Abstract
The proliferation and differentiation of adult stem cells is balanced to ensure adequate generation of differentiated cells, stem cell homeostasis, and guard against malignant transformation. CD48 is broadly expressed on hematopoietic cells but excluded from quiescent long-term murine HSCs. Through its interactions with CD244 on progenitor cells, it influences HSC function by altering the BM cytokine milieu, particularly IFNγ. In CD48-null mice, the resultant misregulation of cytokine signaling produces a more quiescent HSC, a disproportionate number of short-term progenitors, and hyperactivation of Pak1, leading to hematologic malignancies similar to those found in patients with X-linked lymphoproliferative disease. CD48 plays a vital role as an environmental sensor for regulating HSC and progenitor cell numbers and inhibiting tumor development.
Introduction
HSCs maintain lifelong blood production, but the mechanisms regulating them remain largely obscure. Although several genes have been identified that play key cell-autonomous roles in the HSC,1-4 we still have few insights into the manner in which the BM milieu impacts HSC function. Under normal circumstances, the majority of HSCs reside in a quiescent state, with less than 5% in cycle at any given time.5,6 Nevertheless, blood homeostasis is exquisitely effective, in that the progenitor pool and numbers of differentiated blood cells are maintained at a constant level. To ensure coordinated blood cell production, a sensitive feedback mechanism must be in place, so that the number of progeny are sensed by more primitive progenitors allowing for subtle regulation of the HSCs to generate more downstream cells only as needed. Moreover, under hematopoietic stress, the manufacture of differentiated cells is rapidly adjusted, marshaling increased output from both committed progenitors as well as HSCs.7 When the loss of blood cells and committed progenitors is particularly complete, virtually all of the HSCs are transiently drafted, after which most of them return to quiescence to ensure long-term maintenance of the HSC pool.6
CD48 was previously identified as broadly expressed on differentiated hematopoietic cells, but excluded from quiescent long-term HSCs (LT-HSCs).3,8 CD48 is a GPI-linked member of the signaling lymphocyte activation molecule (SLAM) family of proteins. It can act as a ligand for SLAM member CD244 and, depending on the context, either inhibits or stimulates IFNγ production from the target cell.9-12 IFNγ has previously been shown it to be a suppressor of hematopoiesis and at high levels it may lead to BM failure.13 However, IFNγ has also been shown to stimulate progenitor cell proliferation.14-16 Recently, work from our laboratory has shown a lack of IFNγ in vivo leads to a less proliferative HSC, whereas increasing IFNγ increases HSC proliferation.7 Because CD48 offered a context-dependent mechanism for the secretion of IFNγ, which impinges directly on HSCs, we sought to determine its role in maintaining the balance between HSC quiescence, proliferation, differentiation, and the formation of hematologic malignancies.
Methods
Mice and hematopoietic progenitor staining
All mice were housed in a specific pathogen-free barrier facility and fed autoclaved acidified water and mouse chow ad libitum. CD48−/− mice backcrossed to C57BL/6 were obtained from Arlene Sharp's laboratory at Harvard Medical School. Mice used for all these studies were euthanized according to institutional animal care and use committee–approved guidelines. Whole BM was isolated, stained on ice with Abs, and resuspended in propidium iodide, accomplished on live cells with an LSRII (Becton Dickinson; further details are available in supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Transplantation and peripheral blood analysis
After a split dose of 10.5 Gy of whole-body irradiation, C57BL/6-CD45.1 recipients were transplanted by retro-orbital IV injection with whole BM (WBM) at various concentrations that were either CD45.2 CD48−/− or wild-type (WT) cells in combination with CD45.1 WT cells or alone. For peripheral blood analysis, mice were bled, the RBCs were lysed, stained with Abs against CD45.1 and CD45.2 as well as lineage markers (B220, CD4, CD8, GR-1, Mac-1), and analyzed with an LSRII (Becton Dickinson). Nonlinear regression analysis with extra sum-of-squares F test was calculated and the P value reported.
Complete blood counts concurrent aging time course
For the aging study, a large cohort of CD48−/− (n = 56) and WT (n = 82) mice ranging from 8-81 and 17-97 weeks, respectively, was bled the same day. Mice were bled retro-orbitally with EDTA-coated capillary tubes and 180 μL of blood was obtained. Complete blood counts (CBCs) were then determined (Cell-Dyn 3500R; Abbott Laboratories). Nonlinear regression analysis with extra sum-of-squares F test was calculated and the P value reported.
BrdU labeling, Ki-67 staining, and phospho-flow
Mice received an initial IP injection of BrdU (Sigma-Aldrich; 1 mg/6 g mouse weight), followed by inclusion of BrdU in the drinking water at 1 mg/mL for 3 subsequent days, and analyzed as previously described for BrdU and KI-67 status. Differences were calculated by Student t test. For phospho-flow, HSCs were analyzed for phospho-ERK and phospho-PAK1/PAK2 as previously described.17 Differences in protein expression levels were assessed by comparing ratios of median fluorescence intensities (further details are available in supplemental Methods).
Real-time PCR analysis
For real-time analysis, cells were purified by flow cytometry and RNA was isolated with an Illustra RNAspin Mini Kit (GE HealthCare). RT-PCR was performed with Superscript II (Invitrogen) and 100 cell equivalents were used for each real-time reaction. TaqMan master mix and a p21 or CD244 TaqMan probe (Applied Biosystems) were used, and 18s was used as an internal control. The isoforms of CD244 were analyzed by using SYBR green master mix and a primer set previously published.18 GAPDH was used as an external control for all SYBR green real-time reactions. All reactions were run on an ABI prism 7900HT (Applied Biosystems).
5-FU challenge
For 5-FU treatment, mice were injected IV with a single dose of 5-FU (150 mg/kg body weight; Sigma-Aldrich). Six days later mice were killed and the HSCs (SP+Sca-1+B220−, CD4−, CD8−, Gr-1−, and Ter119−) were analyzed by flow cytometry.
BM supernatant preparation and cytokine bead array analysis
BM supernatant was isolated by collection tubes made in house iodide (further details are available in supplemental Methods). Cytokine bead arrays were done according to the manufacturer's protocol and the entire supernatant from one mouse (excluding 1 μL used to measure the protein levels) and analyzed with the use of a BD Facsarray. The concentrations of each cytokine were calculated with the use of R, a statistical programming language, and the ELISA package available from the open-source Bioconductor project.19 Cytokine levels were normalized against the total protein from each mouse and the WT and CD48−/− cytokine levels were compared. A Student t test was used and P value reported.
Cancer study
A cohort of CD48−/− mice (n = 10) and WT mice (n = 14) was followed for 65 weeks. Mice were regularly examined for morbidity and killed at 65 weeks of age. Spleens, sternum, and the thymus were sent for pathology. BM cells and splenocytes were collected for analysis using flow cytometry.
Statistical analysis
All statistical tests were run using Graphpad Prism 5.0 for Windows (www.graphpad.com).
Results
CD48 is excluded only from quiescent LT-HSCs
To understand the role of CD48 in regulation of homeostasis, we needed better characterization of its expression pattern throughout the hematopoietic differentiation hierarchy.3,8 Using established progenitor marker schemes, we examined CD48 expression on multiple hematopoietic progenitors (Figure 1A)20 and found it expressed throughout all the short-term progenitors, but strikingly excluded from the LT-HSCs. When HSCs are stimulated to cycle by systemic administration of the chemotherapeutic agent 5-flurouracil, we had previously observed a transient up-regulation of CD48 on phenotypically defined HSCs.1 To determine the functional significance of the HSCs expressing CD48, we performed a BM transplantation assay; the majority of the functional HSC activity was found within the CD48-negative HSC (supplemental Figure 1), establishing CD48 as a ubiquitous marker of all hematopoietic cells downstream of the most primitive quiescent LT-HSC, likely the most restricted pattern of all of the SLAM-family markers. We obtained CD48-null mice21 to further investigate CD48's role in HSC function. The null allele was previously backcrossed onto the C57Bl/6 background and shown to result in severe defects in CD4+ T-cell activation and proliferation.21 In addition, the CD48-null mouse was shown to also be defective in activating NK cells because of loss of signaling through CD244.9
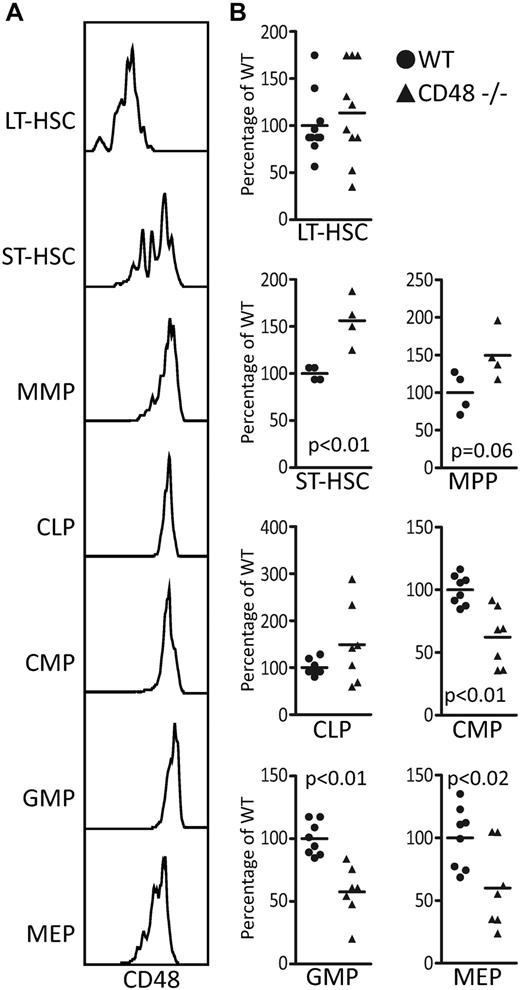
CD48 loss causes the progenitor compartment to lose equilibrium. (A) CD48's expression throughout the hematopoietic progenitor compartment including the LT-HSCs, ST-HSCs, multipotent progenitor (MPP), common lymphoid progenitor (CLP), common myeloid progenitor (CMP), granulocyte/monocyte progenitors (GMP), and the megakaryocyte/erythrocyte progenitors (MEP). (B) The BM of the CD48−/− 8- to 12-week-old mice show significant differences in the proportions of short-term progenitors with the long-term HSCs (SPKLS CD34−Flk2−) remaining stable as determined by a Student t test.
CD48 loss causes the progenitor compartment to lose equilibrium. (A) CD48's expression throughout the hematopoietic progenitor compartment including the LT-HSCs, ST-HSCs, multipotent progenitor (MPP), common lymphoid progenitor (CLP), common myeloid progenitor (CMP), granulocyte/monocyte progenitors (GMP), and the megakaryocyte/erythrocyte progenitors (MEP). (B) The BM of the CD48−/− 8- to 12-week-old mice show significant differences in the proportions of short-term progenitors with the long-term HSCs (SPKLS CD34−Flk2−) remaining stable as determined by a Student t test.
CD48 loss perturbs progenitor cell numbers
With such a distinct expression pattern, we considered the possibility that loss of CD48 would lead to alterations in the proportions of progenitor cells; thus, we compared the relative percentages of each progenitor type between WT and CD48−/− mice21 (Figure 1B, supplemental Figure 2). We first examined the LT-HSC (SPKLS CD34- Flk2-) and found similar numbers in the WT and CD48−/− BM. The short-term HSC (ST-HSC; non-SP, KSL CD34+ Flk2−) showed increased cell numbers in the CD48−/− mice, and the multipotent progenitor (MPP; non-SP KLS CD34+ Flk2+) showed a similar but less significant increase. However, the myeloid progenitor compartment (CMP [cKit+ CD34+ CD16/CD32− Il7ra− Lin−]; GMP [cKit+ CD34+ CD16/CD32+ Il7ra− Lin−]; MEP [cKit+ CD34− CD16/CD32− Il7ra− Lin−]) showed significant decreases in the CD48−/− mice, whereas the common lymphoid progenitor compartment of the CD48−/− mice equivalent to WT. Overall, the composition of the progenitor compartment of the CD48-null mice was distorted indicating a role for CD48 in maintaining the proper numbers of hematopoietic progenitors.
HSC from CD48-null mice exhibit functional defects
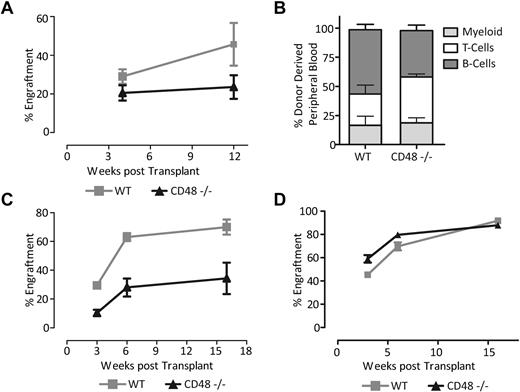
While HSC numbers in CD48-null mice appeared normal, a disturbed progenitor compartment could lead to functional consequences for the HSC because of effects on the HSC niche. To test this, we performed competitive BM transplantation between WT and CD48-null mice. We transplanted 100 000 BM cells from CD48−/− or WT mice into lethally irradiated recipients along with distinguishable WT competitor cells. We then sampled the peripheral blood at 4 and 12 weeks to assess the short- and long-term engraftment and differentiation potential of CD48-null HSCs. We observed significantly lower chimerism in mice transplanted with the CD48 −/− cells compared with their WT counterparts compared with the WT BM (Figure 2A-B). A similar competitive transplant using 300 000 WBM test and competitor cells was also carried out and comparable results were seen (Figure 2C). Finally, we performed noncompetitive transplants to examine the potential of these cells in an environment requiring high proliferation in the absence of normal BM. The CD48−/− cells showed a significantly lower initial engraftment; however, by 16 weeks these HSCs were able to achieve the same level of chimerism as WT cells (Figure 2D). Despite the expansion of the ST-HSC compartment, the CD48−/− cells demonstrate a significantly reduced short-term engraftment in a transplant setting. This suggests a decrease in functionality of not only the LT-HSC but the ST-HSC as well. Another possibility is a decrease in the homing ability of the CD48−/− cells; however, a homing assay showed no significant difference (data not shown). Overall, the CD48−/− cells showed a diminished capacity to reconstitute the hematopoietic system of an irradiated host implying a defect in HSC function or their regulation.
CD48−/− HSCs show a defect in both short-term and long-term activity. (A) Donor chimerism in the peripheral blood measured with CD45.2 expression, from transplants of 100 000 donor WBM cells competed against 100 000 WT WBM cells. CD48−/− HSCs show a substantial transplantation defect (nonlinear regression analysis, P = .02061). (B) Lineage contribution of CD48−/− HSCs at 12 weeks. CD48−/− recipients show a slight but insignificant skewing toward the T-cell lineage at the cost of B cells. (C) Donor chimerism in the peripheral blood measured with CD45.2 expression, from competitive transplants of 300 000 WBM cells from either a CD48−/− mouse or WT mouse. CD48−/− HSCs still exhibit a substantial transplantation defect (nonlinear regression analysis, P < .01) at the higher BM dose. (D) Noncompetitive BM transplant with 300 000 CD48−/− or WT whole BM cells. Shown is the proportion of peripheral blood (CD45.1+) cells generated from the donor cells at the indicated time points (all error bars are SEM).
CD48−/− HSCs show a defect in both short-term and long-term activity. (A) Donor chimerism in the peripheral blood measured with CD45.2 expression, from transplants of 100 000 donor WBM cells competed against 100 000 WT WBM cells. CD48−/− HSCs show a substantial transplantation defect (nonlinear regression analysis, P = .02061). (B) Lineage contribution of CD48−/− HSCs at 12 weeks. CD48−/− recipients show a slight but insignificant skewing toward the T-cell lineage at the cost of B cells. (C) Donor chimerism in the peripheral blood measured with CD45.2 expression, from competitive transplants of 300 000 WBM cells from either a CD48−/− mouse or WT mouse. CD48−/− HSCs still exhibit a substantial transplantation defect (nonlinear regression analysis, P < .01) at the higher BM dose. (D) Noncompetitive BM transplant with 300 000 CD48−/− or WT whole BM cells. Shown is the proportion of peripheral blood (CD45.1+) cells generated from the donor cells at the indicated time points (all error bars are SEM).
CD48−/− HSCs demonstrate a diminished ability to proliferate in vivo
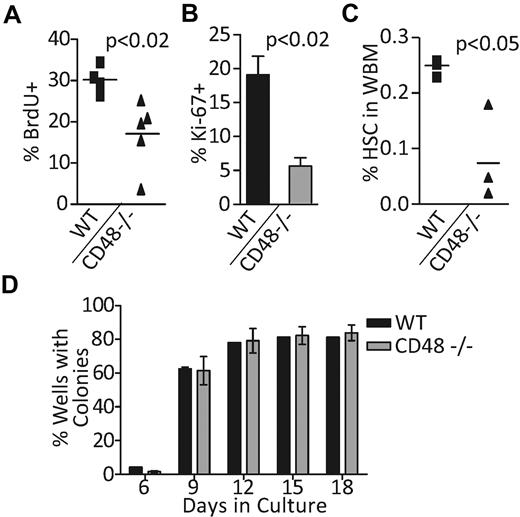
The expansion of the ST-HSC progenitor compartment could indicate higher proliferation or differentiation from LT-HSCs. To examine this, BrdU incorporation was used to determine whether CD48−/− LT-HSCs were more proliferative than the WT. Surprisingly, we found a significant decrease in the number of CD48−/− LT-HSCs completing cell cycle compared with WT cells (Figure 3A). Finally, the CD48−/− HSC population showed a drastic decrease in cells expressing Ki-67 compared with WT cells providing further evidence of the increased quiescence of the CD48−/− HSCs (Figure 3B). These results reveal an inhibition of proliferation in the CD48−/− LT-HSCs in a normal steady-state environment.
CD48−/− HSCs are less proliferative in vivo. (A) The number of HSCs completing the cell cycle was assessed by in vivo BrdU labeling. HSCs (SPKLS) were purified from WT and CD48−/− mice after 3 days of BrdU exposure. (B) HSCs (SPKLS) from CD48−/− and WT mice were isolated by flow cytometry and cells were examined for expression of Ki-67, a marker of actively cycling cells. (C) 5-FU was used to challenge the HSCs (SPSL) in CD48 knockout and WT mice and the cells were examined 6 days post 5-FU. (D) Single HSCs (SPKLS) were sorted into a 96-well plate with methocult to promote stem cell proliferation and colony formation in 3 separate experiments. Wells were inspected for colonies at regular intervals after plating and the percentage of wells with colonies at each time point was recorded. In this assay, CD48−/− cells performed equivalently to WT cells.
CD48−/− HSCs are less proliferative in vivo. (A) The number of HSCs completing the cell cycle was assessed by in vivo BrdU labeling. HSCs (SPKLS) were purified from WT and CD48−/− mice after 3 days of BrdU exposure. (B) HSCs (SPKLS) from CD48−/− and WT mice were isolated by flow cytometry and cells were examined for expression of Ki-67, a marker of actively cycling cells. (C) 5-FU was used to challenge the HSCs (SPSL) in CD48 knockout and WT mice and the cells were examined 6 days post 5-FU. (D) Single HSCs (SPKLS) were sorted into a 96-well plate with methocult to promote stem cell proliferation and colony formation in 3 separate experiments. Wells were inspected for colonies at regular intervals after plating and the percentage of wells with colonies at each time point was recorded. In this assay, CD48−/− cells performed equivalently to WT cells.
To determine whether CD48−/− LT-HSCs could proliferate normally when challenged, we injected mice with the chemotherapeutic agent 5-FU to ablate the cycling progenitor pools and force the HSC to proliferate.1 The LT-HSC compartment was examined 6 days after 5-FU injection, and while the WT HSC population showed a 5-fold increase in size, the CD48−/− HSCs showed only a 2-fold expansion (Figure 3C). To determine whether this lack of a proliferation response was intrinsic to the CD48-null HSCs, we tested the proliferation potential of single purified HSCs plated in vitro. We plated single HSCs into wells of a 96-well plate containing methylcellulose medium with cytokines to force HSC proliferation and differentiation, and we counted macroscopic colonies throughout an 18-day time course (Figure 3D); the CD48−/− HSCs showed the same colony-forming potential as WT HSCs in vitro. With the caveat that the in vitro conditions do not necessarily recapitulate the in vivo situation, these data indicating the CD48−/−HSCs have no or a very slight intrinsic proliferation defect, suggesting instead an environmental contribution to the failure of the CD48−/− HSCs to appropriately proliferate in vivo.
Lower levels of IFNγ in BM of CD48-null mice
CD48 has previously been shown to affect cytokine signaling. Given the influence of cytokines on HSC function, we chose to examine the cytokine levels of the CD48−/− BM environment.9,22,23 After normalizing the cytokine levels to protein levels, the cytokine concentrations of young WT (n = 12) and CD48-null (n = 10) mice were compared. When analyzed as a group, a significant reduction in overall cytokine levels was observed (P < .01, supplemental Figure 3), with IFNγ and Cxcl9 presenting individually significant reductions in the CD48−/− supernatant (Figure 4A). Because Cxcl9 secretion is directly stimulated by IFNγ, the decrease in Cxcl9 is likely a consequence of the decrease in IFNγ.24
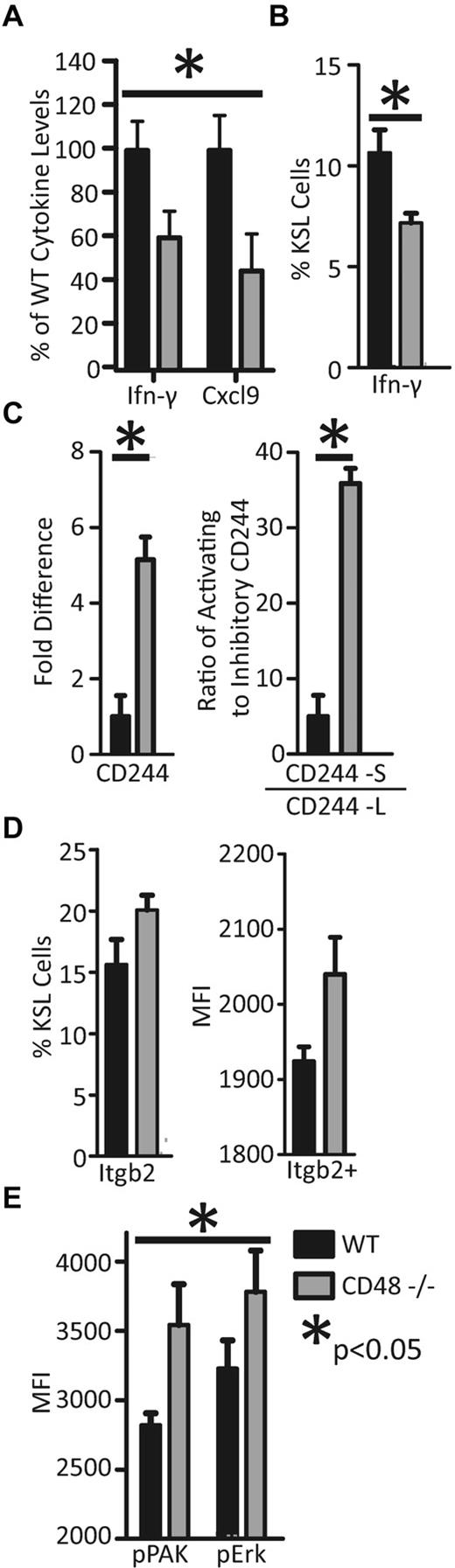
CD48−/− BM microenvironment displays a dysregulation of cytokines. (A) BM supernatant was isolated from 8- to 12-week-old CD48−/− and WT mice, and using cytokine bead arrays we found a severe decrease in IFNγ and Cxcl9. Other cytokines showed a trend of lower levels in the CD48−/− BM (supplemental Figure 3). (B) Intracellular IFNγ was examined by flow cytometry in hematopoietic progenitors (KLS). A small but consistent population of IFNγ+ progenitors was observed, and the CD48−/− mice exhibited a smaller population of IFNγ+ cells in comparison to WT. (C) CD244 expression was examined in short-term progenitors (SPneg KLS) with TaqMan real-time PCR. CD244 was found to be 5-fold higher in the CD48−/− short-term progenitors. SYBR green real-time PCR was used to analyze the CD244 isoforms present in the short-term progenitors (SPneg KLS) and GAPDH was used as an external control. (D) The Itgb2-Vav-Pak-Erk pathway was examined by flow cytometry to determine whether it was up-regulated in the CD48−/− hematopoietic progenitors (KLS). The number of Itgb2+ cells was slightly increased in the CD48−/− KLS cells, moreover CD48−/− cells expressing Itgb2 did so at a higher level. (E) In addition, activation of Pak and Erk, as measured by phosphorylation, was significantly increased in the CD48−/− KLS cells (2-way ANOVA, P < .05).
CD48−/− BM microenvironment displays a dysregulation of cytokines. (A) BM supernatant was isolated from 8- to 12-week-old CD48−/− and WT mice, and using cytokine bead arrays we found a severe decrease in IFNγ and Cxcl9. Other cytokines showed a trend of lower levels in the CD48−/− BM (supplemental Figure 3). (B) Intracellular IFNγ was examined by flow cytometry in hematopoietic progenitors (KLS). A small but consistent population of IFNγ+ progenitors was observed, and the CD48−/− mice exhibited a smaller population of IFNγ+ cells in comparison to WT. (C) CD244 expression was examined in short-term progenitors (SPneg KLS) with TaqMan real-time PCR. CD244 was found to be 5-fold higher in the CD48−/− short-term progenitors. SYBR green real-time PCR was used to analyze the CD244 isoforms present in the short-term progenitors (SPneg KLS) and GAPDH was used as an external control. (D) The Itgb2-Vav-Pak-Erk pathway was examined by flow cytometry to determine whether it was up-regulated in the CD48−/− hematopoietic progenitors (KLS). The number of Itgb2+ cells was slightly increased in the CD48−/− KLS cells, moreover CD48−/− cells expressing Itgb2 did so at a higher level. (E) In addition, activation of Pak and Erk, as measured by phosphorylation, was significantly increased in the CD48−/− KLS cells (2-way ANOVA, P < .05).
The decrease in IFNγ in the BM microenvironment led us to examine intracellular IFNγ staining in the hematopoietic progenitors. We isolated whole BM from WT and CD48−/− mice and used flow cytometry to examine the intracellular IFNγ status of the hematopoietic progenitors (specifically the LT-HSCs, ST-HSCs, MPP, and CLP; which are c-Kit+, Lin-neg, Sca-1+ also known as the KLS compartment) to discover whether they were capable of expressing IFNγ. When we examined the KLS progenitor compartment, we found a small but consistent population of IFNγ-expressing cells. Moreover, we discovered a significantly lower portion of IFNγ-expressing progenitors in the CD48−/− mice (Figure 4B). These results suggest a role for IFNγ signaling in the earliest hematopoietic progenitors.
The CD48:CD244 interaction is well characterized for its effects on IFNγ secretion in the NK cell–mediated cytotoxicity pathway, making CD244 a likely player in the interactions of the hematopoietic cells.11 Because of the decrease in IFNγ secretion in the CD48-null BM, we examined the CD244 status in these cells. We isolated LT-HSCs (SPKLS) and short-term progenitors (KLS–non-SP) by FACS, and used real-time PCR to examine the expression of CD244 in these cell types. In the LT-HSCs, CD244 expression remained undetectable even after 50 cycles. In the WT short-term progenitors, CD244 expression was evident and it was approximately 5-fold higher in the CD48−/− mice (Figure 4C). The CD244:CD48 interaction is known to be either inhibitory or activating depending on the isoform of CD244 being primarily expressed by the cell.18 We used a previously established set of SYBR green real-time primers to examine the ratio of activating (CD244-S) to inhibitory (CD244-L) transcripts in these cells.18,25,26 WT short-term progenitors express 5 activating mRNA molecules to 1 inhibitory mRNA molecule; however, this ratio is ∼ 35 to 1 in the CD48−/− cells (Figure 4C). The increase in CD244 expression and the skewing toward the activating isoform in the CD48−/− cells is likely the result of a feedback loop responding to the overall drop in cytokine signaling.
While IFNγ is lower in the CD48−/− mice, there remains some production, potentially a result of a compensatory pathway. In NK cells, signaling through Itgb2-Vav-Pak-Erk stimulates secretion of the same cytokines as CD48:CD244 signaling, making it a possible mechanism of compensation in the absence of CD48.27,28 We thus examined the expression of Itgb2 to determine whether it was being up-regulated on the progenitors of CD48−/− mice. We indeed found a slight increase in the percentage of Itgb2+ progenitors compared with WT. Furthermore, the cells expressing Itgb2 did so at a higher level relative to WT (Figure 4D). We then examined the activation of Pak and Erk by examining their phosphorylation status using intracellular phospho-flow cytometry. We found an increase in Pak and Erk phosphorylation, overall resulting in a significant increase (Figure 4E, 2-way ANOVA, P < .05) in signaling by this pathway in the CD48−/− hematopoietic progenitors, representing a possible compensation mechanism for the lack of signaling via CD48:CD244.
Mice lacking CD48 develop lymphoma with age
We noted an increased mortality of our CD48−/− mice with time, with nearly all mice deceased by approximately 80 weeks of age (average lifespan of WT C57Bl/6 is 125 weeks29 ), and this appeared to be associated with severe anemia (supplemental Figure 4). We examined CBCs of a large cohort of WT and KO mice over a concurrent time course and observed a general decrease in both WBCs and RBCs (particularly lymphocytes; supplemental Figure 5). We thought the considerable decrease in the lymphocyte counts of the older CD48 mice could be an indicator of a hematologic malignancy, leading us to believe the early mortality of these mice was likely because of development of lymphomas; similar to what is seen in 30% of patients with X-linked lymphoproliferative disease (XLP), a disease associated with defective signaling through SAP, which is downstream of CD48:CD244 signaling.30,31 Thus, we aged a cohort of CD48−/− mice (n = 10) and WT mice (n = 14) for 65 weeks to determine whether lymphomas were the cause of the early mortality in the CD48-null mice.
Morbidity was observed in the first CD48−/− animal at 51 weeks. At 65 weeks, we killed the remaining animals and found 90% of the CD48−/− animals had developed tumors (Figure 5A). For each mouse in the study, the sternum, spleen, and thymus was collected for pathology and stained with H&E. Tumors were observed in spleen and thymus, however, no tumors were seen in the BM of the sternum (Figure 5C). The tumors were primarily lymphomas, with the exception of a histiosarcoma in the spleen of one mouse, however a lymphoma was present in the thymus of this mouse. The lymphomas in general had large cells and some characteristics of follicular B-cell lymphomas.
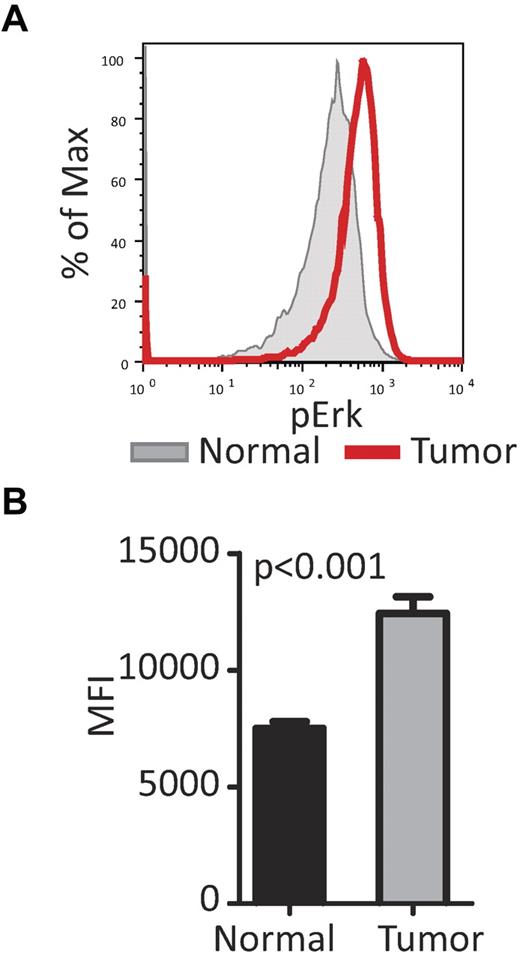
CD48−/− mice develop lymphoma with age. (A) A cohort of CD48−/− (n = 10) and WT mice were followed and monitored closely until 65 weeks of age or until morbidity was observed. The spleen, thymus, and sternum were then collected for pathology. None of the WT mice hade visible tumors in these organs, however, 90% of the CD48-null mice developed lymphoma (log-rank [Mantel-Cox] test, P = .0003). (B) Flow cytometry was performed on spleens collected from WT and CD48−/− mice. The tumors in the CD48−/− mice appeared to be CD19dim, CD44+, Thy1.2− and a large portion of these cells were positive for early B-cell lineage. (C) An example of H&E staining of thymus and spleen from WT and CD48 mice. Large malignant lymphomas can be seen in both organs in the majority of the CD48-null mice, and the lymphomas had characteristics of follicular lymphoma. (D) Mean fluorescence intensity pPAK and pERk for CD19dim cells was collected and a significant increase in the activation of the PAK/Erk pathway was seen in the CD48-null tumor cells (2-way ANOVA, *P < .05). (E) Mean fluorescence intensity pPAK and pERk for early B-cell Ag+ cells was collected and a significant increase in the activation of the PAK/Erk pathway was seen in the CD48-null tumor cells (2-way ANOVA, *P < .05).
CD48−/− mice develop lymphoma with age. (A) A cohort of CD48−/− (n = 10) and WT mice were followed and monitored closely until 65 weeks of age or until morbidity was observed. The spleen, thymus, and sternum were then collected for pathology. None of the WT mice hade visible tumors in these organs, however, 90% of the CD48-null mice developed lymphoma (log-rank [Mantel-Cox] test, P = .0003). (B) Flow cytometry was performed on spleens collected from WT and CD48−/− mice. The tumors in the CD48−/− mice appeared to be CD19dim, CD44+, Thy1.2− and a large portion of these cells were positive for early B-cell lineage. (C) An example of H&E staining of thymus and spleen from WT and CD48 mice. Large malignant lymphomas can be seen in both organs in the majority of the CD48-null mice, and the lymphomas had characteristics of follicular lymphoma. (D) Mean fluorescence intensity pPAK and pERk for CD19dim cells was collected and a significant increase in the activation of the PAK/Erk pathway was seen in the CD48-null tumor cells (2-way ANOVA, *P < .05). (E) Mean fluorescence intensity pPAK and pERk for early B-cell Ag+ cells was collected and a significant increase in the activation of the PAK/Erk pathway was seen in the CD48-null tumor cells (2-way ANOVA, *P < .05).
Flow cytometry was performed for each organ isolated, and the tumor cells appeared to be negative for the T-cell marker Thy1, and to have low expression of the B-cell marker CD19 (a decrease in CD19 expression is a diagnostic marker of follicular lymphoma32 ; Figure 5B). The tumor cells were almost uniformly CD44+ (indicating a minimally or partial follicular lymphoma33 ) and a large fraction were positive for early B-cell lineage Ag, a marker of pre-B cells and pre-B-cell lymphomas.34 Overall, pathology and surface markers of the tumor cells indicate the tumors are B-cell in origin, and most likely a diffuse follicular lymphoma with pre-B-cell traits.
Hyperactivation of PAK is known to be involved in the formation of several cancer types,35-38 and the increased activation of Pak1 in the hematopoietic progenitors from CD48−/− mice led us to speculate increased Pak1 activation may also play a role in the lymphoma development in the CD48-null mice. Splenocytes from WT mice and CD48−/− mice with lymphoma in the spleen were isolated and stained with either CD19 or early B-cell lineage Ag, and the phosphorylation status of Pak1 and Erk was examined (Figure 5D-E). CD19dim cells from CD48-null mice had a slight, but significant, increase in pPak and in pErk; however, there was a striking increase in pPak and pErk in the early B-cell lineage Ag-positive cells from the CD48-null tumors (Figure 5E). This suggests the compensation in IFNγ signaling achieved by the up-regulation of this pathway in young CD48−/− mice may be a double-edge sword; although providing necessary signaling for effective hematopoiesis in young mice, it may lead to hematopoietic malignancies with age.
CD48−/− tumors are able to reconstitute nonirradiated recipients
To determine the malignancy of the CD48−/− tumors, we attempted to reconstitute these tumors in both WT and CD48−/− nonirradiated mice. For this experiment, we pooled 2 tumors freshly isolated from CD48−/− mice and injected either 30 000 (n = 3 per genotype) or 50 000 (n = 5 per genotype) tumor cells into recipients. We then monitored the mice for 20 weeks after which all mice were killed, spleens were collected, and samples were sent for pathology. Of the mice receiving 30 000 cells only one CD48−/− mouse (1 of 3) developed a tumor; however, at the 50 000 cell dose all the CD48−/− mice (5 of 5) developed tumors. WT mice at either dose did not develop tumors indicating a role for the CD48-null environment in reconstituting these tumors. In addition to pathology, we examined the status of Erk activation in the reconstituted tumors (CD19− cells) and found it was still hyperactive (Figure 6A-B) compared with normal cells (CD19− splenocytes) as was seen in the primary tumors.
CD48−/− tumors can reconstitute a nonirradiated host. Eight- to 10-week-old nonirradiated WT or CD48−/− mice were injected with either 30 000 or 50 000 tumor cells. Mice were monitored for 20 weeks and killed for pathology. At 30 000 tumor cells, no WT mice (n = 3) developed tumors; however, 1 of 3 CD48−/− mice (n = 3) developed tumors. At 50 000 tumor cells, no WT mice (n = 5) developed tumors, and all CD48−/− mice (n = 5) developed tumors (P = .007, Fisher exact test). (A) CD19− splenocytes from mice with reconstituted tumors and those without tumors were collected and analyzed for levels of phosphorylated ERK (pERk). Shown here are representative histograms of the analysis of phosphoprotein. (B) CD19− cells from the reconstituted tumors possess significantly higher levels of pERK (P < .001, Student t test) than the normal CD19− splenocytes.
CD48−/− tumors can reconstitute a nonirradiated host. Eight- to 10-week-old nonirradiated WT or CD48−/− mice were injected with either 30 000 or 50 000 tumor cells. Mice were monitored for 20 weeks and killed for pathology. At 30 000 tumor cells, no WT mice (n = 3) developed tumors; however, 1 of 3 CD48−/− mice (n = 3) developed tumors. At 50 000 tumor cells, no WT mice (n = 5) developed tumors, and all CD48−/− mice (n = 5) developed tumors (P = .007, Fisher exact test). (A) CD19− splenocytes from mice with reconstituted tumors and those without tumors were collected and analyzed for levels of phosphorylated ERK (pERk). Shown here are representative histograms of the analysis of phosphoprotein. (B) CD19− cells from the reconstituted tumors possess significantly higher levels of pERK (P < .001, Student t test) than the normal CD19− splenocytes.
Discussion
Here we have explored the role of CD48 in maintaining balanced hematopoiesis and we show a crucial role for CD48 in the regulation of the hematopoietic stem and progenitor cells. CD48 has previously been implicated in initiation of T-cell activation and their subsequent proliferation.21,39,40 It has been proposed that T cells and HSCs may share similar activation mechanisms, and our findings support this idea.3,41 Even though CD48 is essentially excluded from the quiescent long-term HSC population, its loss has a profound effect on the function of the stem cell by altering the cytokine milieu. IFNγ secretion can be directly stimulated or inhibited by the interaction between CD48 and CD244, and the coexpression of these 2 molecules in the short-term progenitor pool suggests this interaction between progenitors is crucial to correctly modifying IFNγ signaling between these cells.
IFNγ has been shown to negatively affect self-renewal, inhibit proliferation, promote survival, increase proliferation, and increase self-renewal depending on the context of the signal.14-16,42-44 Interestingly, IFNα, was recently shown to provoke HSCs from dormancy into an active and proliferating state.45,46 Finally, our laboratory has recently shown a fundamental role for IFNγ in promoting HSC proliferation similar to IFNα,7 thus, the loss of CD48 most likely leads to misregulation of IFNγ signaling resulting in the observed defects.
In young CD48−/− mice, the lack of CD48-CD244 signaling leads to lower levels of IFNγ and more quiescent LT-HSCs, which are unable to appropriately respond to stresses, such as 5-FU treatment. Compensatory signaling through the Itgb2-Vav-Pak-Erk pathway is able to maintain homeostasis in young mice by inducing IFNγ, but this comes at an immense cost. Hyperactivation of Pak coupled with the loss of signaling through SAP via CD48:CD244 interactions could result in malignant lymphomas similar to those seen in a large portion of XLP patients.30,31,47 XLP is a rare and usually fatal disease characterized by defective antiviral and antitumor responses resulting in aberrant lymphoid proliferation.30,31 Approximately 30% of patients develop a lymphoproliferative disorder mostly in the form of a malignant lymphoma, and constitutional defects in SAP, which is critical for successful signaling in CD48:CD244 interactions, have been shown to be causative.48 Up to 30% of patients with an XLP-like syndrome lack mutations in SAP,49 which in light of our data leads to the intriguing possibility mutations in other components of the CD48:CD244 signaling pathway, including CD48 itself, may be found in some of these other patients.
The activation of PAK to compensate for a lack of SAP signaling in the early progenitor compartment could reflect a heretofore unknown possible contributor to lymphoma development and may have a role in XLP patients. PAK inhibitors, currently being developed for clinical use because of the association of Pak activation in solid tumors35 could be considered for treatment of this disease. Interestingly, PAK has also been implicated in T-cell activation and proliferation,50 and the CD48-null cells in our murine transplant recipients gave rise to an increased number of T-cells, similar to that seen in a majority of XLP patients. Thus, we speculate PAK hyperactivation could play a major role in XLP.
Overall, our data show for the first time a molecule expressed on HSC progeny, but excluded from the quiescent HSCs, plays a key role in regulating HSC activity through modulating the cytokine milieu. We illustrate the importance of tightly regulated cytokine levels in the BM to maintain hematopoiesis; alteration of this signaling can lead to drastic effects on homeostasis. Moreover, we show the loss of CD48 leads to increased signaling through Pak1 and Erk to compensate for the loss of signal through CD244 and SAP, which predisposes the animal to hematologic malignancies. Finally, we highlight a possible role for PAK hyperactivation in XLP and related syndromes in patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (DK58192, EB005173, HL08100, P50CA126752, and F30DK082107), as well as the Ellison Foundation and the American Heart Association.
National Institutes of Health
Authorship
Contribution: N.C.B. designed and performed experiments, analyzed the data, and wrote the manuscript; K.K.L. designed and performed experiments and analyzed data; T.V.B. designed and performed experiments and analyzed data; G.L.L. and M.T.B. performed experiments; and M.A.G. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret A. Goodell, One Baylor Plaza, N1030, Houston, TX 77030; e-mail: goodell@bcm.edu.

![Figure 5. CD48−/− mice develop lymphoma with age. (A) A cohort of CD48−/− (n = 10) and WT mice were followed and monitored closely until 65 weeks of age or until morbidity was observed. The spleen, thymus, and sternum were then collected for pathology. None of the WT mice hade visible tumors in these organs, however, 90% of the CD48-null mice developed lymphoma (log-rank [Mantel-Cox] test, P = .0003). (B) Flow cytometry was performed on spleens collected from WT and CD48−/− mice. The tumors in the CD48−/− mice appeared to be CD19dim, CD44+, Thy1.2− and a large portion of these cells were positive for early B-cell lineage. (C) An example of H&E staining of thymus and spleen from WT and CD48 mice. Large malignant lymphomas can be seen in both organs in the majority of the CD48-null mice, and the lymphomas had characteristics of follicular lymphoma. (D) Mean fluorescence intensity pPAK and pERk for CD19dim cells was collected and a significant increase in the activation of the PAK/Erk pathway was seen in the CD48-null tumor cells (2-way ANOVA, *P < .05). (E) Mean fluorescence intensity pPAK and pERk for early B-cell Ag+ cells was collected and a significant increase in the activation of the PAK/Erk pathway was seen in the CD48-null tumor cells (2-way ANOVA, *P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/1/10.1182_blood-2010-12-322339/4/m_zh89991174050005.jpeg?Expires=1769082301&Signature=VWCIhDoWnHDI8RtTGVKISlFSleUfVUaN-ioB1AViHosgPFLl3B4VwJFaeaVUJ3ALTQJz5tRc27jzUtjvTc6zZowRIxKmaJiqayZXJC-TH10reVy8mHdlE6iFyAnUsRLwnzpvwN6gXdX8jBp3vpvIpqPpIcAmpwxvYZ-LVf-O6OqeE0k7XxUsw2wV0WGjyreVA-FB94S8Gu04XeWFMblpIN--PRfYgcUsC6JWinZPqtQuWeGf6JJ0jFF6uYsKXLIZOuQhL11-Qy26C1TqQrwI7LeLdZaNyDJuO7MS4M3cb1AexbBELSEZ0DyZnshFJUfe6L0Ht36sdAtFYQPSUygwyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)