Abstract
In the hematopoietic hierarchy, only stem cells are thought to be capable of long-term self-renewal. Erythroid progenitors derived from fetal or adult mammalian hematopoietic tissues are capable of short-term, or restricted (102- to 105-fold), ex vivo expansion in the presence of erythropoietin, stem cell factor, and dexamethasone. Here, we report that primary erythroid precursors derived from early mouse embryos are capable of extensive (106- to 1060-fold) ex vivo proliferation. These cells morphologically, immunophenotypically, and functionally resemble proerythroblasts, maintaining both cytokine dependence and the potential, despite prolonged culture, to generate enucleated erythrocytes after 3-4 maturational cell divisions. This capacity for extensive erythroblast self-renewal is temporally associated with the emergence of definitive erythropoiesis in the yolk sac and its transition to the fetal liver. In contrast, hematopoietic stem cell-derived definitive erythropoiesis in the adult is associated almost exclusively with restricted ex vivo self-renewal. Primary primitive erythroid precursors, which lack significant expression of Kit and glucocorticoid receptors, lack ex vivo self-renewal capacity. Extensively self-renewing erythroblasts, despite their near complete maturity within the hematopoietic hierarchy, may ultimately serve as a renewable source of red cells for transfusion therapy.
Introduction
In the adult, all blood cells are ultimately derived from hematopoietic stem cells (HSCs) that are primarily quiescent yet capable of extensive self-renewal. The differentiation of HSCs into multipotential and unipotential progenitors is accompanied by a loss both of proliferative capacity and of self-renewal potential. Immature erythroid-restricted progenitors, termed erythroid burst-forming units, have a higher proliferative potential than late-stage erythroid progenitors, termed erythroid colony-forming units (CFU-E).1 CFU-E subsequently generate a cascade of morphologically identifiable erythroid precursors that undergo 3-4 maturational cell divisions as they progress from proerythroblast to basophilic, polychromatophilic, and orthochromatic erythroblast stages.2 Erythroid precursor maturation is characterized by decreased cell size, hemoglobin accumulation, nuclear condensation, and the cell surface expression of Ter119.3 Orthochromatic erythroblasts enucleate and soon thereafter enter the blood stream as reticulocytes.
Red blood cell (RBC) production is regulated by several exogenous factors, including erythropoietin (Epo), cortisol, and stem cell factor (SCF). Erythropoiesis is critically dependent on Epo, a glycoprotein hormone that provides a survival signal to late-stage erythroid progenitors.4,5 Low oxygen levels in tissues stimulate the production of Epo, resulting in the survival of more CFU-E and, in turn, an increase in the number of RBCs. The cellular response to acute hypoxia, termed stress erythropoiesis, is also regulated, in part, by glucocorticoids, because mice with diminished glucocorticoid signaling display a delayed recovery after induction of anemia.6 SCF, a soluble protein that signals through the Kit receptor, which is expressed by erythroid progenitors and immature precursors, is also necessary for erythroid differentiation and the early stages of maturation of erythroid progenitors.7,8
The addition of the synthetic glucocorticoid dexamethasone, along with SCF and Epo, to cultures of mouse bone marrow or fetal liver cells induces the outgrowth and proliferation of erythroid “progenitors” for ∼ 15 days.6,9-14 The proliferative capacity of these cells is restricted to ∼ 102- to 105-fold total expansion. However, cultures initiated from murine embryonic stem cells proliferate for longer periods of time.15 Although this difference in proliferative capacity was ascribed to the embryonic stem cell origin of the cultures, we asked whether the ex vivo proliferative capacity of erythroid progenitors derived from the early embryo may differ from that of their fetal and adult counterparts.
Here, we investigate the ability of erythroid cells cultured from carefully staged mouse embryos to proliferate ex vivo. Surprisingly, definitive erythroid cells derived from the yolk sac and early fetal liver are capable not only of restricted (102- to 105-fold) but also extensive (106- to 1060-fold) proliferation ex vivo, a far greater proliferative potential than previously recognized. Despite prolonged culture, these immature erythroblasts preserve the potential to mature into enucleated RBCs, indicating that they are capable of long-term self-renewal. In contrast, primitive erythroid cells derived from the yolk sac are incapable of either restricted or extensive self-renewal ex vivo. Our findings raise the possibility that definitive erythropoiesis is uniquely characterized by the capacity of immature erythroblasts, lying only 3-4 cell divisions from terminally differentiated RBCs, to undergo self-renewal cell divisions. Extensively self-renewing erythroblasts (ESREs) may ultimately serve as an in vitro source of RBCs for use in transfusion therapy.
Methods
Mice and tissues
All experiments with mice were approved by the University of Rochester's Committee on Animal Resources. Outbred ICR mice (Charles River Laboratories International or Taconic Farms Inc) or C57BL/6J mice (Charles River Laboratories International or The Jackson Laboratory) were mated overnight, and vaginal plugs were examined in the morning (embryonic day 0.3; E0.3). At various gestational ages, mice were killed by CO2 narcosis, and the embryos were dissected in PB2 (Dulbecco phosphate-buffered saline [Invitrogen], 0.1% glucose [Invitrogen], and 0.3% bovine serum albumin [Gemini Bioproducts]). Yolk sacs from E7.5-E9.5 conceptuses were dissociated with 0.008% trypsin (Worthington Biochemical Corporation) and mechanical trituration. Livers from E11.5-E18.5 fetuses, bone marrow, and spleens were mechanically dissociated by pipetting with a 1000-μL pipette.
Acute anemia was induced by injecting adult mice intraperitoneally with 35 mg/kg phenylhydrazine (P26252; Sigma-Aldrich) on days 1 and 2. Bone marrow and spleen cells were harvested from killed mice on day 4 and cultured as described in “Erythroid expansion culture.”
Erythroid expansion culture
Cells from each tissue were resuspended ≤ 2 × 106 cells/mL in a 24-well gelatin-coated tissue culture dish (Gibco/BRL) in serum-free “erythroid expansion media” consisting of either StemPro34 plus nutrient supplement (Gibco/BRL) or StemSpan SFEM (Stem Cell Technologies) supplemented with 2 U/mL human recombinant Epo (Amgen), 100 ng/mL SCF (PeproTech), 10−6M dexamethasone (D2915; Sigma), 40 ng/mL insulin-like growth factor-1 (PeproTech), and penicillin/streptomycin (Pen/Strep; Invitrogen). Cholesterol Mix (Sigma) was added to a final concentration of 0.4% in StemSpan-based erythroid expansion media. The dexamethasone stock solution was stored in the original glass container and never frozen. After 1 and 3 days of culture, the nonadherent cells were filtered through a 70-μm filter (BD Falcon) and transferred to a new gelatin-coated well at a concentration of ≤ 2 × 106 cells/mL. The second transfer was termed day 0 of expansion culture. No adherent cells were present in culture after the 2 well transfers. Total live and dead cell numbers were determined daily by Trypan Blue (Gibco/BRL) exclusion, and the cell concentration was brought to 2 × 106 total cells/mL daily through partial medium changes.
Embryonic stem cell differentiation and culture
PC13 embryonic stem cells (University of Rochester transgenic facility) were quickly thawed at 37°C and washed with DMEM-ES (Dulbecco modified Eagle medium [DMEM; Invitrogen], Pen/Strep, sodium bicarbonate [Sigma], 15% fetal bovine serum [Gemini Bioproducts], monothioglycerol [MTG; Sigma], and glutamine [Invitrogen]). Cells were passaged at least once in DMEM-ES with leukemia inhibitory factor (LIF; Millipore) on a T25 gelatin-coated flask (BD Falcon). Before differentiation into embryoid bodies (EBs), cells were grown overnight in IMDM-ES with LIF (same as DMEM-ES with LIF, except Iscove modified Dulbecco medium [IMDM; Invitrogen] replaced DMEM). Cells were washed once in PB2 and resuspended in 4 mL of EB differentiation media (IMDM, 15% fetal bovine serum [Summit Biotechnology], MTG, ascorbic acid [Sigma], transferrin [Sigma], Protein-Free Hybridoma Media [PFHM-II; Invitrogen], glutamine, and Pen/Strep) at various cell concentrations (2500-20 000 cells) into ultra-low adhesion 60 × 35-mm dishes (Corning). EBs were harvested daily after 5 days of incubation and dissociated with mechanical trituration in phosphate-buffered saline (Invitrogen) containing 1mM EDTA (ethylenediaminetetraacetic acid; Sigma) and 0.008% trypsin. Dissociation was stopped with PB2 containing 10% plasma-derived serum (PDS; Animal Technologies), and the single-cell suspension was cultured in erythroid expansion media.
Erythroid cell maturation
Maturation of erythroid cells proliferating in culture was initiated by washing the cells with PB2 and resuspending them in “erythroid maturation media” (1× IMDM, 2 U/mL Epo, 100 ng/mL SCF, 10% Serum Replacement [Invitrogen], 5% PDS, 1× glutamine, 10% PFHM-II, and 12.7 μL/100 mL 1:10 MTG), at 2 × 106 cells/mL in a gelatin-coated well. Cultures were maintained daily at 2 × 106 cells/mL during maturation.
Erythroid cell evaluation
Cells were cytospun (Shandon II; Thermo-Scientific), Wright-Giemsa stained, premounted, and coverslipped. Images were acquired on a Spot RT Slider camera (Diagnostic Instruments, Inc) attached to an Eclipse 80i microscope (Nikon) with the use of Plan Fluor objective lenses (Nikon, 100× magnification, 1.3 NA). Photoshop CS4 Extended software (Adobe) was used for image processing. Images for supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were acquired on a Digital Sight-U2 camera (Nikon). The maturational stages of the erythroblasts were scored with cell and nuclear size and with nuclear and cytoplasmic staining characteristics. Benzidine staining was performed as previously described.16 The cell surface phenotype of the expanding erythroblasts was analyzed with the use of phycoerythrin (PE)–indocyanine 7 CD117 (Kit), PE CD71, and allophycocyanin (APC) Ter119 antibodies (eBioscience) on an LSR-II flow cytometer (BD Bioscience). Data were analyzed with the use of FlowJo software (Version 8.8.6; TreeStar).
Analysis of gene expression
RNA was isolated with either Trizol, as previously described,17 or the RNeasy kit according to the manufacturer's instructions (QIAGEN). For the latter approach, cells were disrupted with the use of the kit's RLT buffer, and centrifugation through a Qia-shredder column. RNA was then washed several times on the RNeasy column and eluted with RNase-free water. cDNA was constructed with the SuperScript III First Strand kit (Invitrogen). Quantitative reverse-transcription polymerase chain reaction (qPCR) of embryonic (ϵy and βH1) and adult (β1 and β2) globins was performed as previously described with the use of the 18s ribosomal subunit RNA as a control.17 qPCR was also performed with primer pairs for Epo receptor (EpoR; 5′-CCC AAG TTT GAG AGC AAA GC-3′, 5′-TGC AGG CTA CAT GAC TTT CG-3′), Epo (5′-CCA CCC TGC TGC TTT TAC TC-3′, 5′-CTC AGT CTG GGA CCT TCT GC-3′), SCF (5′-CCG TGA CCT TGT GTG GAT GAT TC-3′, 5′-TGG GTT TTC AGC ACT CAG ACG-3′), and cMyb (5′-AAG ACC CTG AGA AGG AAA AGC G-3′, 5′-GTG TTG GTA ATG CCT GCT GTC C-3′), all with an annealing temperature of 57°C, and Kit (5′-CTC ACA TAG CAG GGA GCA CA-3′, 5′-ACA ACT CAC CCA CAC GCA TA-3′) and glucocorticoid receptor (Nr3c1; 5′-AGG CCG CTC AGT GTT TTC TA-3′, 5′-TAC AGC TTC CAC ACG TCA GC-3′), both with an annealing temperature of 60°C.
Carboxyfluorescein diacetate, succinimidyl ester
Carboxyfluorescein diacetate, succinimidyl ester (CFSE) staining was performed according to the manufacturer's directions (Invitrogen). After staining with CFSE, cells were cultured in erythroid expansion media as described in “Erythroid expansion culture.” A portion of the culture was removed daily and stained with antibodies for Kit, CD71, propidium iodide (Molecular Probes) for live/dead discrimination, and Vybrant DyeCycle VioletStain (Invitrogen) or Draq5 (Biostatus Limited) to visualize DNA content. Stained cells were analyzed with an LSR-II flow cytometer, and the data were analyzed with FlowJo software.
Isolation of primary proerythroblasts
Primitive, embryonic-definitive, and adult-definitive proerythroblasts were purified from E9.5 yolk sac, E11.5 fetal liver, and adult bone marrow, respectively, with the use of fluorescent-activated cell sorting. Briefly, live (DAPI− [4′-6′-diamidine-2-phenylindole]; Invitrogen) and Ter119low(APC) primitive proerythroblasts were isolated from the E9.5 yolk sac; large (high forward scatter; FSC), live (DAPI−), and Kithigh(PE)/Ter119low(APC) embryonic-definitive proerythroblasts were isolated from E11.5 fetal liver; and large (high FSC), live (DAPI−), and Kithigh(PE)/Ter119low(APC) adult-definitive proerythroblasts were isolated from bone marrow.
Cortisol measurement
Cortisol levels in E11.5 and E12.5 embryonic tissues as well as adult peripheral blood were determined with the use of an enzyme-linked immunosorbent assay (ELISA), performed according to the manufacturer's protocol (Parameter Cortisol ELISA kit; R&D Systems). Wells were read at 450 nm with the use of an AD340 plate reader (Beckman Coulter). Amniotic fluid was collected from E11.5 and E12.5 embryos with the use of a 30-gauge syringe. Glass micropipettes were used to gently aspirate blood directly from the heart of each embryo. The livers were placed in 25 μL of dilution buffer (ELISA kit) and triturated. More dilution buffer (75 μL) was added, and the samples were centrifuged for 5 minutes at 8000g to collect supernatant for cortisol analysis.
Results
Yolk sac–derived erythroid cells exhibit extensive ex vivo proliferative potential
We previously established that primitive and definitive erythroid progenitors emerge in 2 overlapping waves from the murine yolk sac between E7.5 and E10.5 of gestation.18 To examine the potential of these erythroid lineages to generate ex vivo–proliferating erythroblasts, cells from spatially and temporally defined murine tissues were cultured in the presence of either StemPro34 or StemSpan supplemented with Epo, SCF, insulin-like growth factor-1, and dexamethasone. Consistent with published results,19 cell cultures derived from adult bone marrow showed a restricted (102- to 105-fold) capacity to proliferate ex vivo (Figure 1A). Similarly, cultures derived from E9.5 yolk sac initially underwent a “restricted” phase of exponential growth that resulted in a gradual increase in terminally mature RBCs. Unlike adult marrow–derived cultures, however, the restricted phase of these E9.5 yolk sac–derived cultures was followed by the continued proliferation of erythroblasts for weeks to months (Figure 1A). We routinely obtained cell cultures that underwent extensive (1010- to 1030-fold) expansion. The longest culture resulted in 1064-fold cellular expansion over 203 days, before the culture was electively terminated (data not shown). This extensive ex vivo proliferative potential has not previously been described for cells harvested from wild-type murine embryos.
Cell cultures initiated from embryonic tissues are capable of yielding extensively proliferating erythroid cells. (A) Cells were grown in erythroid expansion media. Erythroid cells derived from adult bone marrow proliferated ∼ 103-fold, whereas those from the 3 independent E9.5 yolk sac cultures each proliferated > 1012-fold. (B) Cells were grown as in panel A but were maintained at indicated cell concentrations. Cells exhibit a slowed growth rate and increased death at concentrations of ≥ 4 × 106 cells/mL or higher, but no changes in kinetics were observed at concentrations < 2 × 106cells/mL. (C) Most of the extensively proliferating cells resemble proerythroblasts (ProE) and basophilic erythroblasts (BasoE). The cultures also contain a small number of polychromatophilic (PolyE) and orthochromatic (OrthoE) erythroblasts, as well as reticulocytes (mean ± SEM; N = 16). (D) Ter119 and Kit levels of ex vivo extensively proliferating erythroblasts, adult bone marrow (BM) cells, and E12.5 fetal liver (FL) cells. Most extensively proliferating erythroblasts are Kithigh/Ter119low. Kithigh/Ter119low cell populations (red circles), with similar forward scatter (FSC) characteristics are found in the adult marrow and E12.5 fetal liver. One of 3 representative experiments is shown. (E) Extensively proliferating erythroid cells express small amounts of adult (β1 and β2), but no embryonic (ϵy and βH1), β globin transcripts (mean ± SEM; N = 3). In contrast, circulating blood cells from E12.5 of gestation, composed predominantly of primitive erythroblasts, express both embryonic and adult β-globin gene transcripts.
Cell cultures initiated from embryonic tissues are capable of yielding extensively proliferating erythroid cells. (A) Cells were grown in erythroid expansion media. Erythroid cells derived from adult bone marrow proliferated ∼ 103-fold, whereas those from the 3 independent E9.5 yolk sac cultures each proliferated > 1012-fold. (B) Cells were grown as in panel A but were maintained at indicated cell concentrations. Cells exhibit a slowed growth rate and increased death at concentrations of ≥ 4 × 106 cells/mL or higher, but no changes in kinetics were observed at concentrations < 2 × 106cells/mL. (C) Most of the extensively proliferating cells resemble proerythroblasts (ProE) and basophilic erythroblasts (BasoE). The cultures also contain a small number of polychromatophilic (PolyE) and orthochromatic (OrthoE) erythroblasts, as well as reticulocytes (mean ± SEM; N = 16). (D) Ter119 and Kit levels of ex vivo extensively proliferating erythroblasts, adult bone marrow (BM) cells, and E12.5 fetal liver (FL) cells. Most extensively proliferating erythroblasts are Kithigh/Ter119low. Kithigh/Ter119low cell populations (red circles), with similar forward scatter (FSC) characteristics are found in the adult marrow and E12.5 fetal liver. One of 3 representative experiments is shown. (E) Extensively proliferating erythroid cells express small amounts of adult (β1 and β2), but no embryonic (ϵy and βH1), β globin transcripts (mean ± SEM; N = 3). In contrast, circulating blood cells from E12.5 of gestation, composed predominantly of primitive erythroblasts, express both embryonic and adult β-globin gene transcripts.
To determine whether cell concentration altered growth rate or cell survival, cultures were maintained at 2 × 102, 2 × 104, 2 × 105, 2 × 106, 4 × 106, and 2 × 107 cells/mL. Cells exhibited decreased growth rate when maintained at 4 × 106 cells/mL, and a cessation of growth combined with massive cell death occurred at the highest concentration (Figure 1B). In contrast, growth rate was not affected by concentrations as low as 200 cells/mL (Figure 1B). No differences in the cellular kinetics of restricted or extensive cellular proliferation were found between StemPro34-based and StemSpan-based erythroid expansion media.
Expansion cultures are composed of immature erythroid precursors
To begin to investigate the cellular identity of the proliferating cells, we examined their morphology after Wright-Giemsa staining. As shown in Figure 1C, the cells constituting the expansion cultures morphologically resemble proerythroblasts and basophilic erythroblasts with a small percentage of more mature polychromatophilic and orthochromatic erythroblasts. Benzidine staining, to identify hemoglobin-containing cells, showed that 13.5% ± 5.6% (mean ± SEM; N = 21) of the cells were benzidine positive, consistent with the morphological observations that the proliferating cultures consisted primarily of immature erythroblasts along with a small percentages of maturing erythroblasts.
We recently determined that proerythroblasts in the murine bone marrow are large cells that coexpress Kit, CD71, and low levels of the erythroid-specific marker Ter119.20 Flow cytometric analysis of the extensively proliferating erythroid cells also showed a high FSC and the cell surface expression of Kit, CD71, and low levels of Ter119, a phenotype similar to primary proerythroblasts in the bone marrow and fetal liver (Figure 1D circled cells).
We also assayed the pattern of β-globin genes expressed by the cells present in extensively proliferating cultures. As shown in Figure 1E, only the adult (β1 and β2) globins were expressed. The globin expression is derived primarily from the small subpopulation of maturing cells (data not shown). Despite the yolk sac origin of these cultures, we could not detect the embryonic (ϵy and βH1) globins that are expressed by primitive erythroid cells.21 These results are consistent with a definitive erythroid identity of the cells in culture.
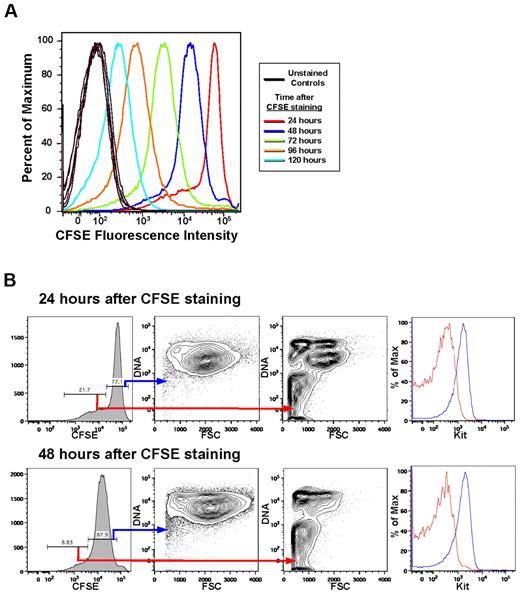
To assess the contribution of individual cells to the expanding culture's overall growth rate, we performed CFSE staining. Evaluation of the culture over several days showed a major cell population undergoing a uniform decrease in CFSE fluorescence intensity each day, as well as a minor cell population undergoing an increased loss of CFSE fluorescence intensity each day (Figure 2A). The major cell population is composed of large (FSChigh), Kithigh, CD71high, DNAhigh cells (Figure 2B). In contrast, the minor cell population is composed of small (FSClow), Kitlow cells, of which 48.2% ± 6.5% are enucleated (compared with the major population which is 1.9% ± 0.3% enucleated; n = 8; Figure 2B). These data indicate that immature erythroblasts, which constitute the majority of the cells in these cultures, are dividing daily and are responsible for the exponential cell growth that occurs in the cultures. Furthermore, these data suggest that the minor cell population consists of maturing erythroid cells that divide more rapidly than once a day as they mature into RBCs.
Most of the cells in the expansion culture divide once daily and maintain their large size and Kithigh phenotype. (A) Staining characteristics of ex vivo extensively expanding erythroid cultures indicate that most of the cells divide once daily. There is also a minor population of cells that divide 2 or 3 times in 24 hours. Results from 1 of 3 experiments are shown. (B) The major cell population that divides only once daily is composed of large (FSChigh), nucleated (DNAhigh), Kithigh cells that retain these characteristics from day to day (blue lines), shown for 24 and 48 hours of culture after CFSE staining. In contrast, the minor, rapidly dividing population is composed of smaller (FSClow), Kitlow cells, many of which are enucleated (DNAlow/−; red lines). These results suggest that the minor population consists of terminally maturing erythroid cells.
Most of the cells in the expansion culture divide once daily and maintain their large size and Kithigh phenotype. (A) Staining characteristics of ex vivo extensively expanding erythroid cultures indicate that most of the cells divide once daily. There is also a minor population of cells that divide 2 or 3 times in 24 hours. Results from 1 of 3 experiments are shown. (B) The major cell population that divides only once daily is composed of large (FSChigh), nucleated (DNAhigh), Kithigh cells that retain these characteristics from day to day (blue lines), shown for 24 and 48 hours of culture after CFSE staining. In contrast, the minor, rapidly dividing population is composed of smaller (FSClow), Kitlow cells, many of which are enucleated (DNAlow/−; red lines). These results suggest that the minor population consists of terminally maturing erythroid cells.
Extensively proliferating erythroblasts mature into enucleated erythrocytes
In vivo, proerythroblast maturation into enucleated RBCs is characterized by the progressive accumulation of hemoglobin.2 To test the ability of extensively proliferating erythroblasts to mature, we cultured them in erythroid maturation media, which contains IMDM, Epo, SCF, serum replacement, PDS, glutamine, PFHM-II, and MTG. After 3 days, > 97% of cells from all cultures (N = 49) were benzidine-positive and consisted primarily of enucleated erythrocytes and a small number of late-stage (orthochromatic) erythroblasts (Figure 3A-B). Consistent with benzidine positivity, qPCR analysis showed an 11.4-fold and 6.3-fold up-regulation of the β1- and β2-globin genes, respectively, after 3 days of maturation. However, no ϵy- or βH1-globin gene expression was detected (data not shown), consistent with the definitive erythroid identity of these cells.17
ESREs are capable of terminal erythroid maturation. (A) Proliferating ESREs transferred from erythroid expansion media to erythroid maturation media mature from proerythroblasts to orthochromatic erythroblasts and enucleated erythrocytes over 3 days. The lower panel is a composite of cells from a single photograph of a cytospin preparation. (B) The kinetics of ESRE maturation. Over the course of 3 days ESREs transition from immature erythroblasts into > 90% enucleated erythrocytes (mean ± SEM; N = 16 independent maturation cultures). (C) Proliferating ESREs have a high FSC and are KithighTer119low cells (upper left). After maturation, the cells decrease in size (FSC; upper right compared with lower right), down-regulate Kit, and up-regulate Ter119 (upper left compared with lower left). One of 3 representative experiments is shown. (D) ESREs grown in StemPro34-based erythroid expansion media, when transferred to erythroid maturation media, yield an 8-fold increase in cell number over the course of 3 days (mean ± SEM; N = 36). ESREs grown in StemSpan-based erythroid expansion media, when transferred to erythroid maturation media, yield a 19-fold increase in cell number over the course of 3 days (mean ± SEM; N = 13).
ESREs are capable of terminal erythroid maturation. (A) Proliferating ESREs transferred from erythroid expansion media to erythroid maturation media mature from proerythroblasts to orthochromatic erythroblasts and enucleated erythrocytes over 3 days. The lower panel is a composite of cells from a single photograph of a cytospin preparation. (B) The kinetics of ESRE maturation. Over the course of 3 days ESREs transition from immature erythroblasts into > 90% enucleated erythrocytes (mean ± SEM; N = 16 independent maturation cultures). (C) Proliferating ESREs have a high FSC and are KithighTer119low cells (upper left). After maturation, the cells decrease in size (FSC; upper right compared with lower right), down-regulate Kit, and up-regulate Ter119 (upper left compared with lower left). One of 3 representative experiments is shown. (D) ESREs grown in StemPro34-based erythroid expansion media, when transferred to erythroid maturation media, yield an 8-fold increase in cell number over the course of 3 days (mean ± SEM; N = 36). ESREs grown in StemSpan-based erythroid expansion media, when transferred to erythroid maturation media, yield a 19-fold increase in cell number over the course of 3 days (mean ± SEM; N = 13).
Maturation of extensively proliferating erythroblasts is characterized not only by structural changes and globin gene up-regulation but also by changes in cell surface phenotype. Analysis by flow cytometry showed marked down-regulation of Kit and up-regulation of Ter119 associated with a decrease in FSC (Figure 3C), changes that characterize the maturation of erythroid precursors in vivo, in both the fetal liver and the postnatal bone marrow.3,20
Proerythroblasts normally undergo 3-4 maturational cell divisions in vivo to generate enucleated erythrocytes.2 We examined the kinetics of proliferating erythroblasts cultured for 3 days in erythroid maturation media. Cells that had originally proliferated in StemPro34-based erythroid expansion media exhibited an 8-fold increase in cell number when placed in erythroid maturation media (Figure 3D). This corresponds on average to 3 maturational cell divisions. Cells that had originally proliferated in StemSpan-based erythroid expansion media exhibited a 19-fold increase in cell number during maturation, corresponding to ∼ 4 maturational cell divisions (Figure 3D). The extra division for StemSpan-expanded cells occurs in the first 24 hours of maturation. These results, taken together, provide functional evidence that the cultures consist of extensively proliferating immature erythroblasts that are, on average, 3-4 divisions from enucleated erythrocytes.
Ex vivo extensively proliferating erythroblasts self-renew
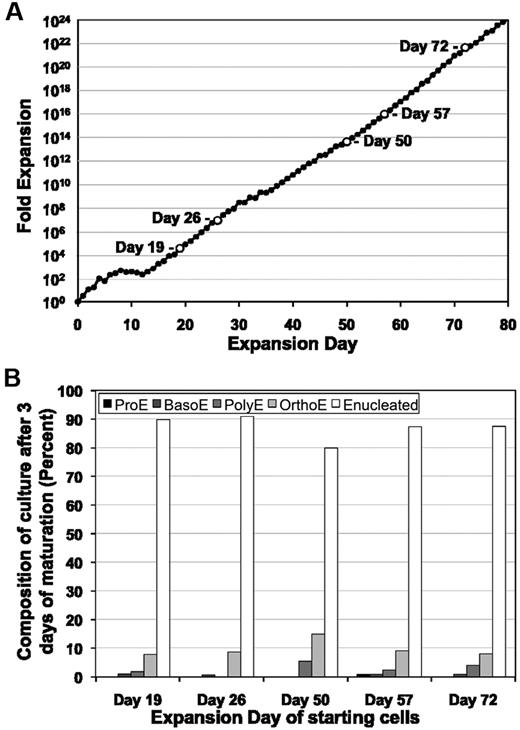
We next asked whether the proliferating erythroblasts maintain the potential to terminally mature throughout the extensive life of the expanding culture. As shown in Figure 4A, we removed cells from a proliferating culture at days 19, 26, 50, 57, and 72 and examined their ability to mature ex vivo. At all time points tested, ≥ 80% of the cells matured into enucleated erythrocytes over the course of 3 days (Figure 4B). These results indicate that maturation potential is preserved despite daily cell divisions over months of ex vivo proliferation. We therefore named these cells extensively self-renewing erythroblasts (ESREs).
ESREs maintain their ability to mature despite extensive proliferationex vivo. (A) The capability of ESREs to mature was tested sequentially (open time points) during continued ESRE culture. (B) ESREs from time points listed in panel A were placed into erythroid maturation media, and the kinetics of erythroid cell maturation was analyzed by morphological evaluation of cytospun cells. ESREs maintain the capability to completely mature with the same kinetics after 3 days in erythroid maturation media despite prolonged culture ex vivo.
ESREs maintain their ability to mature despite extensive proliferationex vivo. (A) The capability of ESREs to mature was tested sequentially (open time points) during continued ESRE culture. (B) ESREs from time points listed in panel A were placed into erythroid maturation media, and the kinetics of erythroid cell maturation was analyzed by morphological evaluation of cytospun cells. ESREs maintain the capability to completely mature with the same kinetics after 3 days in erythroid maturation media despite prolonged culture ex vivo.
ESRE proliferation and survival are cytokine dependent
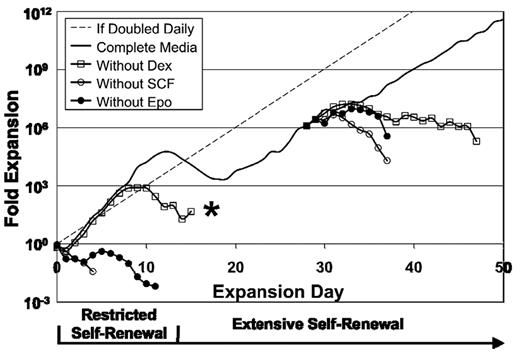
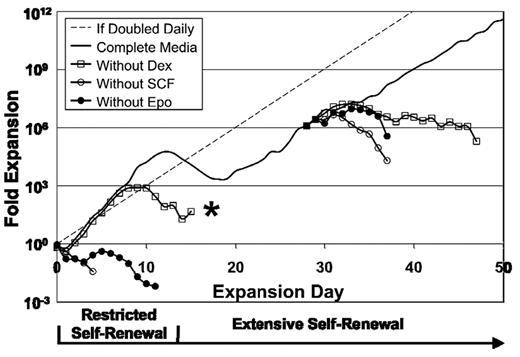
To determine whether Epo, SCF, and dexamethasone are each required for the continued proliferation of ESRE cultures, we removed each factor individually during the restricted and extensive expansion phases of ex vivo culture. Cells deprived of Epo, or of SCF, stopped proliferating within 2 days of cytokine removal, and all cells in the cultures stained Trypan-positive within 5 days (Figure 5). Removal of dexamethasone resulted in a transient increase in cell numbers as the cells completed rapid maturational cell divisions. During restricted self-renewal, the cultures deprived of dexamethasone ultimately favored the slow growth of mast cells after the erythroid cells had matured or died. Our results, taken together, indicate that ESREs remain dependent on Epo, SCF, and dexamethasone for both restricted and extensive ex vivo self-renewal.
Epo, SCF, and dexamethasone are each required both for restricted and for extensive erythroid self-renewal. When Epo, SCF, or dexamethasone is individually removed from the culture during restricted self-renewal or during extensive self-renewal, proliferation is halted because of cell death, decreased proliferation, or terminal maturation. Results of 1 of 3 independent experiments is shown. *Culture was ultimately composed of mast cells.
Epo, SCF, and dexamethasone are each required both for restricted and for extensive erythroid self-renewal. When Epo, SCF, or dexamethasone is individually removed from the culture during restricted self-renewal or during extensive self-renewal, proliferation is halted because of cell death, decreased proliferation, or terminal maturation. Results of 1 of 3 independent experiments is shown. *Culture was ultimately composed of mast cells.
Developmental origin of ESRE potential
Our initial studies indicated that erythroblasts capable of extensive ex vivo self-renewal could be generated from the E9.5 yolk sac and that these ESREs were “definitive” in nature on the basis of their globin expression pattern (Figure 1E). To better define the developmental origin of ESRE potential and to determine whether primitive erythroid precursors can also self-renew when cultured ex vivo, we examined temporally and spatially defined embryonic, fetal, and adult hematopoietic tissues from outbred mice for ESRE potential.
No ex vivo erythroblast proliferation, restricted or extensive in nature, was generated from the E7.5 yolk sac, the time and place during embryogenesis when primitive, but not definitive, erythroid progenitors are present18 (Figure 6). The earliest tissue that generated ex vivo self-renewing erythroblasts was the E8.5 yolk sac, the time point when and the place where definitive erythroid potential is first detected in the murine conceptus.18 A large proportion of cultures established from E8.5 yolk sac, E9.5 yolk sac, and E12.5 fetal liver generated not only restricted but also extensive erythroid self-renewal (38 of 70; Figure 6). ESRE potential from these tissues and developmental time points was more frequently generated with the use of StemSpan-based erythroid expansion media (15 of 16; 94%) than with StemPro34-based erythroid expansion media (23 of 54; 43%). Our results support the hypothesis that ESRE potential is associated with the transient wave of definitive hematopoiesis that emerges from the yolk sac, before HSC emergence, and transitions to the fetal liver. In contrast to fetal tissues, culture of adult bone marrow or adult spleen cells, which are derived from HSCs, resulted in restricted, but almost never in extensive, erythroid self-renewal potential (1 of 26; Figure 6). Tissues from fetal and adult C57BL/6J mice generated cultures of erythroid cells with extensive and restricted self-renewal capacity similar to those from outbred mice (supplemental Table 1).
Generation of erythroid cell cultures with restricted and extensive self-renewal from embryonic, fetal, and adult hematopoietic tissues derived from ICR mice. No restricted or extensively proliferating erythroid cultures could be established from E7.5 yolk sac (YS) cells. Cultures of erythroid cells with restricted self-renewal potential were generated from later YS, fetal liver (FL), spleen, and bone marrow (BM). The highest frequency of erythroid cultures exhibiting extensive self-renewal potential was derived from E8.5-E14.5 embryos, which is associated with the emergence of a transient wave of definitive erythroid potential in the yolk sac and its transition to the early fetal liver. Cultures derived from “stressed” BM and spleen from mice made anemic with phenylhydrazine were only capable of restricted self-renewal.
Generation of erythroid cell cultures with restricted and extensive self-renewal from embryonic, fetal, and adult hematopoietic tissues derived from ICR mice. No restricted or extensively proliferating erythroid cultures could be established from E7.5 yolk sac (YS) cells. Cultures of erythroid cells with restricted self-renewal potential were generated from later YS, fetal liver (FL), spleen, and bone marrow (BM). The highest frequency of erythroid cultures exhibiting extensive self-renewal potential was derived from E8.5-E14.5 embryos, which is associated with the emergence of a transient wave of definitive erythroid potential in the yolk sac and its transition to the early fetal liver. Cultures derived from “stressed” BM and spleen from mice made anemic with phenylhydrazine were only capable of restricted self-renewal.
Embryonic and fetal development takes place in a hypoxic environment, raising the possibility that hypoxia is a determinant of the proclivity for fetal but not adult tissues to give rise to extensive ex vivo erythroblast self-renewal. We therefore asked whether the hypoxic stress after the rapid induction of anemia might lead to the emergence of ESRE potential either in the bone marrow or in the spleen, the site of stress erythropoiesis in the adult mouse. However, the culture of bone marrow and spleen cells from phenylhydrazine-treated anemic mice yielded erythroblasts with restricted, but not extensive, self-renewal capacity (Figure 6). Taken together, our results from both outbred and C57BL/6J mice indicate that ex vivo erythroid self-renewal potential is associated with definitive hematopoiesis, and extensive ex vivo erythroid self-renewal is primarily associated with early definitive hematopoiesis that emerges in the yolk sac and transitions to the fetal liver.
Expression of the receptors for Epo, SCF, and cortisol in primary proerythroblasts
Epo, SCF, and glucocorticoids are each necessary to obtain both restricted and extensive ex vivo self-renewal of definitive erythroblasts. To investigate why primitive erythroid cells fail to self-renew in response to these factors, we examined the mRNA expression levels of their receptors, EpoR, Kit, and glucocorticoid receptor (Nr3c1), in primary primitive and definitive proerythroblasts. Primitive proerythroblasts were isolated from E9.5 yolk sac, fetal-definitive proerythroblasts were isolated from E11.5 livers, and adult-definitive proerythroblasts were isolated from bone marrow. EpoR, Kit, and Nr3c1 transcripts were expressed at similar levels in fetal- and adult-definitive proerythroblasts (Figure 7A). Although primitive proerythroblasts also expressed similar levels of EpoR compared with adult-definitive proerythroblasts, they contained 94-fold and 169-fold lower levels of Kit and glucocorticoid receptor, respectively (Figure 7A). These results suggest that primitive erythroblasts fail to self-renew because they lack the ability to respond both to SCF and to glucocorticoids.
Expression of Epo, SCF, and cortisol and their receptors. (A) Primary definitive proerythroblasts (ProEs) express receptors for Epo (EpoR), glucocorticoids (Nr3c1), and SCF (Kit). In contrast, primary primitive ProEs, derived from E9.5 YS, express similar levels of EpoR but nearly undetectable levels of Nr3c1 and Kit (mean ± SEM; N = 3; *P < .001, t test). (B) ESREs expressed high levels of cMyb transcripts compared with fetal liver and bone marrow definitive proerythroblasts. cMyb transcripts were not detected in primitive proerythroblasts. (C) Epo and SCF transcripts are expressed at higher levels in the mid-gestation fetal liver than in the adult bone marrow (BM) or kidney (mean ± SEM; N = 3). (D) Cortisol levels were detected by ELISA in fluid from embryonic and adult mouse tissues (mean ± SEM; N = 3). BD indicates below detection.
Expression of Epo, SCF, and cortisol and their receptors. (A) Primary definitive proerythroblasts (ProEs) express receptors for Epo (EpoR), glucocorticoids (Nr3c1), and SCF (Kit). In contrast, primary primitive ProEs, derived from E9.5 YS, express similar levels of EpoR but nearly undetectable levels of Nr3c1 and Kit (mean ± SEM; N = 3; *P < .001, t test). (B) ESREs expressed high levels of cMyb transcripts compared with fetal liver and bone marrow definitive proerythroblasts. cMyb transcripts were not detected in primitive proerythroblasts. (C) Epo and SCF transcripts are expressed at higher levels in the mid-gestation fetal liver than in the adult bone marrow (BM) or kidney (mean ± SEM; N = 3). (D) Cortisol levels were detected by ELISA in fluid from embryonic and adult mouse tissues (mean ± SEM; N = 3). BD indicates below detection.
cMyb has been shown to be a target of glucocorticoid receptor signaling.22 Given the extremely low levels of glucocorticoid receptor gene expression in primitive, but not definitive, erythroblasts, we examined the expression of cMyb in primary-primitive, fetal-definitive, and adult-definitive proerythroblasts, as well as in ESREs. Consistent with published data,23 we were unable to detect cMyb transcripts in E9.5 primitive erythroblasts (Figure 7B). In contrast, cMyb transcripts are expressed in fetal liver and bone marrow proerythroblasts. Furthermore, extremely high levels of cMyb transcripts were detected in ESREs (Figure 7B).
Epo, SCF, and cortisol are found in the fetal liver
During murine ontogeny, the erythron undergoes a massive expansion in the fetal liver between E12.5 and E16.5, leading to a 275-fold increase in circulating definitive erythrocytes over the course of these 4 days.21,24 We asked whether Epo, SCF, and cortisol, the exogenous factors driving erythroid precursor self-renewal ex vivo, are present in vivo. Both Epo and SCF transcript levels were markedly higher in the fetal liver than in the kidney and bone marrow, their adult sites of synthesis, respectively (Figure 7C). Cortisol was found in amniotic fluid, fetal liver, and peripheral blood at both E11.5 and E12.5, although at lower concentrations than that found in adult serum (Figure 7D). These results indicate that all 3 factors are present in the fetal liver and raise the possibility that erythroblast self-renewal might occur in vivo.
Discussion
The ex vivo culture of primary erythroid cells from the fetal liver, cord blood, and bone marrow of mammalian species in the presence of Epo, SCF, and dexamethasone results in significant, but ultimately restricted, proliferation of definitive erythroblasts.6,9-15,19,25-28 Here, we report that the yolk sac and early fetal liver of murine embryos give rise to erythroblasts that can proliferate for months ex vivo in serum-free conditions. The majority of the cells divide daily while maintaining their phenotype as large, Kithigh cells. These ESREs retain their ability to terminally mature into enucleated RBCs, even after 106- to 1060-fold expansion, all the while remaining entirely dependent on the combinatorial action of Epo, SCF, and glucocorticoid signaling for their survival and proliferation. ESREs can be grown at very low cell concentrations, indicating that their proliferation is not dependent on a more complex set of exogenous factors.
Erythropoiesis is characterized by the progressive maturation of lineage-committed progenitors, capable of colony formation in semisolid media, to morphologically recognizable precursors. To better define the cellular identity of ESREs along this continuum, we analyzed their morphology, gene expression, cell-surface phenotype, and kinetics of cellular maturation. ESREs are large cells with uncondensed chromatin and basophilic cytoplasm, similar to the morphology of proerythroblasts. Like proerythroblasts, ESREs express moderate-to-high levels of Kit and CD71 and low levels of Ter119 on their cell surface.20 Furthermore, like proerythroblasts, ESRE maturation is associated with an approximate 8- to 16-fold increase in cell number.2 Taken together, these findings indicate that proliferating ESREs are erythroid cells at the immature precursor stage of maturation, just 3-4 cell divisions from an enucleated RBC. It is surprising that immature erythroblasts, cells so close to terminal maturity within the hematopoietic hierarchy, are capable both of restricted and of extensive self-renewal when cultured in vitro.
Our studies indicate that ex vivo erythroid self-renewal is strictly associated with definitive erythroid cells, because ESREs exclusively express adult globins and are similar in size to primary immature definitive erythroblasts. Erythroid ontogeny is characterized by the overlapping development of 3 distinct lineages.29 The first lineage, primitive erythropoiesis, which transiently emerges from the yolk sac of the mouse conceptus at E7.25, generates a semisynchronous wave of erythroblasts that mature in the circulation and uniquely express embryonic globins.21,30 The second lineage is a transient definitive erythroid lineage that emerges from the yolk sac at E8.5-E9.5 as a wave of definitive erythro-myeloid progenitors that are thought to colonize the fetal liver by E10.5.18,31,32 The third erythroid lineage is a continuous, definitive erythroid lineage generated by HSCs that emerge from arterial beds at E10.5, colonize the liver around E12.5, and ultimately colonize the bone marrow in the perinatal period.33-35 Given this backdrop of erythroid ontogeny, we analyzed the emergence of erythroid-restricted and extensive self-renewal potential in murine embryos. The potential for restricted ex vivo erythroblast self-renewal first emerges with that of definitive erythroid progenitors in the E8.5 yolk sac, is found in the liver throughout fetal life, and persists postnatally in the bone marrow and spleen, sites of adult murine erythropoiesis. In contrast, the presence of extensive ex vivo erythroid self-renewal potential is correlated temporally and spatially with the transient definitive erythroid lineage that emerges from the yolk sac and colonizes the early fetal liver.
In contrast to definitive erythroid precursors, primitive erythroid precursors do not self-renew ex vivo in the presence of Epo, SCF, and dexamethasone. Given that these factors are each required in concert for ex vivo self-renewal, it is probable that the lack of primitive erythroid self-renewal potential is because of the extremely low levels of Kit and glucocorticoid receptor expression in primitive erythroblasts. We also found that cMyb is preferentially expressed in primary definitive versus primitive proerythroblasts, consistent with its known role in definitive, but not primitive, erythropoiesis.23,36 cMyb is a target of glucocorticoid receptor signaling and can maintain ex vivo erythroblast proliferation in the absence of glucocorticoid signaling.22,37 Constitutive activation of cMyb blocks the maturation of murine erythroleukemia cells.38 Interestingly, we found high levels of cMyb transcripts in ESREs, suggesting that cMyb may play a role in the self-renewal of definitive erythroblasts. Taken together, these data indicate that there are marked differences in cytokine signaling between primitive and definitive erythropoiesis and that these differences correlate with the ability of definitive, but not primitive, erythroid precursor cells to undergo self-renewal cell divisions. We postulate that induction of self-renewal divisions may serve as a mechanism to regulate definitive erythropoiesis. The rapid increase in immature erythroid precursor cell numbers would ultimately result, after 3-4 rapid maturational cell divisions, in the acute expansion of RBC output.
The frequency with which ESRE cultures are established from embryonic and fetal tissues is influenced by the make-up of the culture media. Erythroid cells in culture were more likely to extensively self-renew if they were grown in StemSpan-based than in StemPro34-based erythroid expansion media. Although there were no differences in the growth kinetics during self-renewal, ESREs cultured in StemSpan-based erythroid expansion media yielded twice as many RBCs after their transfer into erythroid maturation media. For these reasons, we favored the use of StemSpan. However, the proprietary nature of these serum-free media prevented us from exploring the causes for their differential effects.
The emergence of blood in cultures of differentiating embryonic stem cells appears to recapitulate yolk sac hematopoiesis, because both are characterized by overlapping waves of primitive and definitive erythroid progenitors.18,39 Carotta et al15 reported the prolonged outgrowth of erythroid “progenitors” with a definitive erythroid phenotype from days 6-9 EBs. We have generated cultures of ESREs from murine embryonic stem cells that are indistinguishable from ESREs derived from primary embryonic tissues (supplemental Figure 1). These results raise the possibility that ESREs derived from human embryonic stem cells and induced pluripotent stem cells may ultimately serve as a renewable source of RBCs for cell replacement therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Anne Koniski, Paul D. Kingsley, and Michael Bulger for help with cell culture and for helpful discussions throughout these studies.
This work was supported by grants from New York Stem Cell Science (NYSTEM) and from NIH/NIDDK (DK09361).
National Institutes of Health
Authorship
Contribution: S.J.E. designed and performed experiments, analyzed data, and wrote the paper; J.M.F. performed experiments; K.E.M. designed experiments and analyzed data; J.P. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James Palis, University of Rochester Medical Center, Department of Pediatrics, Center for Pediatric Biomedical Research, 601 Elmwood Ave, Rochester, NY 14642; e-mail: james_palis@urmc.rochester.edu.