Over the past decades, cure rates in Hodgkin lymphoma (HL) have steadily improved. This was most obvious in patients with advanced disease after the introduction of multi-agent chemotherapy protocols such as MOPP (mechlorethamine, vincristine, procarbazine, prednisone) and ABVD (adriamycin, bleomycin, vinblastine, dacarbazine).1 More recently, the more intensive BEACOPPescalated (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone) was shown to be significantly superior to previous standard approaches in advanced adult HL. In this issue of Blood, Kelly and colleagues now report that BEACOPPescalated is also feasible and highly effective in high-risk pediatric and adolescent HL patients.2
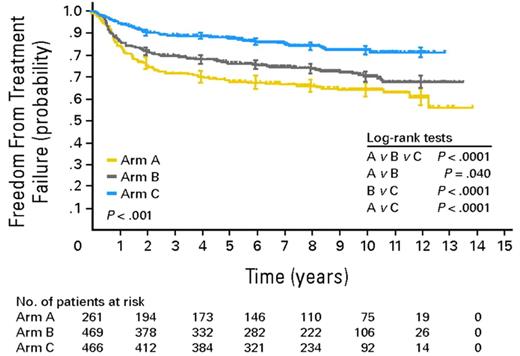
The majority of HL patients can be cured with state-of-the-art first-line treatment. In advanced stages, the pivotal HD9 trial by the German Hodgkin Study Group (GHSG) demonstrated the superiority of 8 cycles of BEACOPPescalated in terms of freedom from treatment failure (FFTF) and overall survival (OS) over 8 cycles of BEACOPP in baseline dose and 8 cycles of COPP (cyclophosphamide, vincristine, procarbazine, prednisone)/ABVD in patients aged 16 to 60 years.3 This superiority became even more evident in the 10-year update analysis of the trial performed in 2009 (see figure).4 Because 8 cycles of BEACOPPescalated represent a highly active treatment but are clearly more toxic than previous standard treatments, the GHSG follow-up trials in advanced-stage HL aimed at de-escalating treatment without compromising efficacy. The HD15 trial showed that positron emission tomography (PET) performed after completion of BEACOPP chemotherapy has a high negative predictive value and can identify those patients who do not need additional radiotherapy.5 In the GHSG HD18 trial, treatment is stratified according to PET performed after 2 cycles of BEACOPPescalated. The major goal of this currently ongoing study is to show whether the total number of cycles can be reduced from 8 to 4 in patients with negative early interim PET.
Freedom from treatment failure (FFTF) in BEACOPPescalated compared with BEACOPPbaseline and COPP/ABVD in adult patients with advanced HL (adopted from Engert et al4 ).
Freedom from treatment failure (FFTF) in BEACOPPescalated compared with BEACOPPbaseline and COPP/ABVD in adult patients with advanced HL (adopted from Engert et al4 ).
The present study by Kelly and et al was conducted in the pre-PET era. Ninety-nine pediatric and adolescent patients between 4 and 21 years of age diagnosed with high-risk HL (stage IIB or IIIB with bulky disease, stage IV) were included. All patients initially received 4 cycles of BEACOPPescalated. Response was then assessed by computed tomography. In those patients with rapid early response (complete or good partial remission) who represented the majority of patients included (74%), treatment was continued with less intensive protocols. Girls received 4 cycles of COPP/ABV (cyclophosphamide, vincristine, procarbazine, prednisone/adriamycin, bleomycin, vinblastine) with no consolidating radiotherapy (RT) to reduce the risk of secondary breast cancer, and boys received 2 cycles of ABVD followed by involved-field RT (IF-RT). Slow early responders (partial remission or stable disease) of both sexes received 4 additional cycles of BEACOPPescalated followed by IF-RT. Overall results after a median follow-up of 6.3 years were excellent with 5-year event-free survival (EFS) and OS rates of 94% and 97%, respectively. Treatment-related acute toxicity mainly consisted of reversible neutropenia, anemia, and thrombocytopenia. During the observation period, 2 patients were diagnosed with fatal secondary myelodysplastic syndrome/acute myeloid leukemia and 13 patients had reduced left ventricular shortening fraction. However, only 1 of them required cardiac medication. Long-term toxicity, mainly the risk of secondary solid tumors, cannot be fully judged yet because the median observation is still too short for reliable conclusions. Other toxicity such as infertility—a relevant problem after BEACOPP—was not consistently documented.
Thus, upfront treatment intensification using BEACOPPescalated followed by a less intensive consolidation in early responders produced impressive results without unexpected acute toxicity in children and adolescents suffering from high-risk HL.2 This finding does not come as a surprise because a significant portion of patients included in this trial (48%) were adolescents aged 15 to 21 years. Adolescent HL patients (usually defined as those between 15 or 16 and 21 years of age) do not differ substantially from young adult HL patients (age up to 45 years) with regard to clinical presentation, distribution of histologic HL subtypes, and other general aspects and are thus candidates for treatment strategies developed for adults.6,7
An approach similar to the one reported by Kelly and colleagues was recently evaluated by Avigdor and coworkers in a group of 45 adult patients diagnosed with advanced HL.8 Patients received early interim PET after 2 cycles of BEACOPPescalated and those with a good response completed treatment with 4 cycles of ABVD. In the PET-negative early responders (31/45), 4-year progression-free survival rate was 87%, indicating that this strategy does not seem to compromise treatment results while side and late effects might be substantially reduced. However, the number of patients included in this analysis is too small for more general conclusions.
In contrast to adolescents, young pediatric HL patients usually show differences in the distribution of histologic subtypes and other more general aspects that might argue for the use of protocols specifically developed for children. Examples include OEPA (vincristine, etoposide, prednisone, adriamycin) and ABVE-PC (adriamycin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide).9,10
In conclusion, the data reported by Kelly and coworkers suggest that BEACOPPescalated represents a viable treatment option in pediatric and adolescent high-risk HL patients. However, to prevent overtreatment, whenever possible, the use of this protocol should be combined with stratification strategies such as interim PET.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■