes. SP8 thymocytes from HLA-A2+ SCID-hu Thy/Liv mice (see Figure 5B) were enriched by CD4 depletion on magnetic beads and stained with MelanA pentamers or control CMV tetramers. (A) Representative examples in each group. (B-D) Frequency of MelanA+ SP8 thymocytes for all thymi (B), correlation with MHC-I up-regulation on DP thymocytes (C), and correlation with CD8α expression in MelanA+ SP8 thymocytes (D). P values were calculated using the Mann-Whitney test for group analysis and by Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
Generation of CD8low SP8 thymocytes correlates with reduced frequency of Melan-A–specific thymocytes. SP8 thymocytes from HLA-A2+ SCID-hu Thy/Liv mice (see Figure 5B) were enriched by CD4 depletion on magnetic beads and stained with MelanA pentamers or control CMV tetramers. (A) Representative examples in each group. (B-D) Frequency of MelanA+ SP8 thymocytes for all thymi (B), correlation with MHC-I up-regulation on DP thymocytes (C), and correlation with CD8α expression in MelanA+ SP8 thymocytes (D). P values were calculated using the Mann-Whitney test for group analysis and by Spearman rank correlation for correlation test. R2 from the Pearson coefficient of correlation is also indicated.
Discussion
Functional impairments of CD8+ T cells have been characterized in many persistent viral infections, including HIV and hepatitis C virus (HCV) infection in humans, SIV infection in macaques, and lymphocytic choriomeningitis virus (LCMV) infection in mice.4,29 In all cases, such impairment has been manifest at the level of differentiated, memory/effector CD8+ T cells. Likewise, reports of functional defects in CD8+ T-cell signaling, cytokine production, granzyme/perforin production, and proliferation have all been studied in the context of cell subpopulations with an effector phenotype.4
Here, we show that functional defects may also be observed in the naive compartment of CD8+ T cells. In both the HIV-infected human with progressive disease and the HIV-infected SCID-hu Thy/Liv mouse, naive CD8+ T cells with low expression levels of CD8 were found to have defects in TCR signaling on stimulation with polyclonal activators such as anti-CD3, SEB, or PHA. Indeed, TCR signaling of both CD8low SP8 thymocytes from HIV-infected thymi and naive CD8low T cells from HIV-infected progressors was functionally impaired in vitro in calcium flux response, phosphorylation of p38MAPK, production of IL-2, and proliferation. These findings suggest that HIV infection generates CD8low SP8 thymocytes and peripheral CD8low naive T cells that are dysfunctional in the calcium/calmodulin/calcinurin/NFAT and MAPK pathways involved in IL-2 secretion and proliferation. Such defects were observed across the entire naive CD8+ T-cell compartment as suggested by the unimodal distribution of low CD8 cell-surface expression on naive CD8+ T cell from HIV-infected progressors. When studied at the antigen-specific level (eg, in the context of specific T cells), defects in TCR signaling were associated with an absolute loss of antigen-specific T cells and with the selection of SP8 thymocytes with low CD8 expression. Because the memory and effector CD8+ T-cell compartment is sustained in part by recruitment of naive CD8+ T cells,30 these observations suggest that functional impairment of the naive CD8+ T-cell compartment is related to HIV disease progression and may represent an underlying lesion of the immune system.
The loss of CD8+ naive T cells has been consistently observed during HIV progression in patients2 and in SIV-infected pathogenic primate models.31 Such losses have been attributed to the inability to produce such cells from progenitors in the bone marrow, thymus, and peripheral lymphoid system or to continuous recruitment to compensate for accelerated destruction of memory/effector cells in the periphery (eg, through chronic immune activation).1 Although we do not show evidence in this article that MHC-I up-regulation occurs in HIV-infected human tissues, we previously found sustained MHC-I up-regulation in all B, T, and monocytic cell compartments of lymphoid organs (eg, peripheral and mediastinal lymph nodes, spleen, and gut-associated lymphoid tissue) and in the thymus of SIV-infected macaques with progressive disease (D.F., R. Reyes, unpublished observations, April 2005) as well as increased IFN-signaling in rectosigmoid biopsies of HIV-infected patients.21 These observations suggest that MHC-I is also up-regulated in tissues from humans with progressive HIV disease. Here, and in a manner consistent with the “activation-threshold tuning” hypothesis,32 we speculate that CD8low naive T cells may be selected in the thymus or in secondary lymphoid organs through high avidity interactions with antigen presenting cells that have unusually high levels of MHC-I.
Indeed, several studies have demonstrated that pDCs are activated through TLR7 and TLR9 after exposure to HIV or to HIV-infected CD4+ cells, culminating in the secretion of IFNα and maturation to antigen-presenting cells.33 In this and a previous study,11 we have shown that HIV infection of the thymus also results in IFNα secretion which, in turn, is associated with up-regulation of MHC-I expression on thymocytes and thymic epithelial cells. In addition, we show here that administration of IFNα alone leads to MHC-I up-regulation and to the generation of CD8low SP8 thymocyte in SCID-hu Thy/Liv mice (Figure 5B). Finally, we show that SP8 thymocytes reactive against the self-peptide MelanA displayed a CD8low SP8 phenotype and that their frequency was reduced by half after the up-regulation of MHC-I thymic expression by productive HIV infection or IFNα treatment (Figure 7). These observations are consistent with, but do not prove, the hypothesis that MHC-I up-regulation on TECs leads to high-avidity interactions with developing thymocytes that may favor the selection of CD8low SP8 thymocytes of lower avidity.34 An alternative possibility is that increased TCR signaling induced by enhanced MHC-I expression may lead to “coreceptor tuning” among SP8 thymocytes, with down-regulation of CD8 expression in positively selected thymocytes.35 Although it is difficult to discriminate between these possibilities, a “true” selection of preexisting CD8low SP thymocytes is supported by studies of high-avidity mouse models of T-cell development.13,36
The above observations also raise a related question about the origin of naive CD8low T cells in secondary lymphoid organs: could these cells arise through de novo selection in peripheral lymphoid tissues?37 This possibility is supported by transgenic and avidity mouse models in which naive (but not memory) CD8+ T cells are critically dependent on TCR/MHC-I interactions for their survival and homeostatic expansion.37-39 Although it remains to be established whether such increased avidity interaction and selection of CD8low naive T cells could also occur as a consequence of generalized MHC-I up-regulation in the periphery during HIV disease, this possibility would extend the relevance of these observations beyond pediatric infection (wherein the thymus is likely active) to include adult infections as well (wherein the thymus may often be less active, if active at all).
At last, could naive CD8low T cells result from chronic T-cell activation that could directly “de-activate” and tune down CD8 expression on activated cells, independent of TCR signaling defect? Historically, nonfunctional T cells have been observed before and interpreted as a sign of HIV-induced immune deficiency.40 Such diminished reactivity in vitro and ex vivo41 that can be “rescued” after cell culture in the presence of IL-2 or IL-15 has been related to “chronic immune activation” defined, in its restricted sense, as a state of hyperimmune reactivity involving differentiated CD4+ and CD8+ T cells, and to some degree, naive T cells.6,19,20,42 In our study, naive CD8low T cells were prevalent in the peripheral circulation of HIV-infected progressors who also demonstrated signs of chronic immune activation. Interestingly, however, activation of the naive CD8+ T-cell pool, as measured by expression of HLA-DR and CD38 as well as the percentage of Ki67+ cells, was clearly reduced compared with that observed in differentiated memory and effector T cells, and the level of T-cell activation was actually not significantly different between CD45RAhighCD27high naive CD8+ T cells from nonprogressors and those from progressors (data not shown; see supplemental Figure 5 for an example of Ki67+ expression in subjects from study B, P > .05). These observations suggest that T-cell immune activation is not directly related to the CD8low phenotype, as observed in naive CD8+ T cells during HIV disease progression. Although this observation does not solve the cause and effect conundrum, it suggests that the decline of naive CD8+ T cells in patients with progressive HIV disease might be due both to decreased production and enhanced recruitment1 as well as to impaired survival and limited homeostatic proliferation. The latter defect could be the result of increased immune activation and inflammation, in particular sustained IFNα response and MHC-I up-regulation, and the generation of dysfunctional CD8low naive T cells though high-avidity interactions.
Of note, although the sustained inflammatory syndrome associated with HIV disease progression, in particular IFNα response, is pathognomonic of pathogenic lentiviral infection,10 clinical experience suggests that administration of IFNα (at least 1 of the 13 IFNα isoforms in the form of pegylated interferon alfa-2b) does not seem sufficient alone to cripple the immune system in virologically controlled HIV patients with concomitant HCV infection,43 not to mention in HIV-negative HCV-infected patients or those with multiple sclerosis. IFNα treatment in small cohorts of (non-HCV) HIV-infected patients also suggested some benefit in primary antibody responses and controlling VL.44,45 These “phenomenologic” observations should, however, be balanced by the high occurrence of autoimmune adverse effect in IFNα-treated patients and the well-established relationship between IFN and the pathology of systemic autoimmunity, including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, dermato/polymiositis, and Sjogren syndrome.46 It is probable that a delicate balance exist in vivo between the beneficial effects of IFNα on controlling viral load and/or coinfections (HCV and others) and detrimental side effects on immune activation, depending of viral load itself and to multiple innate factors, including pDCs.47,48
Altogether, we have shown here that HIV disease progression correlates with the occurrence of dysfunctional naive CD8low T cells in the setting of chronic systemic immune activation. Although the origin and the fate of these cells remain to be determined, it is tempting to extrapolate that a functionally impaired naive CD8low T-cell compartment in vivo may further cripple T-cell immune responses during the course of HIV disease progression.49 If so, then future maneuvers to sustain immune responses and to restore high-avidity CD8+ T-cell responses in HIV-infected patients50 may also focus on preventing the generation of such naive CD8low T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Bigos, J. Rivera, M. B. Moreno, J. Bare, and S. Galkina for expert assistance.
This work was supported in part by National Institutes of Health (NIH) award R37 AI40312 (J.M.M.); American Foundation for AIDS Research award 106383-3-RFGN and Elizabeth Glaser Pediatric AIDS Foundation award 77 510-29-PF (D.F.); NIH contract N01-AI05418 (C.A.S.); the UCSF AIDS Research Institute; and the Harvey V. Berneking Living Trust. The clinical cohorts were supported by grants from the NIH (AI069994), UCSF CFAR (P30 MH59037), the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), and AmFAR (106710-40). J.M.M. is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research and the NIH Director's Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant number DPI OD00329.
National Institutes of Health
Authorship
Contribution: D.F. designed and performed the research, analyzed data, and wrote the paper; C.A.S. was involved in study design, specimen collection, and data management of SCID-hu Thy/Liv samples, and edited the manuscript; B.E. provided FACS data from subjects in study D; R.H. was involved in subject recruitment and specimen collection of SCOPE samples; J.N.M. was involved in subject recruitment, specimen collection, and data management of SCOPE samples; F.M.H. was involved in study design, subject recruitment, specimen collection, and data management of Options samples; S.G.D. was involved in study design, subject recruitment, and specimen collection of SCOPE samples, and edited the manuscript; and J.M.M designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph M. McCune, Chief, Division of Experimental Medicine, University of California at San Francisco, Box 1234, San Francisco, CA 94143-1234; e-mail: m.mccune@ucsf.edu.