Abstract
Few large, international series of enteropathy-associated T-cell lymphoma (EATL) have been reported. We studied a cohort of 62 patients with EATL among 1153 patients with peripheral T-cell or natural killer (NK)–cell lymphoma from 22 centers worldwide. The diagnosis was made by a consensus panel of 4 expert hematopathologists using World Health Organization (WHO) criteria. Clinical correlations and survival analyses were performed. EATL comprised 5.4% of all lymphomas in the study and was most common in Europe (9.1%), followed by North America (5.8%) and Asia (1.9%). EATL type 1 was more common (66%) than type 2 (34%), and was especially frequent in Europe (79%). A clinical diagnosis of celiac sprue was made in 32.2% of the patients and was associated with both EATL type 1 and type 2. The median overall survival was only 10 months, and the median failure-free survival was only 6 months. The International Prognostic Index (IPI) was not as good a predictor of survival as the Prognostic Index for Peripheral T-Cell Lymphoma (PIT). Clinical sprue predicted for adverse survival independently of the PIT. Neither EATL subtype nor other biologic parameters accurately predicted survival. Our study confirms the poor prognosis of patients with EATL and the need for improved treatment options.
Introduction
Enteropathy-associated T-cell lymphoma (EATL) is a primary intestinal lymphoma that is often, but not always, associated with celiac sprue.1-9 It is a rare disease in most parts of the world, with an annual incidence rate of 0.5-1 per million.10,11 Because EATL is rare, large systematic studies of this lymphoma are scarce. EATL occurs predominantly in middle-aged men and is localized primarily in the small bowel. This lymphoma is strongly associated with celiac sprue, with a variable time lapse of a few months to several decades between the diagnosis of celiac sprue and the onset of lymphoma.10,12 EATL has a poor prognosis due to treatment resistance and sepsis or perforation of the bowel at diagnosis or during the course of treatment.
Recent studies indicate that EATL consists of 2 diseases that are morphologically and genetically distinct and differ with respect to their frequency of association with celiac sprue.13-20 The first type of EATL (type 1) is characterized genetically by chromosome 9q31.3 gain or 16q12.1 deletion and is strongly associated with celiac sprue and the HLA-DQ2 haplotype. Interestingly, these lymphomas frequently have a large-cell or pleomorphic cytology and may express CD30. In contrast, EATL type 2 is characterized by chromosome 8q24 gain and, less commonly, by 1q and 5q gains. EATL type 2 is less frequently associated with celiac sprue and the HLA-DQ2 haplotype13,16 and is characterized by monomorphic cytology with frequent expression of CD56.
In an effort to assess the clinical applicability of the World Health Organization (WHO) classification of peripheral T- and natural killer (NK)–cell lymphomas, and to evaluate the efficacy of the therapies used, a large international study was conducted.21 The clinical and pathologic findings of the EATL cases included in the study are reported here.
Methods
Case selection and collection of clinical data
The cases in this study are part of the International Peripheral T-Cell Lymphoma Project. The study was approved by the institutional review boards of all participating centers, and an overview of the entire project has been published previously by Vose et al.21 Briefly, the cases were collected from 22 centers in North America, Europe, and Asia. All patients were adults (> 19 years) with a primary diagnosis of peripheral T-cell lymphoma (PTCL) or NK-cell lymphoma made between January 1, 1990 and December 31, 2002. The clinical features of the patients were required for all cases and data were collected as described by Vose et al.21 Cases of mycosis fungoides and Sézary syndrome were excluded.
Histology review
Local pathologists submitted representative diagnostic materials, including a paraffin-embedded tissue block, to a regional center for more detailed and standardized immunophenotyping and review. The immunophenotyping consisted of stains for CD20, CD3, CD4, CD5, CD8, CD30, CD56, TCR-beta, TIA-1, and Ki67. In situ hybridization for EBV was also performed. Clonality analyses or FISH studies for defined genetic aberrations were performed as necessary to classify the cases according to the 2001 WHO classification.22 Panels of 4 expert hematopathologists drawn from the contributing local sites and regional centers traveled to the regional centers to review the cases. The composition of the panels differed at the various regional centers. A consensus diagnosis was rendered when at least 3 experts in the respective panel agreed on the diagnosis.
In total, 1314 cases were reviewed and a diagnosis of PTCL or NK-cell lymphoma was confirmed in 1153 cases. A diagnosis of enteropathy-type TCL was made when the patient presented with a tumor in the intestine composed of EBV-negative lymphoma cells with a mature T-cell immunophenotype. Cases diagnosed as enteropathy-type TCL were renamed EATL and were subsequently further classified by 2 of the hematopathologists (D.D.W. and J.D.) into 2 subtypes according to the 2008 WHO classification.23 This subdivision was based on histologic analysis and did not take into account clinical evidence of celiac sprue. The first subtype is called EATL type 1 for the purposes of this study, although this terminology is not used in the WHO classification. EATL type 1 shows a variable histology consisting of either a monomorphic infiltrate of medium-sized to large lymphoma cells with irregular nuclear contours and a variable amount of cytoplasm or large anaplastic lymphoma cells. EATL type 1 may also show a marked polymorphism with variable numbers of eosinophils, histiocytes, small lymphocytes, and plasma cells. For EATL type 1, histologic features of celiac sprue in the adjacent mucosa not infiltrated with lymphoma were investigated. However, adjacent noninfiltrated mucosa was not present in all cases. Celiac sprue was diagnosed when increased numbers of intra-epithelial lymphocytes (> 30 of 100 enterocytes) along with either crypt hyperplasia or villus atrophy was noted, as reviewed by Green et al.24 EATL type 2 is characterized by a monomorphic infiltrate of small- to medium-sized lymphoid cells with round, hyperchromatic nuclei having a stippled chromatin pattern. We also investigated whether intra-epithelial infiltration of the mucosa by lymphoma cells was present.
Statistical analysis
Treatment outcomes were measured by failure-free survival (FFS) and overall survival (OS). FFS was defined as the time from initial diagnosis to the first occurrence of progression, relapse after response, or death from any cause. Follow-up of patients not experiencing one of these events was censored at the date of last contact. OS was measured from the time of initial diagnosis to death from any cause, with surviving patient follow-up censored at the last contact date. Estimates of FFS and OS were determined using the method of Kaplan and Meier,25 and time-to-event distributions were compared using the log-rank test.26 The Cox proportional hazards regression model was used to test whether pathologic or clinical features or a history of celiac sprue predicted for FFS or OS. This was done after controlling for clinical prognostic indices such as the International Prognostic Index (IPI)27 and the Prognostic Index for PTCL, unspecified (PIT),28 respectively.
Results
Histology
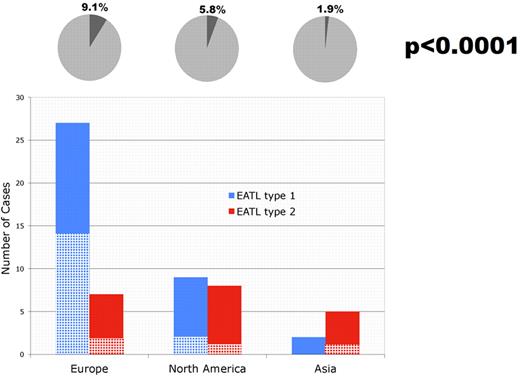
Of the 68 cases in the study cohort in which a local diagnosis of EATL was submitted, only 62 cases were considered to be EATL after review. Three cases were diagnosed as PTCL, unspecified; 1 as anaplastic large-cell lymphoma; and 2 as extranodal NK-/T-cell lymphoma, nasal type. In total, 5.4% of the cases (62 of 1153) were considered to be EATL after review, thus representing the seventh largest category of PTCL in the cohort. The expert agreement with the consensus diagnosis of EATL was 79%. Significant regional differences were noted in the relative frequency of EATL, which represented 1.9%, 5.8%, and 9.1% of PTCL in Asia, North America, and Europe, respectively (P < .0001). EATL type 1 constituted 66% (38 of 58) and EATL type 2 constituted 34% (20 of 58) of the cases available for subclassification. In 4 cases, the materials were no longer available for subclassification at the time this analysis was performed. The typical histology of EATL type 1 and type 2 is illustrated in Figures 1 and 2, respectively, and the immunophenotypes are given in Table 1. As expected, EATL type 2 expressed CD8 and CD56 more often than EATL type 1. EATL type 1 was more common than type 2 in Europe, whereas the latter was more common in Asia. EATL types 1 and 2 were equally frequent in North America (Figure 3). An association with a clinical history of celiac sprue was noted for both EATL type 1 and type 2, representing 16 of 33 cases and 4 of 20 cases, respectively (Table 2). Histological features of celiac sprue were seen in 11 of 28 cases of EATL type 1 in which the mucosa adjacent to the tumor could be investigated. European cases were most frequently associated with celiac sprue when the clinical history and histologic features were combined (Figure 3). All 13 cases of EATL type 2 in which adjacent mucosa was included in the biopsy showed intra-epithelial lymphoma with or without villous atrophy. This feature precluded histological evaluation for celiac disease in EATL type 2.
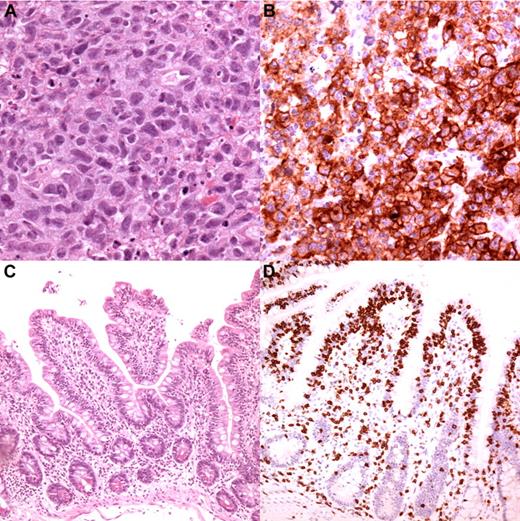
Typical EATL type 1 histology from one of the cases in the study. (A) The cells are large with irregularly shaped or angulated nuclei and a variable amount of cytoplasm (magnification, 250×). (B) The cells in this case expressed CD30 (magnification, 250×). The mucosa adjacent to the tumor had shortened villi (C; magnification, 100×) and “top-heavy” intra-epithelial infiltration by T cells (D; CD3 staining, magnification, 100×).
Typical EATL type 1 histology from one of the cases in the study. (A) The cells are large with irregularly shaped or angulated nuclei and a variable amount of cytoplasm (magnification, 250×). (B) The cells in this case expressed CD30 (magnification, 250×). The mucosa adjacent to the tumor had shortened villi (C; magnification, 100×) and “top-heavy” intra-epithelial infiltration by T cells (D; CD3 staining, magnification, 100×).
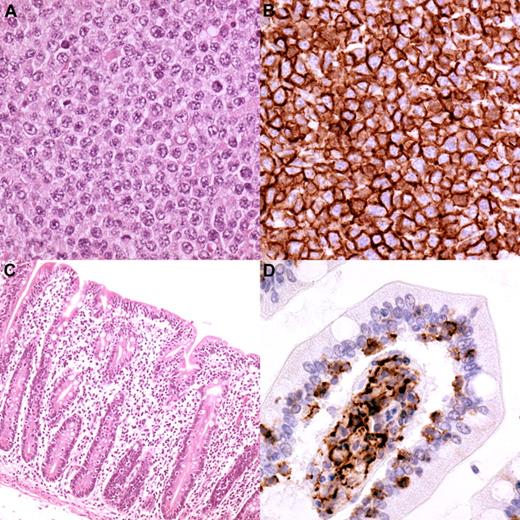
Typical EATL type 2 histology from one of the cases in the study. (A) The lymphoma consists of monotonous, medium-sized cells with round nuclei having a stippled chromatin pattern (magnification, 250×). (B) The cells express CD56 (magnification, 250×). The mucosa adjacent to the lymphoma, shows villous atrophy (C; magnification, 100×) and intra-epithelial infiltration by lymphoma cells (D; CD56 staining, magnification, 400×).
Typical EATL type 2 histology from one of the cases in the study. (A) The lymphoma consists of monotonous, medium-sized cells with round nuclei having a stippled chromatin pattern (magnification, 250×). (B) The cells express CD56 (magnification, 250×). The mucosa adjacent to the lymphoma, shows villous atrophy (C; magnification, 100×) and intra-epithelial infiltration by lymphoma cells (D; CD56 staining, magnification, 400×).
EATL and celiac sprue. The percentage of EATL cases (dark area) among all PTCL cases (circle) in the 3 geographic regions is depicted on top. The number of cases of EATL type 1 (blue columns) and type 2 (red columns) is also depicted for the different regions. The crossed areas of the columns represent the number of cases with a clinical history of celiac sprue.
EATL and celiac sprue. The percentage of EATL cases (dark area) among all PTCL cases (circle) in the 3 geographic regions is depicted on top. The number of cases of EATL type 1 (blue columns) and type 2 (red columns) is also depicted for the different regions. The crossed areas of the columns represent the number of cases with a clinical history of celiac sprue.
Clinical data
The clinical features of the cases are given in Table 3. EATL occurred most frequently in the sixth decade, with an almost equal distribution among the sexes. Abdominal pain, fatigue, and anorexia were the most frequent symptoms. Approximately one-fifth of the patients presented with signs of infection, but there was no clinical information on perforation of the bowel available in the study. Whereas the clinical disease stage was usually advanced at diagnosis, bone marrow involvement was a rare occurrence. Except for tumor size at diagnosis, no significant clinical differences were noted between EATL types 1 and 2, including a clinical history of celiac sprue (P = .13). A tumor of ≥ 5 cm at diagnosis was reported in 11 of 24 patients with EATL type 1 versus 15 of 18 with EATL type 2 (P = .02). There was no correlation between the tumor size at diagnosis and a history of celiac sprue. As expected, the small intestine was the most commonly involved site at diagnosis (90%), followed by the large intestine (16%), with frequent involvement of mesenteric (35%) and para-aortic or iliac lymph nodes (11%; Table 4). In one case, EATL type 1 involved the stomach but not the small intestine. In 3 cases, EATL involved the large intestine without involving the small intestine; 2 of these cases were EATL type 2 but the slides of the third case were not available for subtype review. The small and large intestine were simultaneously involved in 7 cases, of which 3 were EATL type 1 and 4 were type 2.
Treatment was administered in 56 of the patients, whereas no treatment could be given in 5 patients because of their general condition. In one patient, no information on treatment could be retrieved. Initial treatment in 52 patients consisted of combination chemotherapy containing anthracycline; of these patients, 27 received CHOP, 8 CHOP-like, 3 MACOP-B, 2 ACVB, 2 CEOP, and 2 CHO chemotherapy. The remaining 8 patients received a diverse combination of chemotherapy regimens. Complete response, partial response, or no response was observed in 40%, 16%, and 38% of the patients, respectively, whereas no data were available for the other patients. None of the patients received consolidative autologous stem cell transplantation in first remission. No data with respect to salvage therapy was recorded for this study.
Survival analysis
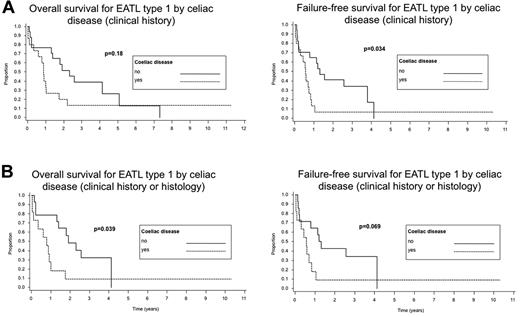
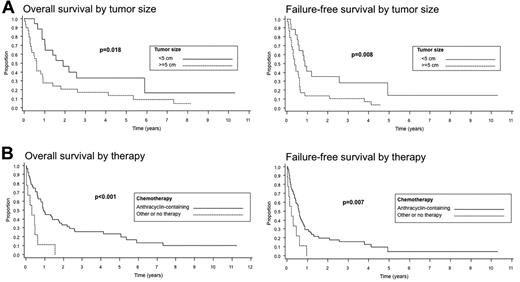
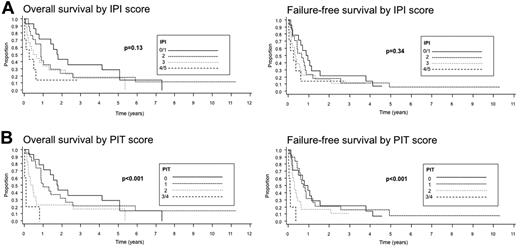
Survival data were available for 61 of the 62 patients. The median follow-up was 10.5 months. The median OS was only 10 months and the FFS was only 6 months. The 5-year OS was 20%, but the 5-year FFS was only 4%. In univariate analysis, there was a significantly decreased FFS in cases with a history of celiac sprue compared with those without sprue documented in the clinical records (P = .02); however, there were no significant differences in OS between these 2 groups (P = .17). When survival with respect to a history of celiac sprue was analyzed only for EATL type 1, similar results were obtained; a lower FFS was seen in the patients with celiac sprue (P = .03), but no significant difference in OS was noted (P = .18; Figure 4A). The survival analysis for EATL type 1 was repeated to include cases with either a clinical history or histological evidence of celiac disease (Figure 4B). Interestingly, a significantly decreased OS was observed for patients with celiac sprue (P = .039), whereas only a borderline decrease in FFS was seen (P = .069). The number of EATL type 2 cases with a clinical history of celiac sprue was too small (n = 4) to perform survival analysis. Disease stage (stage III or IV) and age ≥ 60 years did not predict for OS or FFS (P = .95 and P = .73, P = .34 and P = .72, respectively), but a large tumor mass (≥ 5 cm) at diagnosis (Figure 5A), a nonambulatory performance status, an elevated serum LDH level, and increased C-reactive protein (CRP) were adverse predictors of survival (P values for OS are P = .018, P < .005, P = .008, and P = .003 for OS, and P = .008, P < .001, P < .001, and P = .01 for FFS, respectively). The use of anthracycline-containing chemotherapy improved the OS and FFS compared with other therapies or no therapy (P < .001 and P = .007, respectively), although the number of patients not receiving an anthracycline-containing regimen was small (n = 9) compared with those receiving such a regimen (n = 51; Figure 5B). No analysis of second-line treatment could be performed because this information was not recorded in the study. The IPI did not stratify patients well with regard to OS or FFS (P = .13 and P = .34, respectively), but the PIT was a better predictor of survival (P < .001 and P < .001, respectively; Figure 6).
Survival of patients with EATL type 1 according to the presence of celiac disease. (A) Celiac disease as evidenced by review of the clinical records alone. (B) Celiac disease as evidenced either by review of the clinical records or review of the histology.
Survival of patients with EATL type 1 according to the presence of celiac disease. (A) Celiac disease as evidenced by review of the clinical records alone. (B) Celiac disease as evidenced either by review of the clinical records or review of the histology.
Survival of patients with EATL according to tumor size (A) and therapy with or without an anthracycline (B).
Survival of patients with EATL according to tumor size (A) and therapy with or without an anthracycline (B).
Survival of patients with EATL according to IPI scores (A) and PIT scores (B).
In multivariate analysis, only a clinical history of celiac sprue predicted FFS when controlling for the PIT (P = .03). A history of celiac sprue did not predict OS (P = .17). A history of celiac sprue also predicted FFS when only EATL type 1 was considered (P = .04), but did not predict OS (P = .15). None of the other clinical features of EATL predicted for OS or FFS when controlling for the PIT.
None of the immunohistochemical markers, including CD8, CD56, CD30, or TIA1, were predictors of OS (P = .80, P = .49, P = .16, and P = .55, respectively) or FFS (P = .73, P = .46, P = .33, and P = .47, respectively) in univariate analysis. When using several cutoff scores at 10% intervals for the proliferation-associated marker Ki67, no significant correlation with survival was found (P = .41 for OS and and P = .16 for FFS for a 70% cutoff value). In addition, there were no significant differences in OS (P = .28) or FFS (P = .22) between patients with EATL type 1 and type 2.
Discussion
As seen in previously published studies of EATL7,13,29,30 and corroborated by the present study, it is evident that EATL as defined by the WHO classification has distinctive histological and clinical characteristics. This lymphoma usually involves the small intestine, and most patients present with abdominal pain or signs of intra-abdominal sepsis. The majority of EATL patients have advanced-stage disease and their prognosis is dismal. The latter may be explained, at least in part, by frequent perforation of the bowel and infection related to tumor progression or treatment. Three previous studies7,29,30 consisting of 30, 31, and 80 patients, respectively, reported a significant predominance of men affected by EATL. However, our series of 62 patients, as well as the study of 70 patients by Chott et al,13 showed only a slight predominance of men. Agreement between the expert hematopathologists and the consensus diagnosis of EATL was relatively high (79%), indicating that criteria for the diagnosis of EATL in the WHO classification are well established. The 2001 WHO classification was used in this study because the pathology review was done before 2008. The 2001 criteria are similar to those in the 2008 classification, with the exception of recognition of EATL type 2 in the latter. However, EATL type 2 would still have been diagnosed as EATL in the 2001 WHO classification.
EATL comprised only 5.4% of all PTCL in our study, which makes it rather rare, and there were notable regional differences in its relative frequency. In our study, EATL was 5 times more frequent in Europe than in Asia, whereas the frequency in North America was intermediate. These data are interesting but need to be interpreted with caution in view of the fact that our study was not population based, but rather institution based, which may lead to a biased selection of cases. Data from another institution-based study from Taiwan31 of 600 PTCL cases showed EATL to have a relative frequency of 8.1%, which is almost as high as we observed in Europe. Additional studies of this disease in different parts of the world are needed to clarify this issue.
Previous studies have identified 2 subtypes of EATL.13-20 EATL type 1 consists of medium-sized to large or pleomorphic cells and usually develops in the background of celiac sprue. As with celiac sprue, EATL type 1 is usually associated with the HLA-DQ2 or, less frequently, with the HLA-DQ8 haplotype. EATL type 1 may sometimes develop from an in situ lesion associated with celiac sprue that is refractory to therapy. EATL type 2 consists of monomorphic small- to medium-sized cells that usually express CD8 and CD56, and is not strongly associated with either celiac sprue or with the HLA-DQ2 or HLA-DQ8 haplotypes. A precursor lesion is not described for this subtype, although an intra-epithelial component of T cells with an immunophenotype similar to the tumor cells is frequently observed,23 as confirmed by our study.
The two subtypes of EATL also have different patterns of genetic aberrations.14-16,18,20 In this international series, EATL type 1 was the most frequent subtype (66%). Previous reports indicate that EATL type 1 has a high frequency in Western countries, comprising approximately 80% of cases.13 In contrast, EATL type 2 has been reported to be the predominant subtype in Asia, representing > 90% of cases.18-20 The predominance of EATL type 1 in the West and type 2 in the East is confirmed by our study, although the number of cases from the East was low. Celiac sprue is a known predisposing factor for EATL, and has been reported to be especially associated with EATL type 1, as reviewed by Van de Water et al.12 However, our data show that EATL type 2 can also arise in patients with celiac sprue. This latter association does not seem to show a regional variation despite the observed differences in the frequency of EATL (Figure 3). These observations need to be confirmed by a study consisting of a larger number of well-documented cases from Asia. Our data also show that patients with EATL types 1 and 2 do not display significantly different clinical features, with the exception of larger tumor masses in those with EATL type 2. In addition, EATL type 1 and type 2 have an equally poor prognosis, which is in agreement with a previous study.13
Our study showed a significant correlation between a clinical history of celiac sprue and FFS in EATL that was independent of the PIT. Such a correlation could not be shown for OS. However, because celiac sprue is often undiagnosed,32 it was likely underestimated in our series. Celiac sprue is the most common genetic disease in North America and Europe, affecting 0.5% to 1% of the population. As many as 90% of cases are undiagnosed because of the diversity of symptoms with which patients present.32 Therefore, the association of EATL with celiac sprue is likely to underestimated in most studies. Future studies should include more sensitive methods for the detection of celiac sprue, such as measurement of anti-endomysial and anti-tissue transglutaminase antibodies in addition to endoscopic small bowel biopsy, to determine the true incidence of celiac sprue among EATL patients and to determine more accurately its correlation with survival.
A large tumor mass (≥ 5 cm), nonambulatory performance status, and elevated serum LDH and CRP levels are risk factors significantly associated with a worse OS and FFS in EATL. A large tumor mass often results in complications such as perforation or infection, and nonambulatory patients may have disease complications or not be able to receive aggressive therapy. Similarly, a high serum LDH level is usually a reflection of high tumor burden. CRP is produced by hepatocytes secondary to inflammatory cytokines such as IL6, TNFα, and IL1β and is a marker of inflammation. High serum CRP levels may reflect extensive tissue damage, ulceration, perforation, and secondary inflammation in EATL, and therefore may explain the adverse prognosis. Interestingly, the IPI27 did not stratify patients well into prognostic subgroups, whereas the PIT28 was more predictive of survival. This may have been due to the fact that age, the number of extranodal sites, and tumor stage, 3 of the 5 components of the IPI, are irrelevant for EATL prognosis. Indeed, our study indicates that tumor size rather than tumor stage defines the prognosis in EATL. In contrast, the PIT uses only 4 parameters: LDH, age, bone marrow involvement, and performance status. Of these, only bone marrow involvement may not be relevant to prognosis in EATL, because only a few patients (3.2%) had this feature in our study.
Patients treated with anthracycline-based chemotherapy regimens had better survival rates than those treated with other forms of therapy (including surgery) or no therapy at all. However, these results can be explained by the likely possibility that patients not receiving anthracycline-based chemotherapy either died before any therapy could be given or shortly after surgery for advanced or complicated disease. In addition, the number of patients not receiving anthracycline-based chemotherapy (n = 9) was rather low. It is clear that novel treatment strategies are needed for patients with EATL. A recent study by Sieniawski et al30 showed that patients treated with high-dose ifosfamide, etoposide and epirubicin/methotrexate followed by autologous stem cell transplantation had a significantly improved survival. However, this might have been due, at least in part, to the selection of patients who better tolerate this intensive therapy. Nevertheless, high-dose chemotherapy with stem cell transplantation was well tolerated in this patient group, and it was estimated that approximately 70% of all EATL patients may benefit from this therapy.30 In addition, the early detection of celiac sprue using improved screening methods and close clinical follow-up would likely result in earlier detection of EATL and perhaps better survival rates.
It is increasingly clear that celiac disease is also associated with lymphomas other than EATL, in particular diffuse large B-cell lymphoma.33-35 However, the latter was not studied in the International Peripheral T-Cell Lymphoma Project.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Martin Bast for the data collection; Ventana Medical Systems, Inc. (Tucson, AZ) for donation of the use of a Vantana XT immunostainer for the project; and Dr Inger-Nina Farstad for carefully reading the manuscript.
Authorship
Contribution: J.D. collected the data, contributed materials, participated in the pathology review, analyzed and interpreted the data, and wrote the manuscript; H.H., K.J.S., J.M.C., B.C., M.F., K.T., W.Y.A., R.L., E.M., and E.P.H collected and interpreted the data; J.M.V. designed the research and collected and interpreted the data; F.U. analyzed the data; E.S.J., L.R., N.L.H, K.M.-H., T.R., R.D.G., F.B., and B.N. contributed materials, participated in the pathology review, and interpreted the data; S.P. contributed materials and reagents, participated in the pathology review, and interpreted the data; J.O.A. designed the research and collected, analyzed, and interpreted the data; and D.D.W. designed the research, collected the data, contributed materials and reagents, participated in the pathology review, and analyzed and interpreted the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the participants in the International Peripheral T-Cell Lymphoma Project appears in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Jan Delabie, MD, PhD, Department of Pathology, The Norwegian Radium Hospital, University of Oslo, Montebello N-0310, Oslo, Norway; e-mail: jan.delabie@medisin.uio.no.