Abstract
Romiplostim, a thrombopoietin-mimetic peptibody, increases and maintains platelet counts in adults with immune thrombocytopenia (ITP). In this first study of a thrombopoietic agent in children, patients with ITP of ≥ 6 months' duration were stratified by age 1:2:2 (12 months-< 3 years; 3-< 12 years; 12-< 18 years). Children received subcutaneous injections of romiplostim (n = 17) or placebo (n = 5) weekly for 12 weeks, with dose adjustments to maintain platelet counts between 50 × 109/L and 250 × 109/L. A platelet count ≥ 50 × 109/L for 2 consecutive weeks was achieved by 15/17 (88%) patients in the romiplostim group and no patients in the placebo group (P = .0008). Platelet counts ≥ 50 × 109/L were maintained for a median of 7 (range, 0-11) weeks in romiplostim patients and 0 (0-0) weeks in placebo patients (P = .0019). The median weekly dose of romiplostim at 12 weeks was 5 μg/kg. Fourteen responders received romiplostim for 4 additional weeks for assessment of pharmacokinetics. No patients discontinued the study. There were no treatment-related, serious adverse events. The most commonly reported adverse events in children, as in adults, were headache and epistaxis. In this short-term study, romiplostim increased platelet counts in 88% of children with ITP and was well-tolerated and apparently safe.
The trial was registered with http://www.clinicaltrials.gov as NCT00515203.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder of children and adults characterized by accelerated platelet destruction as well as suboptimal platelet production.1,2 The resulting thrombocytopenia may be asymptomatic and incidentally detected or manifested by bleeding ranging in severity from petechiae and ecchymoses to intracranial hemorrhage.1-5 In the United States and Europe, ITP affects children at a rate of 1.9-6.4 per 100 000 children-years6-9 but is infrequently chronic in children. At 6 months after diagnosis, between 53% and 77% of patients ages 4 months to 16 years will have recovered from ITP, depending on the age of the patients.10 Twelve months after diagnosis, a further 26% will have recovered, leaving 17%-34% with chronic ITP, only some of whom will require treatment.11 Because only 5%-10% of all pediatric ITP patients will have severe, chronic, and/or refractory disease, experience with treating these children is quite limited and there are no randomized clinical trials of treatments for these patients. Therapeutic agents for childhood ITP—corticosteroids, intravenous immunoglobulin, and anti-D immunoglobulin12-16 ; azathioprine and rituximab17-19 ; and splenectomy15,17,20-24 —may be effective but may have limitations with respect to long-term efficacy and/or safety.12-17,19-24
Two new thrombopoietin (TPO) receptor agonists that stimulate platelet production, romiplostim and eltrombopag, are now approved for the treatment of adults with chronic ITP in the United States, Europe, Australia, Japan, and elsewhere. Romiplostim is an Fc-fusion protein (also known as a peptibody)25 that increases platelet production by a mechanism similar to that of eTPO.26 Romiplostim has no amino acid sequence homology with endogenous TPO (eTPO),27 which reduces the probability that antibodies to romiplostim will bind to eTPO and cause thrombocytopenia, as was seen with first-generation agents.28 Eltrombopag is a small molecule that has also been tested in ITP. Both romiplostim and eltrombopag have been shown to be highly efficacious, well tolerated relative to other ITP treatments, and apparently safe in several trials.27,29-32 In a long-term, open-label extension study in adults, the administration of romiplostim increased platelet counts in most patients for up to almost 5 years and had an acceptable safety profile.33,34 No studies of these thrombopoietic agents have been performed to date in children.
This report describes the first (randomized) study conducted to evaluate romiplostim in the treatment of children with ITP. The primary objective of the study was to evaluate the safety and tolerability of romiplostim in the treatment of thrombocytopenia in pediatric patients with ITP persisting for 6 months or longer. Evaluation of the efficacy of romiplostim and characterization of its pharmacokinetics were secondary objectives.
Methods
Study design
This was a phase 1/2, multicenter, randomized, double-blind, placebo-controlled study in pediatric patients who had been diagnosed with ITP at least 6 months previously. Patients were enrolled from 10 centers in the United States, Spain, and Australia between July 19, 2007 (first patient enrolled), and March 3, 2009 (last patient's end-of-study visit). Patients received study treatment for 12 weeks. At the end of the 12-week treatment period, patients who had had a platelet count of 20 × 109/L or greater above baseline for 2 consecutive weeks in the absence of rescue therapy at any point during the treatment period (responders) were requested to enter a 4-week pharmacokinetics assessment period, during which they continued blinded treatment. Children who completed this trial were eligible to enter an open-label extension study to monitor the long-term safety and efficacy of romiplostim treatment.
The study protocol was approved by the institutional review board or independent ethics committee at the participating center before any patients enrolled in the study at that site. Written informed consent was obtained from the patient or from a legally acceptable representative (eg, parent) before the patient underwent any protocol-specific screening or study procedures. All authors had access to clinical data. The trial was registered with http://www.clinicaltrials.gov (NCT00515203).
Patients
Boys and girls from 12 months to 18 years of age who had been diagnosed with ITP according to American Society of Hematology guidelines15 at least 6 months before screening were eligible for the study if the average of 2 platelet counts taken within 21 days before enrollment was 30 × 109/L or less and no single count exceeded 35 × 109/L. Previous therapy (successful or failed) was not a requirement for study entry. Patients were excluded if they had undergone splenectomy within 8 weeks of the screening visit, if they were receiving any treatment for ITP except for corticosteroids, or if they had received rituximab within 14 weeks before the screening visit, alkylating agents within 8 weeks before the screening visit, hematopoietic growth factors within 4 weeks before the screening visit, or intravenous immunoglobulin or anti-D immunoglobulin within 2 weeks before the screening visit. Also excluded were patients who had a history of a BM stem cell disorder, venous or arterial thrombotic or thromboembolic event, systemic lupus erythematosus, or Evans syndrome.
Treatment
Romiplostim (Amgen Inc) and placebo were provided as lyophilized, white powders in glass vials and reconstituted as described previously.32 Patients were stratified by age into groups of children ages 12 months to younger than 3 years, 3 to younger than 12 years, and 12 to younger than 18 years in a 1:2:2 ratio and randomly assigned to treatment with romiplostim or placebo in a 3:1 ratio. Study treatment (romiplostim or placebo) was administered by study-center personnel once weekly at a starting dose of 1 μg/kg as determined by the patient's weight recorded at screening. Both study-site personnel and patients were blinded to treatment assignments. To achieve the target platelet count of 50 × 109/L to 250 × 109/L, doses of study drug were adjusted according to the algorithm in Table 1.35 The permitted doses ranged from 1 to 10 μg/kg.
Throughout the study, ITP medications were administered at the discretion of the investigators with the exception of the following prohibited medications: alkylating agents, cytotoxic drugs, pegylated recombinant human megakaryocyte growth and development factor, recombinant human thrombopoietin, rituximab, or any medication known or suspected to affect platelet production. Recommended rescue medications were intravenous immunoglobulin, platelet transfusions, and corticosteroids. Rescue medication was permitted in the case of bleeding or wet purpura, or if the investigator believed that the patient was at immediate risk (eg, preoperatively). An increase in dose or frequency of concurrent ITP medication above levels at study entry was also considered rescue medication. Reduction in baseline corticosteroids was allowed when platelet counts exceeded 50 × 109/L not as a result of rescue medication.
Assessments and outcome measures
All patients underwent a screening evaluation within 21 days before enrollment and returned to the study center weekly through week 12. Responders continued both treatment and additional weekly visits to the study center during the 4-week pharmacokinetics assessment period (weeks 13 through 16).
Physical examinations were performed at screening and at the end of the study. Vital signs, platelet counts, and adverse events were assessed before treatment and at each weekly visit. Height and weight were measured, and samples were taken for hematology and blood chemistry testing at screening and every 4 weeks throughout the study. Health-related quality of life was assessed at screening and periodically during the study with the Kids' ITP Tool.36 Results of health-related quality of life assessments have been reported elsewhere in abstract form37 ; details will be provided in a full manuscript (R. J. Klaassen, S. D. Mathias, L.R.B., et al, manuscript in preparation). Blood samples to test for antibodies to romiplostim were taken at week 1 and at the end of the study for all patients and also at week 13 for responders who entered the pharmacokinetics assessment period. Antibody assessments were performed as previously described.38
Blood samples for pharmacokinetics assessments were collected at week 12 for all patients and twice each week during the 4-week pharmacokinetics assessment period for patients who responded to study treatment. Samples were drawn before treatment injection and 2 days thereafter. Serum romiplostim concentrations were determined with a modification of a validated ELISA with a lower limit of quantification of 15 pg/mL.25
Safety was the primary outcome measure of the study and was assessed on the basis of the incidence of adverse events, including antiromiplostim antibody formation and anti-TPO antibody formation. Adverse events were collected continuously and captured on a standard case report form. Each investigator graded the severity of adverse events for their patients, including bleeding, according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.39
Efficacy was a secondary outcome measure and was assessed on the basis of the number of weeks platelet counts were ≥ 50 × 109/L during the treatment period, the total number of bleeding events with a grade of 2 or greater on the hemorrhage grading instrument developed by Buchanan and Adix for each patient during weeks 2-13,40 the percentage of patients who achieved a platelet count of 50 × 109/L or greater for 2 consecutive weeks during the 12-week treatment period, the percentage of patients who achieved an increase in platelet count of 20 × 109/L or greater above baseline for 2 consecutive weeks during the 12-week treatment period (treatment response), and the percentage of patients who received rescue therapy during the 12-week treatment period.
Statistical analysis
The nature of the analysis in this study was descriptive and the planned sample size of 15 in the romiplostim group and 5 in the placebo group was determined by the ability to recruit pediatric patients according to the required age distribution within the specified time frame. On the basis of the assumption that 80% of patients in the romiplostim group and 0% of patients in the placebo group would achieve a platelet count of 50 × 109/L or greater for 2 consecutive weeks during the first 12 weeks of the study, the power of Fisher exact test would be 93% at significance level .05.
Categorical end points were summarized by the number and percentage of patients in each category. Continuous end points were summarized by number of patients, mean, median, standard deviation, 25th and 75th percentiles, minimum, and maximum. To discount the possible effect of rescue medications, patients were considered to have had no platelet response for 4 weeks after any administration of rescue medications. The treatment groups were compared with respect to key efficacy end points on an ad hoc basis with the Fisher exact test or the Cochran-Mantel-Haenszel test. Summary statistics of key efficacy end points were reported within each age group.
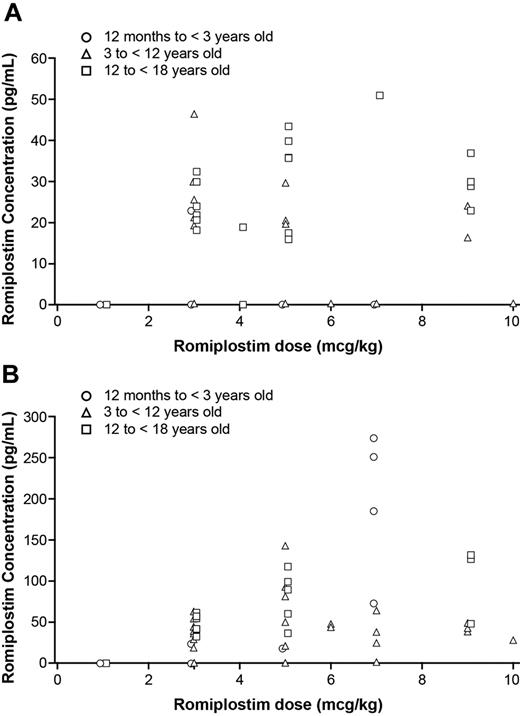
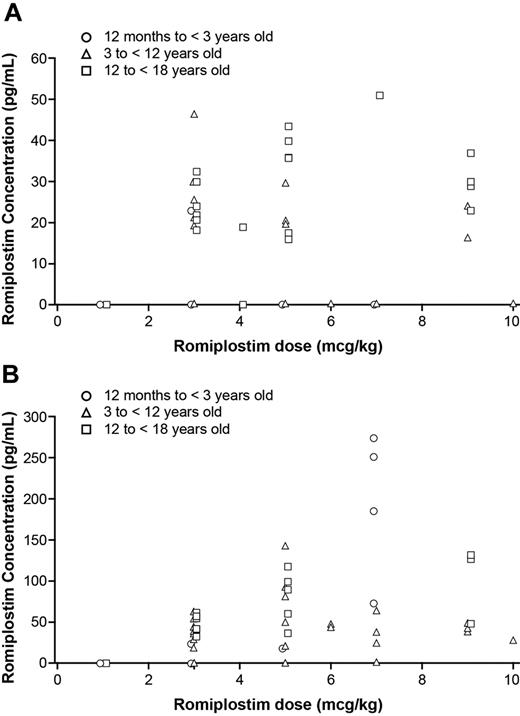
The relationship between romiplostim dose and serum concentration was evaluated and graphically displayed (Figure 5). Romiplostim dose was plotted against romiplostim serum concentration for each patient in the romiplostim group at the same time point predose and 2 days postdose during the pharmacokinetics assessment period. Romiplostim serum concentrations below the lower limit of quantification (15 pg/mL) were set to zero. The data were analyzed by statisticians used by the sponsor.
Results
Patient disposition
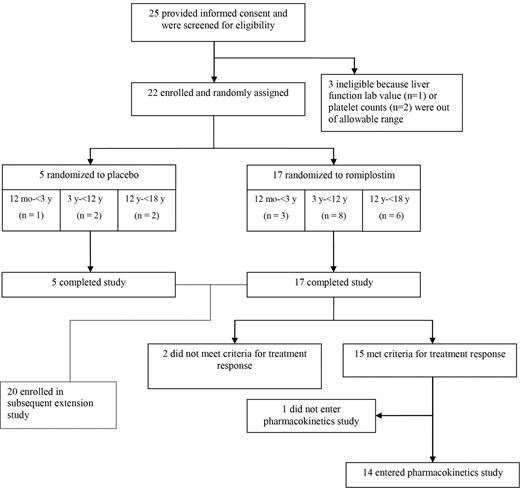
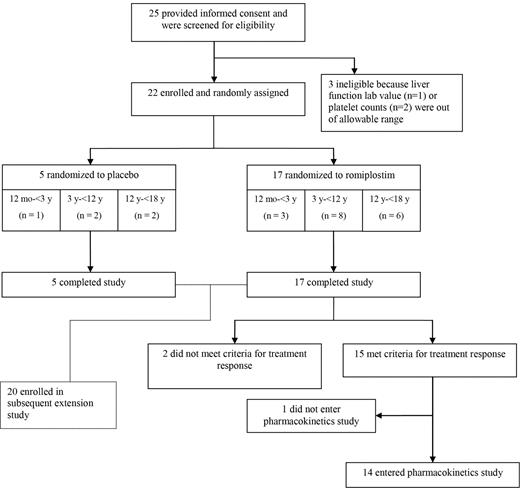
Of the 25 patients screened for this study, 3 failed screening because platelet counts (n = 2) or liver function values (n = 1) were outside the acceptable range. Twenty-two patients passed screening and were enrolled. Of these, 17 were randomized to the romiplostim group and 5 to the placebo group (Figure 1). All 22 patients received at least 1 dose of the study treatment and completed the study. Fifteen of 17 romiplostim-treated patients but none of the 5 placebo-treated patients met the criteria for response. Fourteen of the 15 responders entered the pharmacokinetics assessment study.
Disposition of pediatric patients with ITP of at least 6 months' duration who were treated with placebo or romiplostim for up to 16 weeks.
Disposition of pediatric patients with ITP of at least 6 months' duration who were treated with placebo or romiplostim for up to 16 weeks.
Patient demographics and clinical characteristics
The romiplostim and placebo groups were generally balanced with respect to demographic and disease characteristics at baseline (Table 2). Exceptions were that the median body weight and duration of ITP tended to be lower, whereas the percentages of black and Hispanic/Latino patients and median number of previous ITP treatments tended to be greater in the romiplostim group than in the placebo group.
The median age of the patients was 10 (range, 1-17) years. The median baseline platelet count was 13 (range, 2-29) × 109/L. One patient in the romiplostim group had a history of intracranial bleeding, which occurred more than 30 days before enrollment. All but 1 patient (95%) had received previous ITP treatments. The median number of different previous ITP treatments (drugs, blood transfusions, or splenectomy) was 5 (range, 0-9). Eight (36%) patients had previously undergone splenectomy, and the median time since splenectomy was 5.0 years (range, 0.4-12.0 years).
Safety evaluation
At least 1 adverse event was reported during the treatment period in 16 of the 17 (94%) patients in the romiplostim group and in all 5 patients in the placebo group (Table 3).39 The most frequently reported adverse events were headache and epistaxis (Table 4). During the treatment period, treatment-related adverse events were reported for 3 (18%) patients in the romiplostim group (pyrexia in 2 and headache in 1), and 1 (20%) patient in the placebo group (headache). No treatment-related adverse events were considered serious or led to discontinuation of the study treatment. No patient died during the study.
Most adverse events were mild to moderate in severity. Severe or life-threatening (CTCAE grade ≥ 3) adverse events were reported in 1 patient in each group (6% romiplostim, 20% placebo); neither event was considered to be treatment related. CTCAE grade 4 thrombocytopenia (platelet count of 13 × 109/L) was reported in 1 patient in the romiplostim group. Platelet counts in this patient increased from 14 × 109/L at baseline to 106 × 109/L at week 5, 85 × 109/L at week 6, and 48 × 109/L at week 7. The patient missed his week 8 dose, and 1 week later, his platelet count decreased to 13 × 109/L, and he experienced grade 1 epistaxis of 1 day's duration. He was given intravenous immunoglobulin, and his platelet count increased to 309 × 109/L. Despite being graded 4 (life-threatening), this adverse event was not “serious” in the absence of hospitalization or major hemorrhage. Severe hypersensitivity (allergic reaction), which resolved with medication on the same day, was reported in 1 placebo-treated patient.
One serious adverse event was reported in a romiplostim-treated patient. A splenectomized 16-year-old boy was hospitalized because of influenza and later developed sepsis. Neither event was considered treatment related, but both met criteria to be serious (hospitalization).
Adverse events of hemorrhage, embolism or thrombosis, malignancy, worsening thrombocytopenia after drug withdrawal, thrombocytosis, BM reticulin, and immunogenicity were of special interest because of the mechanism of action of romiplostim. Because of the short duration of treatment in this study (12-16 weeks), no BM examinations were performed and therefore reticulin deposition was not assessed. Of these events, only hemorrhage was observed during this study. During the treatment period, 12 (71%) romiplostim-treated patients and 2 (40%) placebo-treated patients had bleeding adverse events. The 12 romiplostim-treated patients had CTCAE grade 1 bleeding events, and 1 patient also had CTCAE grade 2 events (epistaxis, contusion, petechiae). No bleeding adverse events were serious or considered treatment related. Most bleeding adverse events in the romiplostim-treated patients occurred in the first 6 weeks of the treatment period, and 14 of the 17 occurred at a platelet count < 30 × 109/L. The bleeding events in the 2 placebo-treated patients were CTCAE grade 1. The rate of bleeding was 7.3 (95% confidence interval 4.3-11.7) events per 100 patient-weeks in the romiplostim group and 11.9 (95% confidence interval 5.2-23.5) events per 100 patient-weeks in the placebo group.
A total of 8 of 14 (57%) patients in the romiplostim group who entered the pharmacokinetics assessment period had at least 1 adverse event. Most adverse events reported during this period were mild to moderate in severity and each was reported in only 1 patient; 2 (lymphadenitis and skin laceration) were severe but neither was considered treatment related.
No trends in serum chemistry or hematology parameters were noted other than increases in platelet counts, and no clinically significant, treatment-related changes were observed in vital signs or body weights. No patient had positive test results for neutralizing antibodies to either romiplostim or TPO.
Efficacy evaluation
The median number of weeks that patients maintained a platelet count of 50 × 109/L or more was significantly greater in the romiplostim group (7 weeks; range. 0-11 weeks) than in the placebo group (0 weeks; range. 0-0 weeks' P = .0019; Figure 2A). The incidence of bleeding events of grade 2 or greater as assessed with the Buchanan-Adix semiquantitative grading system for hemorrhage was an efficacy end point in this study. Only 1 patient, a 16-year-old girl in the romiplostim group, had hemorrhage of grade 2 or greater during the treatment period. This patient, who had a history of petechiae, ecchymoses, epistaxis, and menorrhagia, had moderate (Buchanan-Adix grade 3 [CTCAE grade 1]) hemorrhage (mucosal bleeding not requiring medical treatment) 22 days after initiation of treatment with romiplostim. No bleeding events of grade 2 or greater were noted in the placebo group during the treatment period.
Platelet responses during the treatment period in all placebo- and romiplostim-treated patients and in patients by age cohort. (A) Median (25th [Q1] and 75th [Q3] percentiles) number of weeks that platelet counts were 50 × 109/L or greater during the treatment period. Platelet counts within 4 weeks of administration of rescue medication were excluded from these analyses. (B) Percent of patients who had platelet counts of 50 × 109/L or greater for 2 consecutive weeks in the absence of rescue medication. (C) Percent of patients who had an increase in platelet counts of 20 × 109/L or greater above baseline for 2 consecutive weeks in the absence of rescue medication.
Platelet responses during the treatment period in all placebo- and romiplostim-treated patients and in patients by age cohort. (A) Median (25th [Q1] and 75th [Q3] percentiles) number of weeks that platelet counts were 50 × 109/L or greater during the treatment period. Platelet counts within 4 weeks of administration of rescue medication were excluded from these analyses. (B) Percent of patients who had platelet counts of 50 × 109/L or greater for 2 consecutive weeks in the absence of rescue medication. (C) Percent of patients who had an increase in platelet counts of 20 × 109/L or greater above baseline for 2 consecutive weeks in the absence of rescue medication.
When platelet counts determined within 4 weeks of administration of rescue medication were excluded from the analyses, 15 of the 17 (88%) patients in the romiplostim group achieved the efficacy end points of a platelet count of 50 × 109/L or greater for 2 consecutive weeks and an increase in platelet count of 20 × 109/L or greater above baseline for 2 consecutive weeks (Figure 2B-C). In contrast, none of the patients in the placebo group met either of these response criteria. The percentages of patients in the romiplostim group who achieved each of these 2 end points were significantly greater than those in the placebo group (P = .0008 for each end point). Platelet responses to treatment were similar across the 3 age groups of romiplostim-treated patients (Figure 2A-C).
A total of 2 of 17 (12%) patients in the romiplostim group and 2 of 5 (40%) in the placebo group received rescue medication during the treatment period. Both romiplostim-treated patients were responders; one received rescue medication on day 1 of the study and responded thereafter, and the second responded but subsequently missed a dose, developed epistaxis, and received rescue medication (description in “Safety evaluation”).
Throughout the first half of the study, the median weekly platelet count in the romiplostim group increased with time, and from 7 weeks onward was near or > 50 × 109/L (Figure 3A). In contrast, the median weekly platelet count in the placebo group remained stable at approximately 10 × 109/L throughout the study (Figure 3A). A review of platelet counts for individual patients showed that 12 (71%) patients in the romiplostim group had platelet counts > 100 × 109/L in the absence of rescue medication. In contrast, only 1 (20%) patient in the placebo group achieved a platelet count > 100 × 109/L, but this was because of rescue medication that led to an increase in platelet count to 457 × 109/L 4 days after its administration. Only one patient in the romiplostim group had a platelet count > 400 × 109/L.
Platelet counts and romiplostim doses by study week. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. In the romiplostim group, the median weekly platelet count increased with time on study and was near or > 50 × 109/L after 6 weeks of treatment, whereas the counts in placebo group remained at or near 10 × 109/L. Platelet counts within 4 weeks of administration of rescue medication were excluded. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim and placebo administered during the study. The dose of romiplostim increased slowly though week7 and then remained stable at approximately 5 μg/kg. The dose of placebo increased steadily throughout the treatment period. The median romiplostim dose decreased after the start of the pharmacokinetics assessment period because the 2 patients who did not respond to romiplostim did not enter this phase of the trial. Both nonresponding patients had been receiving romiplostim at 10 μg/kg at week 12.
Platelet counts and romiplostim doses by study week. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. In the romiplostim group, the median weekly platelet count increased with time on study and was near or > 50 × 109/L after 6 weeks of treatment, whereas the counts in placebo group remained at or near 10 × 109/L. Platelet counts within 4 weeks of administration of rescue medication were excluded. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim and placebo administered during the study. The dose of romiplostim increased slowly though week7 and then remained stable at approximately 5 μg/kg. The dose of placebo increased steadily throughout the treatment period. The median romiplostim dose decreased after the start of the pharmacokinetics assessment period because the 2 patients who did not respond to romiplostim did not enter this phase of the trial. Both nonresponding patients had been receiving romiplostim at 10 μg/kg at week 12.
In parallel with the platelet count, the median dose in romiplostim-treated children increased slowly until week 7 and thereafter remained stable at 5 μg/kg. In the placebo-treated children, the median dose increased steadily throughout the treatment period (Figure 3B).
Six (35%) romiplostim-treated patients and 2 (40%) placebo-treated patients had undergone splenectomy before enrollment in this study. Both nonresponders in the romiplostim-treated group had had a previous splenectomy and were receiving 10 μg/kg of romiplostim at week 12. Among patients receiving romiplostim, the 6 splenectomized patients received slightly greater romiplostim doses than the 11 nonsplenectomized patients. Platelet counts did not differ substantially between the splenectomized and nonsplenectomized groups (Figure 4A-B). The patient with a history of intracranial hemorrhage, who had not undergone splenectomy, was a responder to romiplostim.
Platelet counts and romiplostim doses by study week in romiplostim-treated splenectomized and nonsplenectomized patients. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. Median platelet counts in splenectomized and nonsplenectomized patients increased with time on study, although counts varied from week to week and at several time points were lower in splenectomized patients. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim administered during the study. The dose of romiplostim increased through week 7 in splenectomized patients and through week 9 in nonsplenectomized patients. The median dose in splenectomized patients was consistently slightly greater than that in nonsplenectomized patients.
Platelet counts and romiplostim doses by study week in romiplostim-treated splenectomized and nonsplenectomized patients. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. Median platelet counts in splenectomized and nonsplenectomized patients increased with time on study, although counts varied from week to week and at several time points were lower in splenectomized patients. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim administered during the study. The dose of romiplostim increased through week 7 in splenectomized patients and through week 9 in nonsplenectomized patients. The median dose in splenectomized patients was consistently slightly greater than that in nonsplenectomized patients.
Fourteen patients in the romiplostim group entered the pharmacokinetics assessment period. Of these, 6 had bleeding events during this period, one of which was Buchanan-Adix grade 3 mucosal bleeding of 5 days' duration that did not require medical treatment.
Pharmacokinetics
Romiplostim pharmacokinetics was assessed during weeks 13-16. Romiplostim doses ranged from 1 to 7 μg/kg for the children ages 12 months to younger than 3 years, 3-10 μg/kg for children ages 3 to younger than 12 years, and 1-10 μg/kg for children ages 12 to younger than 18 years. The observed romiplostim serum concentrations ranged from 16 to 51.1 pg/mL at predose and from 17.7 to 274 pg/mL at 2 days postdose. Romiplostim concentrations were below the limit of quantification in 15 of 59 predose samples and in 39 of 71 postdose samples. The data showed no apparent differences across the 3 age cohorts and no apparent relationship between serum romiplostim concentrations and dose (Figure 5A-B).
Scatter plots showing individual trough serum romiplostim concentration versus romiplostim dose in romiplostim-treated patients. (A) Serum romiplostim concentration versus dose before administration of romiplostim at weeks 13, 14, 15, and 16 during the pharmacokinetics assessment period and (B) 2 days after dosing at each week during the pharmacokinetics assessment period. The data showed no correlation of dose with concentration.
Scatter plots showing individual trough serum romiplostim concentration versus romiplostim dose in romiplostim-treated patients. (A) Serum romiplostim concentration versus dose before administration of romiplostim at weeks 13, 14, 15, and 16 during the pharmacokinetics assessment period and (B) 2 days after dosing at each week during the pharmacokinetics assessment period. The data showed no correlation of dose with concentration.
Discussion
In this short-term study, romiplostim treatment was well tolerated and apparently safe and increased and maintained platelet counts in 88% of pediatric patients with chronic ITP, many of whom had severe and some of whom had refractory disease. After completing this study, 20 of the 22 patients entered the extension study, suggesting that patients, caregivers, and physicians believed that the benefits of treatment outweighed any inconvenience or actual or potential side-effects. Bleeding, usually mild, was the only adverse event of interest observed, and its occurrence was correlated with platelet count. Thromboembolic events or neutralizing antibodies to TPO or romiplostim were not reported. No serious adverse events considered related to study drug were observed (BM biopsies were not performed because this was a short-term pilot study). Overall, the adverse events most commonly reported by these pediatric patients (Table 4) were generally similar to those reported by adults, and the safety profile of romiplostim in the children in this study was not overtly different from that that previously reported in adults.27,32,33
Overall, the response of pediatric patients to romiplostim was similar to that of adults observed in 2 phase 3 studies of romiplostim in patients with ITP.32 In both children and adults, platelet counts in the placebo group remained near baseline levels throughout the study, whereas stable doses of romiplostim maintained platelet counts for most patients within the target ranges. In children, romiplostim led to similar platelet responses in all 3 age cohorts at similar doses of medication per kilogram body weight. Although the platelet count after onset of treatment increased more slowly in children than in adults (week 7 in this study compared with week 4 in the studies in adults32 ), this difference is likely because of the more gradual dose adjustment algorithm used in this pediatric study (Table 1). Adjusting the romiplostim dose on a weekly basis when platelet counts are < 50 × 109/L may help children achieve and maintain platelet counts > 50 × 109/L more quickly and consistently than the dosing schedule used in this study. However, given the occasionally delayed effects of treatment, the dosing algorithm used in this study prevented thrombocytosis.
Serum concentrations of romiplostim varied among children, and there was no apparent correlation between romiplostim dose and serum concentration. These findings were similar to those observed in adult patients with ITP (Amgen, Inc, data on file, 2010).41 The romiplostim half-life in adults with ITP ranges from 1 to 34 days (median, 3.5 days); the sparseness of the data in this study precluded calculation of romiplostim half-life in children.35 The lack of overt relationship between romiplostim dose and serum concentration is because of the combination of the pharmacodynamic response to romiplostim (increased platelet counts) and the receptor-mediated clearance of romiplostim by platelets, which is similar to that observed for TPO.42,43 Although drug serum concentrations traditionally guide dosing, the complex interplay between the romiplostim dose, serum concentration, and platelet count results in a nonlinear relationship between the romiplostim serum concentration and platelet count. Furthermore, the short half-life of platelets means that perturbations in platelet homeostasis (because of infections or use of rescue medications, etc) can increase the variability of platelet counts. Therefore, in children as in adults it appears that romiplostim dose titration needs to be determined by the patient's platelet counts during treatment to maintain platelets within the target range.
This study had limitations beyond the small sample size. Although both patients and study site personnel were blinded to treatment assignments, the substantial increases in platelet counts may have led patients receiving romiplostim (and their physicians) to suspect that they had been assigned to the active treatment arm. The romiplostim and placebo groups were generally similar with respect to demographic and clinical characteristics, but, as might be expected from the small sample size, some minor differences occurred with respect to weight, duration of disease, race, and number of previous treatments. Although race, duration of disease, and previous use of other ITP therapies have not been shown to affect the response to romiplostim treatment in adults, lower weight was significantly associated with an increased response rate in one study in adults.32 A separate issue is that administration of rescue medications could have confounded assessment of bleeding events, although platelet responses that occurred within 4 weeks of administration of rescue medications were excluded from the analysis. Finally, the inclusion in the pharmacokinetics period of only those patients who responded to treatment partly limits the conclusions that can be drawn from safety data collected during this 4-week period.
In summary, this is the first study of a TPO receptor agonist in children with ITP. It was performed as a randomized, double-blind trial in a group of patients with relatively severe disease. Although it is fortunate that few children with ITP are as severely affected as many of the patients treated here, the demonstration of substantial efficacy and safety in this study is encouraging for those children most in need of treatment. Certainly all of these results need to be confirmed in a larger, longer study that would better explore safety issues such as thromboembolism and reticulin fibrosis. The long-term safety of romiplostim in children will continue to be monitored in an ongoing extension study and in a planned, longer-term study. Other specific issues that could be explored include a slightly more aggressive dosing algorithm for children maintaining very low platelet counts despite receiving an initial dose of 1 μg/kg and some estimation of how long treatment should be continued. Overall, the outcome of this study is very encouraging, given the high response rate in romiplostim-treated patients, no response at all in placebo patients, and the absence of important adverse events.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from Amgen Inc. Mary Royer, a medical writer supported by Amgen Inc, and James O'Kelly, an employee of Amgen Inc, provided writing and editorial support to the authors during preparation of this manuscript, as did Chris Yurchuck at Cornell.
Authorship
Contribution: J.B.B. performed the research, interpreted the data, and wrote the paper; G.R.B. designed the trial, performed the research, interpreted the data, and edited the paper; D.J.N. performed the research, interpreted the data, and edited the paper; D.J.G. performed the research, interpreted the data, and edited the paper; L.R.B. designed the trial, performed the research, interpreted the data, and edited the paper; V.S.B. designed the trial, interpreted the data, and edited the paper; Y-M.W. collected, analyzed, and interpreted the data, and edited the paper; K.N. collected, analyzed, and interpreted the data, and edited the paper; S.J. analyzed and interpreted the data, and edited the paper; and all authors reviewed and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: J.B.B. has received research support from Amgen Inc, Eisai Inc, Shionogi, Sysmex, Ligand, Immunomedics, GlaxoSmithKline, Cangene, and Genzyme; has served on an advisory board for Amgen Inc, Ligand, GlaxoSmithKline, Shionogi, and Eisai Inc; and holds equity in Amgen Inc and GlaxoSmithKline. G.R.B. has received research support from Amgen Inc. Y-M.W., K.N., and S.J. are employees of and hold stock options in Amgen Inc.
Correspondence: James B. Bussel, Weill Medical College of Cornell University, P609, 525 E 68th St, New York, NY; e-mail: jbussel@med.cornell.edu.

![Figure 2. Platelet responses during the treatment period in all placebo- and romiplostim-treated patients and in patients by age cohort. (A) Median (25th [Q1] and 75th [Q3] percentiles) number of weeks that platelet counts were 50 × 109/L or greater during the treatment period. Platelet counts within 4 weeks of administration of rescue medication were excluded from these analyses. (B) Percent of patients who had platelet counts of 50 × 109/L or greater for 2 consecutive weeks in the absence of rescue medication. (C) Percent of patients who had an increase in platelet counts of 20 × 109/L or greater above baseline for 2 consecutive weeks in the absence of rescue medication.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-313908/4/m_zh89991172640002.jpeg?Expires=1767895845&Signature=3UhcEmR0VPXbmh08wWt5OxazGkI7UVc6UIA-fZr5iWLa3nDm4A1McMqLMCzoYbEP4592UukOcJVJEMLv64W9peb6pfJkA1fQIZqtAH278bTt2dubmmVFI4cNunTafSySitU-YPLSwqT95lkxGQ0O2VaHsvVVnfvujh7HiivjORIHDy894ye~lQB7-LUSYRG3CZTvV1FP7rJ4BlDUh53MCSJnL~xpN9briQQKmd6SnIoumdsbUsfeFH02FG8DWzK7HKV8VMasGITjJ17QuYTLfWblmQIPHt5XgVGuyZReOjIAws8jq13mVVSIge8BPvlvzbfJ0EOq7V~eIQdRx0eIVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Platelet counts and romiplostim doses by study week. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. In the romiplostim group, the median weekly platelet count increased with time on study and was near or > 50 × 109/L after 6 weeks of treatment, whereas the counts in placebo group remained at or near 10 × 109/L. Platelet counts within 4 weeks of administration of rescue medication were excluded. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim and placebo administered during the study. The dose of romiplostim increased slowly though week7 and then remained stable at approximately 5 μg/kg. The dose of placebo increased steadily throughout the treatment period. The median romiplostim dose decreased after the start of the pharmacokinetics assessment period because the 2 patients who did not respond to romiplostim did not enter this phase of the trial. Both nonresponding patients had been receiving romiplostim at 10 μg/kg at week 12.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-313908/4/m_zh89991172640003.jpeg?Expires=1767895845&Signature=uTLPaCy7ne8v9EuJ9~E83FF5kMTvWP7tr2zVYKTC-LB~FuO976a-4PLe9~yeG06oMOinAkUrlCGXxBzN2W1vfg9-ot2n8ZGGije81lwq9J0bOKU405xdL-5eRkCOdHjrWgfEUcOFz7aXZIhsyg6sVuZXTksAurHATKcWv16oO78VkJ5fJnVWIFhPzeEUr2EhpPU-JqzN4jcAJvh1QfGpdizegM2A9WsVRZG0N~3v689MBJn99TCrAqH~i0mWAgcp0pATnutszwcJshIF0ewAyhiBzH5AOmOCwxcFEUNFvtYfJHTded8wN0QMveqROBVFRxzR7keQ0kaGLtVsn0-qEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Platelet counts and romiplostim doses by study week in romiplostim-treated splenectomized and nonsplenectomized patients. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. Median platelet counts in splenectomized and nonsplenectomized patients increased with time on study, although counts varied from week to week and at several time points were lower in splenectomized patients. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim administered during the study. The dose of romiplostim increased through week 7 in splenectomized patients and through week 9 in nonsplenectomized patients. The median dose in splenectomized patients was consistently slightly greater than that in nonsplenectomized patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-313908/4/m_zh89991172640004.jpeg?Expires=1767895845&Signature=OeQu9Fgz2eAcscJx5~SM3m9DfhsyJox0fjDBtLeqIosLmZ1H243Erpgtvs6VxqMrWy69GkG5A0SZA6-5E5NdRQ9dXKpPBhFuBv3LL95H1n-suTsoTqPh1Lth-uDXerxLy7sRG3DlbbgmB80hkVFmYATvlgFDUYBXtPnJbFvduW9iPl9BWF-iNklygizRqcSLrxbCtWIapP-lsXJBuiUD0V5YxKa17a73zw5~im71kZw6TMrJxRYudeAvdpl3jYmXYzD87gGXZvPUqvKOks8GGOsAG342XofXkfo8lekh2DVgeJaoMOXAyUzKBvelhni8gP915rCKcrmVwUWCHZV9MA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Platelet responses during the treatment period in all placebo- and romiplostim-treated patients and in patients by age cohort. (A) Median (25th [Q1] and 75th [Q3] percentiles) number of weeks that platelet counts were 50 × 109/L or greater during the treatment period. Platelet counts within 4 weeks of administration of rescue medication were excluded from these analyses. (B) Percent of patients who had platelet counts of 50 × 109/L or greater for 2 consecutive weeks in the absence of rescue medication. (C) Percent of patients who had an increase in platelet counts of 20 × 109/L or greater above baseline for 2 consecutive weeks in the absence of rescue medication.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-313908/4/m_zh89991172640002.jpeg?Expires=1767895846&Signature=TowlOe2HyVx0cSO6HQcLsQPewZTkEwpsmG7kT9ypYjxrXkO4nY7~he~DiC9WORzq6dPinJ32Snhv8OqT3LFntltn1fKYxVNssy8GKhYd9~PS1GjRwDEIlJ-d5g9VOq~z~D~~F8X0GEaxkTBB5DuZyap8kLKNNboJHUr9fiXnsbIitdlqtxvDIxJwIfXS9pemSxe648IbxdgsNh7qgI4UDh-DQxv6THa0SqU4wDcgEkUA7mqH6KBus7sYB9n9MMJglIycQuWRzTysE6faDc0MhsK4NazhPER1MhwQHJ8vSDGo~Rm8p584GnwYdGBcnXU7vmYzd4S8W9QHPe5mIE2ZQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Platelet counts and romiplostim doses by study week. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. In the romiplostim group, the median weekly platelet count increased with time on study and was near or > 50 × 109/L after 6 weeks of treatment, whereas the counts in placebo group remained at or near 10 × 109/L. Platelet counts within 4 weeks of administration of rescue medication were excluded. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim and placebo administered during the study. The dose of romiplostim increased slowly though week7 and then remained stable at approximately 5 μg/kg. The dose of placebo increased steadily throughout the treatment period. The median romiplostim dose decreased after the start of the pharmacokinetics assessment period because the 2 patients who did not respond to romiplostim did not enter this phase of the trial. Both nonresponding patients had been receiving romiplostim at 10 μg/kg at week 12.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-313908/4/m_zh89991172640003.jpeg?Expires=1767895846&Signature=U5-Zny3b6vHlDl-6Fdtro5J6n2yqEGG~wS6ZIaUBP8Rw4uZ8wvGNwoFgCXjF5TmZ97xkYyXNtyvJM3CHLLEjZk4V0L~GbeSUpKK00zaS~hx7WDMsTjU7rDj0WYg65NWfrlZrPfFqpHP~PJMbevaLaFeqOCOikRovaDkcgwHhrgtDJfBMZtrzcrX2N0ul5YL6r3HhYYrEX8HRkM2UtZjHEv17HHo8~~fn0HITlPQf8C0IWxi4Kejn0shuKEiwUJKKPiemXuNZr7gybEjOxEiFtLNsYOlKy7h7faHQQEtsV6lOpQe0kNaAMST-zDIB7S2feDF0tWQc1TjE31IkcFGk0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Platelet counts and romiplostim doses by study week in romiplostim-treated splenectomized and nonsplenectomized patients. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts by study week. Median platelet counts in splenectomized and nonsplenectomized patients increased with time on study, although counts varied from week to week and at several time points were lower in splenectomized patients. (B) Median (25th [Q1] and 75th [Q3] percentiles) weekly doses of romiplostim administered during the study. The dose of romiplostim increased through week 7 in splenectomized patients and through week 9 in nonsplenectomized patients. The median dose in splenectomized patients was consistently slightly greater than that in nonsplenectomized patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-10-313908/4/m_zh89991172640004.jpeg?Expires=1767895846&Signature=e4w6OF8xX~3486-833HgECzTd2MmeWu5~ZfiDkiJuWeJ-Q-E1R7B50T9SYWc1HTVi1rt3QpE0mtG92d8A3fJRCoNsGhjB~le3oaXyocKhknAPOTvGacYK0h0xi6xM84qiLQnlNBl5IMyTj1gLoSo8nrevvMjjzn6gbf-stieWVsb48n0dNCH~IzG7Y4RERUjp3TJuWV0LYnhjKmIdEfudjaUr4eToMqSTSCMDuigvBuEtDNHujqx7Ma6o67N7PQL0NH1oKzWmPh5MzKJFmixl6BgrXxT5~MXYTFujtHHlhtLhh1kBf0s4CKxUIxHtHi-GJLrWZtBPYkMnsbmBmWCCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)