Abstract
Endoglin (Eng), an accessory receptor for the transforming growth factor β (TGF-β) superfamily, is required for proper hemangioblast and primitive hematopoietic development. However the mechanism by which endoglin functions at this early developmental stage is currently unknown. Transcriptional analyses of differentiating eng−/− and eng+/+ ES cells revealed that lack of endoglin leads to profound reductions in the levels of key hematopoietic regulators, including Scl, Lmo2, and Gata2. We also detected lower levels of phosphorylated Smad1 (pSmad1), a downstream target signaling molecule associated with the TGF-β pathway. Using doxycycline-inducible ES cell lines, we interrogated the TGF-β signaling pathway by expressing activated forms of ALK-1 and ALK-5, type I receptors for TGF-β. Our results indicate that ALK-1 signaling promotes hemangioblast development and hematopoiesis, as evidenced by colony assays, gene expression and FACS analyses, whereas signaling by ALK-5 leads to the opposite effect, inhibition of hemangioblast and hematopoietic development. In Eng−/− ES cells, ALK-1 rescued both the defective hemangioblast development, and primitive erythropoiesis, indicating that ALK-1 signaling can compensate for the absence of endoglin. We propose that endoglin regulates primitive hematopoiesis by modulating the activity of the Smad1/5 signaling pathway in early stages of development.
Introduction
During vertebrate embryogenesis, the establishment of the hematopoietic system is highly complex, initiating with mesoderm formation and patterning followed by specification of the hematopoietic lineage. This multistep process involves several key anatomical sites, cellular interactions as well as intrinsic and extrinsic regulators. The first wave of hematopoiesis is observed at approximately 8 days after fertilization in the blood islands that arise in the extraembryonic mesoderm of yolk sac. Blood islands consist of endothelial sacs encapsulating clusters of primitive erythroid progenitors, which produce embryonic globin. The close spatial association between primitive hematopoietic progenitors and endothelial cells prompted the hypothesis, more than a century ago, that these progenitor cells may derive from a common precursor, the so-called hemangioblast.1,2 In addition to their proximity, these 2 lineages also share common expression of crucial regulatory genes and antigenic determinants such as Scl,3 Flk-1,4 endoglin,5 PECAM,6 and CD34.7
Because the in vitro differentiation of embryonic stem (ES) cells into embryoid bodies (EBs) recapitulates early events of embryonic hematopoiesis, this system has proven extremely useful to study early cell fate decisions as well as the molecular mechanisms controlling these processes. A cell with properties of the hemangioblast has been identified using the ES/EB system.8 This precursor, referred to as the blast colony-forming cell (BL-CFC) forms in response to vascular endothelial growth factor (VEGF), stem cell factor (SCF), and thrombopoietin (TPO), and represents a transient population of cells that stand at the juncture of the endothelial and hematopoietic lineages.8,9 More recently, Keller and colleagues have been able to detect hemangioblast precursors within the mouse embryo, at maximum frequency in the posterior streak (PS) region of the late primitive streak stage embryos (E7.5).10 The average number of hemangioblasts detected per embryo varied between one and 5. These results indicate that bipotent progenitors exist in low frequency during embryogenesis, and that the initial stages of hematopoietic and endothelial commitment occur before, and not during, blood island development in the yolk sac, as previously thought.11
Through the use of the ES/EB system, several signaling pathways have been demonstrated to be involved distinctively in these early embryonic processes, including development of the proper PS, induction of Flk-1+ mesoderm, and subsequent hematopoietic specification.12-15 For instance, while Activin/Nodal, Wnt, bone morphogenetic protein (BMP), and Notch signaling pathways regulate the induction of Flk-1+ mesoderm,12,14,15 only Activin/Nodal and Wnt are essential for PS development,13,14 whereas commitment of the Flk-1+ population to the hematopoietic lineage requires VEGF/Flk-1 and Wnt signaling pathways.12,14
We have previously shown that endoglin (also known as CD105), an ancillary type III receptor of the TGF-β signaling pathway,16,17 is an important regulator of early stages of hemangioblast specification and hematopoietic commitment.5 In addition to these early functions, endoglin also has been linked to definitive hematopoiesis as it is coexpressed with CD45 in hematopoietic cells obtained from ES/OP9 cocultures,18 and it enriches for the long-term repopulating hematopoietic stem cells (HSCs) in adult bone marrow.19 Nevertheless, most of what is known about endoglin relates to its expression and function in endothelial cells,16 in which this receptor binds to TGF-β isoforms 1 and 3 in combination with the signaling complex of TGF-β receptors types I and II.17,20 On ligand binding, TβRII recruits and phosphorylates a TGF-β type I receptor (TβRI). Endothelial cells express 2 types of TβRI, activin receptor-like kinase 5 (ALK-5)21 and ALK-1.20,22-24 Activated ALK-5 transduces the signal to the nucleus by phosphorylating Smad 2/3 proteins, whereas ALK-1 signals through Smad1/5.25 In endothelial cells, it has been suggested that endoglin is required for efficient TGF-β/ALK-1 signaling,24,26,27 and may act as a modulator of TGFβ-dependent activation of ALK-1 (stimulatory signal) versus ALK-5 (inhibitory signal) to maintain a balance between the Smad1/5 and Smad2/3 signaling pathways, respectively.25,27 Mutations in both endoglin and ALK-1 have been associated with an autosomal dominant vascular disorder termed hereditary hemorrhagic telangiectasia,28 further indicating that these receptors function in the same pathway. Mice lacking endoglin (eng−/−) fail to progress beyond 10.5 days postcoitum (dpc) due primarily to vascular and cardiac abnormalities.29,30 Analysis of 9.5 dpc eng−/− embryos reveal anemia of the yolk sac, suggesting that endoglin is required for blood development. This hypothesis was confirmed by our studies using differentiating eng−/− ES cells which demonstrated that lack of endoglin leads to defective primitive hematopoiesis, as evidenced by a dramatic reduction in the number of primitive erythroid colonies (EryP) as well as decreased levels of globins and several hematopoietic transcription factors.5 Analysis of day 3 EBs using the BL-CFC assay indicated that endoglin also plays a key role in the specification of the hemangioblast progenitor.5
To gain insight into the molecular mechanisms by which endoglin regulates early hemangioblast/hematopoietic development in the context of the TGF-β signaling pathway, we investigated the global transcriptional effects of endoglin deficiency and determined the role of ALK-1 and ALK-5 signaling in the function of endoglin during these early developmental stages.
Methods
Generation of inducible ES cell lines
HA-expressing vectors encoding constitutively active and wild-type ALK-1 kinase (Q201D and WT, respectively), generously provided by Dr K. Miyazono (University of Tokyo, Japan), and constitutively active ALK-5 (T204D), generously provided by Dr L. Attisano (University of Toronto, Canada), were used to generate inducible ES cell lines. Inducible CA-ALK-1 and CA-ALK-5 ES cell lines were generated in ZX1 ES cells, an improved version of A2Lox,31 in which a second generation nonleaky tet response element replaces the original TRE, and cre is present at the doxycycline-inducible locus before recombination and catalyzes its replacement by the gene of interest. Inducible expression of these genes was confirmed by real time RT-PCR and Western blotting for HA tag (Santa Cruz). To generate iALK-1:Eng−/− ES cells, ES cells were transduced with a lentiviral vector expressing the reverse tet-transactivator (rtTA),32 followed by a vector enabling inducible ALK-1 expression. The cDNA for WT ALK-1 was subcloned into pSAM2, a lentiviral construct containing the transactivator, a tet-responsive element (TRE), which allows the expression of the target gene on doxycycline induction, and internal ribosome entry site (IRES)-EGFP, which allows confirmation of integration and inducible expression. Viruses for these plasmids, rtTA and TRE/ALK-1/IRES.GFP, were generated through transfection in 293T cells using the Fugene method. Eng−/− ES cells were initially infected with rtTA, followed by infection with TRE/ALK-1/IRES.GFP 24 hours later. Eng−/− ES cells containing the ALK-1 insert were purified by FACS based on GFP expression after an overnight incubation with doxycycline (dox) at 0.5μg/mL. After one passage, cells were subjected to a second round of FACS, in which the brightest 3% of GFP+ cells were single-cell sorted onto single wells of a 96-well dish pre-plated with MEFs. Wells with single ES cell colonies were harvested and expanded into clonal cell lines. 6 clones were tested individually for GFP expression during an EB time course. A clone that maintained high levels of GFP after differentiation was selected for rescue studies.
Growth and differentiation of ES cells
Wild-type E14, eng−/− ES cells18,29 (generously provided by Dr J. C. Zuniga-Pflucker from the University of Toronto), and the newly generated ES cell lines described in “Generation of inducible ES cell lines” were used in this study. ES cells were maintained on irradiated mouse embryonic fibroblasts (MEFs) in DMEM (Sigma-Aldrich) supplemented with 1000 U/mL LIF (leukemia inhibitory factor; Chemicon), 15% inactivated FBS (Gemini Bio-Products), 0.1 mM nonessential amino acids (Sigma-Aldrich), and 0.1 mM of β-mercaptoethanol (Sigma-Aldrich). For differentiation cultures, ES cells were trypsinized, resuspended in embryoid body differentiation (EBD) medium, IMDM supplemented with 15% FBS (Stem Cell Technologies), 4.5 mM monothioglycerol (Sigma-Aldrich), 50μg/mL ascorbic acid (Sigma-Aldrich), and 200μg/mL iron-saturated transferrin (Sigma-Aldrich), and plated onto fresh gelatin coated T25 flasks for 30 minutes to allow MEFs to adhere. Non-adhering cells (ES cells, depleted of MEFs) were then plated as hanging drops at a concentration of 100 cells per 10 μL drop in an inverted bacterial Petri dish. After 48 hours in culture, EBs were collected and recultured in 10 mL of EBD medium in low adherence 10 cm Petri dishes on a slowly swirling table rotator (set up inside of the tissue culture incubator). At day 4, EBs were fed by exchanging half of their spent medium for fresh EBD medium. To induce appropriate ALK expression during EB differentiation, doxycycline (Sigma-Aldrich) was added to the cultures at 0.75μg/mL.
BL-CFC assay
EBs were collected at 3.25 days after differentiation, washed with PBS and dissociated with 0.25% trypsin-EDTA for 3 minutes at 37°C. EBs were disrupted to single cells by repeated pipetting and plated at 5 × 104 cells in 1 mL of methylcellulose medium (M3120, Invitrogen) with 10% FBS, 50 μg/mL ascorbic acid, 200 μg/mL iron-saturated transferrin, 4.5 × 10−4 M MTG, in the presence of TPO (25 ng/mL; Peprotech), VEGF (5 ng/mL; Peprotech) and SCF (100 ng/mL; Peprotech), as previously described.8,9 Plated cells were cultured in a humidified incubator at 37°C in an environment of 5% CO2. 5 days after plating, BL-CFCs were enumerated.
Generation of hematopoietic cells
Cells from day 4.25 and 6.25 EBs were plated into methylcellulose media containing IL-3, IL-6, erythropoietin (EPO) and SCF (M3434; StemCell Technologies). Cultures were maintained as described in “Growth and differentiation of ES cells.” EryP and definitive hematopoietic colonies were scored 6 and 10 days after plating, respectively.
Flow cytometry
We used PE-conjugated anti–mouse CD41 (also known as ITGA2B and GPIIB; Mouse Genome Informatics) and APC-conjugated anti–mouse c-Kit (eBioscience). EB cells were collected after a short incubation with 0.25% trypsin-EDTA, washed twice, first with IMDM 10% FBS and then with blocking buffer (PBS with 1% FBS). Cells were then resuspended in the blocking buffer containing 0.25 μg/106 cells of Fc block (Pharmingen) and incubated on ice for 5 minutes. Staining antibody was added at 1 μg/106 cells and incubated at 4°C for 30 minutes before washing with blocking buffer. We analyzed stained cells on a FACS Aria instrument (BD Biosciences) after adding propidium iodide (Pharmingen) to exclude dead cells.
Incorporation of BrdU and FACS analyses
Day 3.25 and 6.25 iALK1 and iALK5 EBs, that had been induced with 750 ng/mL doxycycline since day 2, were incubated with 10μM BrdU (BD Biosciences) for 60 minutes. After washing, labeled cells were then counter-stained with c-Kit–APC and CD41-PE (for day 6.25 EBs). Incorporation of BrdU was detected using FITC conjugated Anti-BrdU antibody, according to the protocol provided by the FITC BrdU Flow Kit (BD Biosciences). Samples were analyzed using FACS Aria II and FlowJo Version 7.6.3 software.
RT-PCR analysis
Total RNA was isolated using RNeasy Mini Kit (QIAGEN) as described by the manufacturer. cDNA was synthesized using Thermoscript reverse transcriptase (Invitrogen) with Oligo dT priming. For real-time PCR, all probe sets were from Applied Biosystems. For globins, we designed customized primer/probe sets (all shown 5′-3′): Beta-major F, AGGGCACCTTTG CCAGC; Beta-major R, GGCAGCCTCTGCAGCG; Beta-major probe, 6FAM-CGTGATTG TGCTGGGCCACCACCT-TAMRA. Embryonic F, CCTCAAGGAGACCTTTGCTCAT; Embryonic R, CAGGCAGCCTGCACCTCT; Embryonic probe, 6FAM-CAACATGTTGG TGATTGTCCTTTCT-TAMRA. To obtain the relative expression, we first calculated the gene expression levels relative to Gapdh (the fold change to Gapdh). Then we normalized to the level of wild-type or nondox-treated samples.
Western Blotting
Day 3.25 EB cell lysates were prepared using 1X RIPA Buffer (ThermoScientific) in combination with Complete Protease Inhibitor Cocktail (Roche) and PhosSTOP (Roche). Protein concentration was measured against a wavelength of λ595, and 25μg samples were prepared using 2X Laemmli Buffer (BioRad). Samples were then denatured on a heat block at 100°C for 10 minutes. SDS-PAGE was performed with an 8% bis-acrylamide separating gel and 4% Bis-acrylamide stacking gel. Proteins were transferred to a nitrocellulose membrane (Immobilon-P; Millipore) over the course of 2 hours at 400 mAmp at 4°C. Subsequently, the membranes were blocked for 1 hour in 5% BSA (1X TBS-Tween20). The following primary antibodies were applied at the indicated dilution in 5% BSA (1X TBS-Tween20). Primary antibodies included a 1:1000 dilution of phosphorylated Smad1/5/8 (Cell Signaling), 1:1000 dilution of Smad2 (Cell Signaling), 1:1000 dilution of ALK-1 (Abcam), 1:1000 dilution of ALK-5 (R&D Systems), 1:3000 dilution of HA tag (Santa Cruz Biotechnology), 1:10 000 dilution of GAPDH (Abcam). Smad1 (Abcam) and phosphorylated Smad2 (Millipore) were diluted at 1:3000 and 1:1500, respectively, using Primary Antibody Signal Boost Immunoreaction Enhancer (Calbiochem). All primary antibodies were incubated for 12 hours at 4°C on a shaker. The membranes were washed for 3 × 20 minutes in 1X TBS-Tween20, before secondary antibody application. Secondary antibodies included ECL peroxidase-labeled anti–mouse antibody (GE Biosciences) and ECL peroxidase-labeled anti–rabbit antibody (GE Biosciences). Both secondary antibodies were applied at a 1:20 000 dilution for 1 hour on a shaker at room temperature. Again, the membranes were washed 3 × 20 minutes in 1X TBS-Tween20. To detect the HRP signal, SuperSignal West Pico Chemiluminescent Substrate (ThermoScientific) was added to the membranes and exposed to film in the darkroom. The western blots were quantified using ImageJ (http://imagej.nih.gov/ij/index.html) and data for each antibody were normalized to the value of GAPDH. The results were then plotted as fold relative to WT cells (or no dox group) with the relative standard error. Experiments were repeated at least 3 times.
Microarray analysis
Total RNA from Eng−/− and E14 day 3 EBs was purified using TRIzol (Invitrogen, CA), following the manufacturer's protocol. Total RNA was amplified using the TotalPrep RNA Amplification Kit (Ambion), which amplifies RNA across a range from 50 to 500 ng. The amplified RNA (aRNA) then incorporated a biotinylated UTP nucleotide. An aliquot of aRNA was hybridized to an Illumina BeadChip (Mouse Genome 430 2.0 Array) using proprietary buffers at 65°C for thirty minutes, then overnight at 58°C. After hybridization, the arrays were washed and then stained with Cy3-streptavidin. The array was then washed again and scanned on an Illumina BeadStation bead array scanner. Microarray results were analyzed with GeneSpring GX Version 11.0 software. Differences of gene expression were determined by applying > 1.4 fold change and using a baseline to experimental intensity difference of > 100. The Gene Expression Omnibus accession number for the corresponding microarray data is GSE29300, titled “The Effect of Endoglin Loss on Embryoid Bodies.”
Statistical analysis
Differences between samples were assessed using the Student t test.
Results
Lack of endoglin perturbs TGF-β signaling
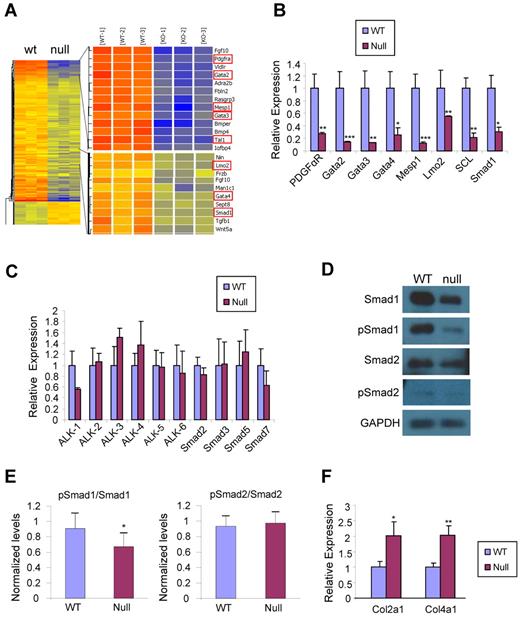
To investigate the mechanism by which endoglin regulates early mesoderm development, eng−/− and eng+/+ ES cells that had been differentiated as EBs for 3 days were subjected to global microarray analyses. A total of 128 genes were found down-regulated to a 1.5 fold change cut-off and P value < .05. Among these genes, we identified a specific group related to mesoderm and hematopoietic development, that were down-regulated, including PDGFαR, Gata2, Gata3, Gata4, Mesp1, Tal1 (Scl), and Lmo2 (Figure 1A). Smad1, one of the transcriptional modulators of the TGF-β signaling pathway was also found down-regulated in eng−/− EBs (Figure 1A). These results were confirmed by real time PCR which showed that genes identified in the microarray were indeed down-regulated in the absence of endoglin (Figure 1B). Based on the observation of reduced levels of Smad1 in eng−/− EBs, we decided to screen the expression levels of other signaling molecules associated with the TGF-β superfamily. Overall, no significant changes were detected (Figure 1C). Although eng−/− EBs showed lower expression levels of ALK-1 mRNA (Figure 1C), quantification of Western blot signals did not show significant differences between wild-type and eng−/− EBs (supplemental Figure 1A, available on Blood Web site; see the Supplemental Materials link at the top of the online article). Nevertheless, clear reduction in the levels of both Smad1 and p-Smad1 were found in the absence of endoglin (Figure 1D-E). While no changes were observed in the levels of ALK-5 or Smad2 (supplemental Figure 1A and Figure 1D-E), 2 target genes of the ALK-5/Smad2 TGF- β1 signaling pathway, Col2a1 and Col4a1,33 were found up-regulated in eng−/− EBs (Figure 1F).
Transcriptional profile of day 3 EBs in the absence of endoglin. (A) Microarray heat map representing up- and down-regulated genes in day 3 eng−/− (null) EBs compared with wild-type (WT). Results represent 3 biologic replicates. (B) Confirmatory real time PCR analyses for selected down-regulated genes. Transcripts are normalized to Gapdh. Error bars indicate SE from 3 independent experiments performed in duplicate. (C) Real time RT-PCR expression analysis for several TGF-β signaling molecules on day 3 WT and eng−/− EBs. Transcripts are normalized to Gapdh. Error bars indicate SE from 3 independent experiments performed in duplicate. (D) Western blot analyses of day 3 WT and eng−/− EBs for TGF-β signaling molecules, including Smad1, Smad2, phosphorylated Smad1/5/8 (pSmad1/5/8), and phosphorylated Smad2 (pSmad2). (E) Quantification of phosphorylated Smad1 and phosphorylated Smad2. After normalization to Gapdh levels, results were plotted as ratio between phosphorylated Smad and total Smad. (F) Real time RT-PCR expression analysis for 2 target genes of the ALK-5/Smad2 pathway on day 3.75 WT and eng−/− EBs. Transcripts are normalized to Gapdh. Error bars indicate SE from 3 independent experiments performed in duplicate. *P < .05, **P < .01, ***P < .001.
Transcriptional profile of day 3 EBs in the absence of endoglin. (A) Microarray heat map representing up- and down-regulated genes in day 3 eng−/− (null) EBs compared with wild-type (WT). Results represent 3 biologic replicates. (B) Confirmatory real time PCR analyses for selected down-regulated genes. Transcripts are normalized to Gapdh. Error bars indicate SE from 3 independent experiments performed in duplicate. (C) Real time RT-PCR expression analysis for several TGF-β signaling molecules on day 3 WT and eng−/− EBs. Transcripts are normalized to Gapdh. Error bars indicate SE from 3 independent experiments performed in duplicate. (D) Western blot analyses of day 3 WT and eng−/− EBs for TGF-β signaling molecules, including Smad1, Smad2, phosphorylated Smad1/5/8 (pSmad1/5/8), and phosphorylated Smad2 (pSmad2). (E) Quantification of phosphorylated Smad1 and phosphorylated Smad2. After normalization to Gapdh levels, results were plotted as ratio between phosphorylated Smad and total Smad. (F) Real time RT-PCR expression analysis for 2 target genes of the ALK-5/Smad2 pathway on day 3.75 WT and eng−/− EBs. Transcripts are normalized to Gapdh. Error bars indicate SE from 3 independent experiments performed in duplicate. *P < .05, **P < .01, ***P < .001.
Effect of ALK-1 induction in early EB development
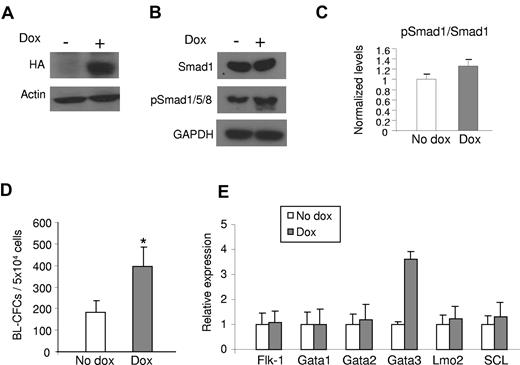
Based on the finding that the ALK-1/Smad1 TGF-β1 signaling pathway is disturbed in the absence of endoglin, and thus may play a role in the defective hemangioblast phenotype observed in eng−/− ES cells, we assessed the effect of ALK-1 gain-of-function during early EB differentiation by generating an ES cell line with inducible expression of HA-tagged constitutively active ALK-1 (iALK-1). Conditional expression of ALK-1 in this cell line was confirmed by Western blotting (Figure 2A). Because we have used human ALK-1 for these gain-of-function experiments, we were able to distinguish the levels of exogenous and endogenous ALK-1 using specific RT-PCR primers for both human and mouse, respectively. The relative expression of exogenous versus endogenous ALK-1 was approximately8-fold (supplemental Figure 1D). As expected, induction of ALK-1 led to increased levels of pSmad1/5/8 (Figure 2B-C), while levels of nonphosphorylated Smad1 remained the same (Figure 2B). No changes were observed for Smad2 and pSmad2 levels, downstream targets of the ALK-5 pathway (supplemental Figure 1B).
Effect of ALK-1 signaling on hemangioblast development. iALK1 ES cells were differentiated into EBs. Dox was added to the culture medium from day 2 of EB differentiation. Cells were characterized at day 3.25 as follows: (A) Western blot for HA confirms induction of CA-ALK-1 in iALK-1 ES cells. (B) Western blot for Smads. Induction of CA-ALK-1 leads to increased levels of phosphorylated Smad1/5/8 (pSmad1/5/8), downstream targets of ALK1. Actin and GAPDH were used as loading control. (C) Quantification of phosphorylated Smad1. After normalization to GAPDH levels, results were plotted as ratio between phosphorylated Smad1/5/8 and total Smad1. (D) iALK-1 ES cells were assayed for hemangioblast activity in BL-MCM, which consists of methylcellulose containing VEGF, SCF, and TPO. Error bars indicate SE from 3 independent experiments performed in duplicate. (E) Gene expression analyses for Flk-1, Gata1, Gata2, Lmo2, and SCL. Error bars indicate SE from 2 independent experiments performed in duplicates. *P < .05, **P < .01
Effect of ALK-1 signaling on hemangioblast development. iALK1 ES cells were differentiated into EBs. Dox was added to the culture medium from day 2 of EB differentiation. Cells were characterized at day 3.25 as follows: (A) Western blot for HA confirms induction of CA-ALK-1 in iALK-1 ES cells. (B) Western blot for Smads. Induction of CA-ALK-1 leads to increased levels of phosphorylated Smad1/5/8 (pSmad1/5/8), downstream targets of ALK1. Actin and GAPDH were used as loading control. (C) Quantification of phosphorylated Smad1. After normalization to GAPDH levels, results were plotted as ratio between phosphorylated Smad1/5/8 and total Smad1. (D) iALK-1 ES cells were assayed for hemangioblast activity in BL-MCM, which consists of methylcellulose containing VEGF, SCF, and TPO. Error bars indicate SE from 3 independent experiments performed in duplicate. (E) Gene expression analyses for Flk-1, Gata1, Gata2, Lmo2, and SCL. Error bars indicate SE from 2 independent experiments performed in duplicates. *P < .05, **P < .01
Next we examined the effect of ALK-1 induction on hemangioblast development by plating iALK-1 ES cells that had been differentiated as EBs for 3.25 days, in the presence or absence of dox from day 2 of EB differentiation, for blast colony formation. As observed in Figure 2D, ALK-1-induced EBs generated a significantly higher number of BL-CFCs than their control counterparts (no dox). Real time PCR analyses of day 3.25 EBs showed that ALK-1 induction does not change Flk-1 expression in these cells (Figure 2E). At this time point, no changes were observed in the expression levels of hematopoietic transcription factors, including Gata1, Gata2, Scl, and Lmo2 (Figure 2E).
ALK-1 signaling promotes hematopoiesis
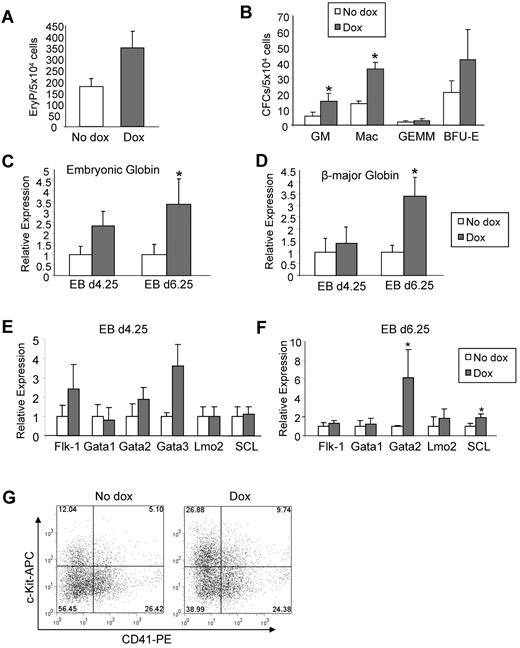
To investigate the effect of ALK-1 gain-of-function in hematopoietic development, iALK-1 ES cells were assayed for primitive erythropoiesis and definitive hematopoietic development by plating day 4.25 and 6.25 EBs, respectively, in methylcellulose medium containing IL-3, IL-6, SCF, and Epo. Induction of ALK-1 during EB differentiation resulted in increased numbers of EryPs (Figure 3A) as well as definitive burst forming unit-erythroid (BFU-E), CFU-granulocyte/macrophage (GM), and CFU-macrophage (Mac; Figure 3B). The stimulatory effect of ALK-1 on erythroid development was further corroborated by the increased levels of embryonic (Figure 3C) and β-major (Figure 3D) globins. This phenotype was observed consistently in multiple clones (supplemental Figure 2B-C). Flk-1 and the examined hematopoietic transcription factors remained unchanged (Figure 3E-F), except for GATA2 and SCL, which were significantly up-regulated on day 6.25 of EB differentiation (Figure 3F). FACS analysis of embryonic hematopoietic progenitors on day 6.25 EBs revealed an approximately 2-fold increase in the CD41+c-Kit+ double positive fraction on ALK-1 induction (Figure 3G), supporting the premise that ALK1 activation has a stimulatory effect on hematopoietic development.
ALK-1 gain-of-function stimulates hematopoiesis. iALK-1 ES cells were differentiated as EBs in the presence or absence of Dox for 4.25 and 6.25 days, and assayed for primitive erythroid (A) and definitive CFCs (B), respectively. Error bars indicate SEs of 3 independent experiments performed in duplicate. Gene expression analyses for embryonic globin (C) and β major globin (D) at days 4.25 and 6.25 of EB differentiation. Transcripts are normalized to Gapdh. Error bars indicate SE from 2 independent experiments performed in duplicate. (E-F) Relative levels of Flk-1, Gata1, Gata2, Lmo2, and SCL in 4.25 and 6.25 EBs, respectively. Transcripts are normalized to Gapdh. (G) A representative FACS profile for CD41 and c-Kit in induced and noninduced iALK-1 cells at EB day 6.25. Fluorescence intensity for c-Kit is indicated on the y axis and CD41 on the x axis. *P < .05, ***P < .001
ALK-1 gain-of-function stimulates hematopoiesis. iALK-1 ES cells were differentiated as EBs in the presence or absence of Dox for 4.25 and 6.25 days, and assayed for primitive erythroid (A) and definitive CFCs (B), respectively. Error bars indicate SEs of 3 independent experiments performed in duplicate. Gene expression analyses for embryonic globin (C) and β major globin (D) at days 4.25 and 6.25 of EB differentiation. Transcripts are normalized to Gapdh. Error bars indicate SE from 2 independent experiments performed in duplicate. (E-F) Relative levels of Flk-1, Gata1, Gata2, Lmo2, and SCL in 4.25 and 6.25 EBs, respectively. Transcripts are normalized to Gapdh. (G) A representative FACS profile for CD41 and c-Kit in induced and noninduced iALK-1 cells at EB day 6.25. Fluorescence intensity for c-Kit is indicated on the y axis and CD41 on the x axis. *P < .05, ***P < .001
Effect of ALK-5 induction in early EB development
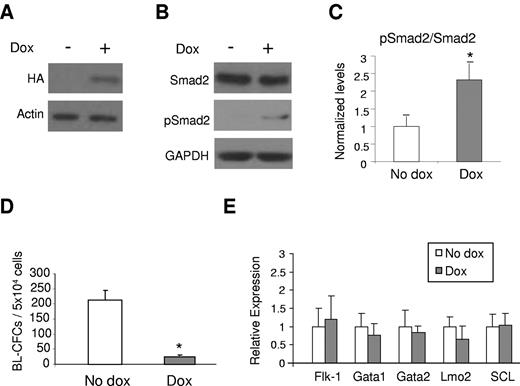
To examine the effect of the other type I TGF-β receptor on hemangioblast and blood development, we generated an ES cell line with inducible expression of constitutively active ALK-5 (iALK-5). Conditional expression of ALK-5 in this cell line was confirmed by Western blotting to the HA-tag (Figure 4A). The relative expression of exogenous (human) versus endogenous (mouse) ALK-5 was approximately 3-fold (supplemental Figure 1D). As expected, induction of ALK-5 led to phosphorylation of Smad2 (Figure 4B-C), but no changes were observed in the levels of nonphosphorylated Smad2 (Figure 4B) or Smad1/5/8/pSmad1/5/8 levels, downstream targets of the ALK-1 signaling pathway (supplemental Figure 1C).
ALK-5 gain-of-function impairs hemangioblast development. (A) Western blot against HA confirming the induction of CA-ALK-5. (B) Western blot for Smads. Induction of ALK-5 leads to increased levels of phosphorylated Smad2 (pSmad2), downstream target of ALK-5. Actin and Gapdh were used as loading control. (C) Quantification of phosphorylated Smad2. After normalization to Gapdh levels, results were plotted as ratio between phosphorylated Smad2 and total Smad2. (D) iALK-5 ES cells were assayed for hemangioblast activity in BL-MCM on day 3.25 of EB differentiation. The graph represents 3 experiments performed in duplicate. Bars indicate SE. (E) Relative levels of Flk-1, Gata1, Gata2, Lmo2, and SCL in day 3.25 EBs cultured in the presence or absence of Dox. Graph represents data from 2 independent experiments performed in duplicate. Bars indicate SE. *P < .05
ALK-5 gain-of-function impairs hemangioblast development. (A) Western blot against HA confirming the induction of CA-ALK-5. (B) Western blot for Smads. Induction of ALK-5 leads to increased levels of phosphorylated Smad2 (pSmad2), downstream target of ALK-5. Actin and Gapdh were used as loading control. (C) Quantification of phosphorylated Smad2. After normalization to Gapdh levels, results were plotted as ratio between phosphorylated Smad2 and total Smad2. (D) iALK-5 ES cells were assayed for hemangioblast activity in BL-MCM on day 3.25 of EB differentiation. The graph represents 3 experiments performed in duplicate. Bars indicate SE. (E) Relative levels of Flk-1, Gata1, Gata2, Lmo2, and SCL in day 3.25 EBs cultured in the presence or absence of Dox. Graph represents data from 2 independent experiments performed in duplicate. Bars indicate SE. *P < .05
Opposite to the ALK-1 gain-of-function, induction of ALK-5 during EB development significantly inhibited blast colony formation (Figure 4D). This reduction does not seem to correlate with improper mesoderm development as the Flk-1 positive population was not reduced in ALK-5-induced EBs (Figure 4E). To further assess the expression of transcriptional regulators of hematopoiesis, we examined the expression levels of Gata1, Gata2, Lmo2, and Scl by real time PCR analysis. No significant changes were detected for these genes on Dox induction (Figure 4E).
ALK-5 gain-of-function causes defective hematopoiesis
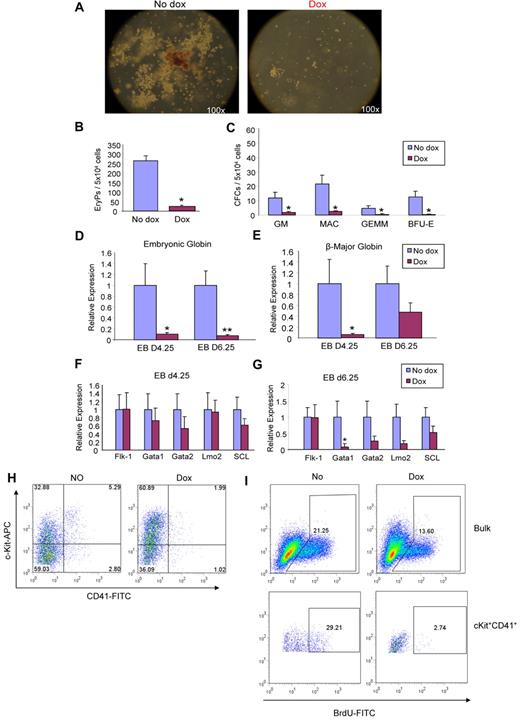
The effect of ALK-5 induction on hematopoietic development was assessed by plating day 4.25 and 6.25 EBs for EryP and definitive CFUs, respectively. Dox was added to the medium from day 2 of EB differentiation. We observed a dramatic reduction in the number and size of hematopoietic colonies derived from ALK-5–induced EBs (Figure 5A). Overall there was a significant decrease in all types of CFCs, including primitive EryPs (Figure 5B) as well as definitive CFCs, such as GMs, Macs, GEMMs, and BFU-Es (Figure 5C). Accordingly, expression levels of embryonic globin (Figure 5D) and β-major globin (Figure 5E) were significantly reduced. This inhibitory effect was observed consistently in multiple clones (supplemental Figure 2B,D). Expression levels for hematopoietic transcription factors, Gata1, Gata2, Lmo2, and SCL were reduced in ALK-5– induced EBs, in particular on day 6.25 of EB differentiation (Figure 5F-G). These findings were corroborated by FACS analysis for c-Kit and CD41 at this time point, which demonstrated that the double positive fraction was reduced by 2-fold (Figure 5H). Taken together, these data suggest that ALK-5 is an inhibitory factor, counter-balancing the stimulatory effect of ALK-1 on hemangioblast and hematopoietic development.
ALK-5 gain-of-function leads to defective hematopoiesis. iALK-5 ES cells were differentiated as EBs in the presence or absence of Dox, and assayed for hematopoietic colonies. (A) Representative images of noninduced and induced ALK-5 cultures. (B-C) Frequency of primitive erythroid (B) and definitive CFCs (C) at days 4.25 and 6.25 of EB differentiation, respectively. Error bars indicate SE of 3 independent experiments performed in duplicate. Gene expression analyses for embryonic globin (D) and β major globin (E) at days 4.25 and 6.25 of EB differentiation. Transcripts are normalized to GAPDH. Bars indicate SE from 3 independent experiments performed in duplicate. (F-G) Relative levels of Flk-1, Gata1, Gata2, Lmo2, and SCL in 4.25 and 6.25 EBs, respectively. Transcripts are normalized to GAPDH. (H) A representative FACS profile for CD41 and c-Kit in induced and noninduced iALK-5 cells at EB day 6.25. Fluorescence intensity for c-Kit is indicated on the y axis and CD41 on the x axis. *P < .05, **P < .01. (I) A representative FACS profile for BrdU incorporation in induced and noninduced iALK-5 cells at EB day 6.25. iALK-5 day 6.25 EBs were labeled with 10μM BrdU and then stained with c-Kit–APC and CD41-PE. The incorporation of BrdU was visualized using FITC conjugated anti-BrdU antibody.
ALK-5 gain-of-function leads to defective hematopoiesis. iALK-5 ES cells were differentiated as EBs in the presence or absence of Dox, and assayed for hematopoietic colonies. (A) Representative images of noninduced and induced ALK-5 cultures. (B-C) Frequency of primitive erythroid (B) and definitive CFCs (C) at days 4.25 and 6.25 of EB differentiation, respectively. Error bars indicate SE of 3 independent experiments performed in duplicate. Gene expression analyses for embryonic globin (D) and β major globin (E) at days 4.25 and 6.25 of EB differentiation. Transcripts are normalized to GAPDH. Bars indicate SE from 3 independent experiments performed in duplicate. (F-G) Relative levels of Flk-1, Gata1, Gata2, Lmo2, and SCL in 4.25 and 6.25 EBs, respectively. Transcripts are normalized to GAPDH. (H) A representative FACS profile for CD41 and c-Kit in induced and noninduced iALK-5 cells at EB day 6.25. Fluorescence intensity for c-Kit is indicated on the y axis and CD41 on the x axis. *P < .05, **P < .01. (I) A representative FACS profile for BrdU incorporation in induced and noninduced iALK-5 cells at EB day 6.25. iALK-5 day 6.25 EBs were labeled with 10μM BrdU and then stained with c-Kit–APC and CD41-PE. The incorporation of BrdU was visualized using FITC conjugated anti-BrdU antibody.
To examine whether these inhibitory effects of ALK-5 on hematopoiesis were a consequence of reduced cell proliferation, we performed BrdU incorporation assays in EBs obtained from iALK-5 and iALK-1 (control) ES cells. After 12 hours of dox treatment, BrdU incorporation in iALK-1 day 3.25 and 6.26 EBs remained the same as the nontreated control (supplemental Figure 3). However, BrdU incorporation at EB day 6 was decreased 2-fold by ALK-5 gain-of-function, indicating that ALK5 signaling inhibits cell proliferation in day 6.25 EBs (Figure 5I top panel). The cKit+/CD41+ population showed even greater than 10-fold reduction in incorporation of BrdU (Figure 5I bottom panel), indicating that this cell population has minimal proliferation ability.
ALK-1 rescues the Eng−/− defective phenotype
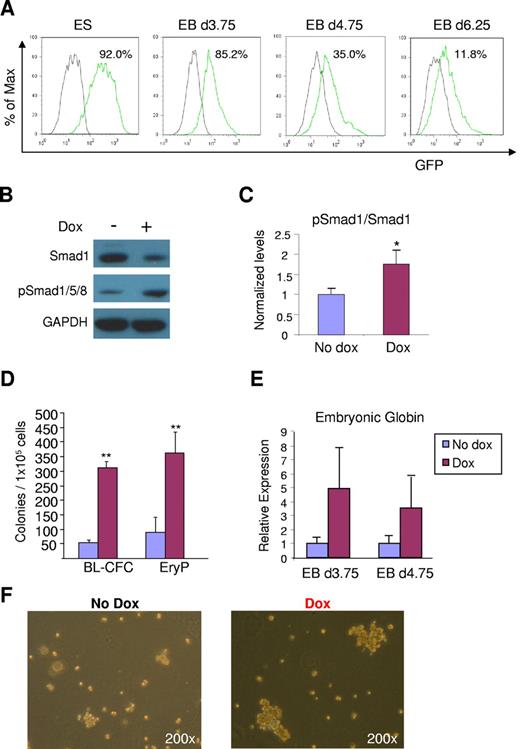
Based on the stimulatory effect of ALK-1 gain-of-function on hemangioblast and blood development, and the observation that the ALK-1/Smad1 signaling pathway is disrupted in Eng−/− EBs, we examined the ability of ALK-1 to rescue the eng−/− phenotype by inserting an inducible lentiviral ALK-1 transgene into eng−/− ES cells (iALK-1:Eng−/−). Expression of the transgene was detected by an IRES-GFP reporter downstream of the ALK-1 gene (Figure 6A). Further confirmation of ALK-1 activation in these cells was provided by Western blot analyses, which showed increased levels of pSmad1/5/8 on dox induction (Figure 6B-C). At the same time, these samples presented slightly lower levels of total Smad1 (Figure 6B). As expected, levels of Smad2/pSmad2 remained unaltered with ALK-1 activation (data not shown), as observed on ALK-1 induction in the wild-type background (supplemental Figure 1B).
ALK-1 rescues defective hemangioblast and primitive erythroid development in Eng−/− ES cells. (A) Flow cytometry analysis of inducible iALK-1:Eng−/− ES cells. FACS analysis was performed to detect GFP expression, which indicates the induction of ALK-1. Green line represents levels of GFP in undifferentiated ES cells, as well as cells from EBs differentiated for 3.75, 4.75, or 6.25 days cultured in the presence of dox. The gray line denotes controls (no dox). (B) Western blot analyses of Smads at day 3.75 nonsorted EBs. 25μg proteins were subjected to SDS-PAGE and Western blotting. pSmad1/5/8 indicates phosphorylated Smad1/5/8. (C) Quantification of phosphorylated Smad1/5/8. After normalization to Gapdh levels, results were plotted as ratio between phosphorylated Smad1/5/8 and total Smad1. (D) iALK-1:Eng−/− ES cells were assayed for BL-CFC and primitive erythroid development by plating 1 × 105 cells from EBs differentiated for 3.75 and 4.75 days, respectively. Error bars indicate SE from 3 independent experiments performed in duplicate. (E) Gene expression analyses for embryonic globin. Transcripts are normalized to Gapdh. Error bars indicate standard errors from 3 independent experiments performed in duplicate. (F) Representative morphology of blast colonies obtained from Eng−/− and rescued iALK-1:Eng−/− ES cells. Colonies are shown at the same magnification (200×). **P < .01.
ALK-1 rescues defective hemangioblast and primitive erythroid development in Eng−/− ES cells. (A) Flow cytometry analysis of inducible iALK-1:Eng−/− ES cells. FACS analysis was performed to detect GFP expression, which indicates the induction of ALK-1. Green line represents levels of GFP in undifferentiated ES cells, as well as cells from EBs differentiated for 3.75, 4.75, or 6.25 days cultured in the presence of dox. The gray line denotes controls (no dox). (B) Western blot analyses of Smads at day 3.75 nonsorted EBs. 25μg proteins were subjected to SDS-PAGE and Western blotting. pSmad1/5/8 indicates phosphorylated Smad1/5/8. (C) Quantification of phosphorylated Smad1/5/8. After normalization to Gapdh levels, results were plotted as ratio between phosphorylated Smad1/5/8 and total Smad1. (D) iALK-1:Eng−/− ES cells were assayed for BL-CFC and primitive erythroid development by plating 1 × 105 cells from EBs differentiated for 3.75 and 4.75 days, respectively. Error bars indicate SE from 3 independent experiments performed in duplicate. (E) Gene expression analyses for embryonic globin. Transcripts are normalized to Gapdh. Error bars indicate standard errors from 3 independent experiments performed in duplicate. (F) Representative morphology of blast colonies obtained from Eng−/− and rescued iALK-1:Eng−/− ES cells. Colonies are shown at the same magnification (200×). **P < .01.
Next we investigated the ability of ALK-1-induced eng−/− ES cells to generate BL-CFCs and EryPs, which were found previously to be significantly reduced in the absence of endoglin.5 Induction of ALK-1 signaling during EB development rescued the eng−/− phenotype, as evidenced by the 6- and 4-fold increase in the number of BL-CFCs and EryPs, respectively (Figure 6D-F). Corroborating this rescue, higher levels of embryonic globin were detected in ALK-1–induced eng−/− EBs (Figure 6E). These findings indicate that ALK-1 can compensate for the lack of endoglin during early stages of EB development.
Discussion
During embryonic development, endoglin is widely expressed in endothelial cells between E8.5 and E10.5,34 however it is also detected earlier in the amniotic fold and developing allantois at E6.5,35 as well as in the early extraembryonic mesoderm from gastrulating embryos at E7.5-E8.5,34-36 suggesting a potential function for endoglin in earlier developmental stages. Using the ES/EB system, we have recently shown a dramatic reduction in the frequency of hemangioblast and primitive erythroid (EryPs) precursors in the absence of endoglin.5 These findings concur with the anemia observed in the yolk sac of 9.5 dpc eng−/− embryos,30 which was hypothesized to result from the defective vasculature found in these embryos. Our results point instead to a direct effect of endoglin on mesoderm development and hematopoiesis, as further evidenced by the microarray analyses shown in this study (Figure 1A-B). In addition to the down-regulation of several key mesodermal and hematopoietic transcription factors, reduced levels of Smad1 were observed in the absence of endoglin. Further gene expression and Western blot analyses confirmed that the ALK-1/Smad1 signaling pathway is disrupted in Eng−/− early EBs. Although there were no changes in pSmad2, we observed up-regulation of target genes associated with the ALK-5/Smad2 pathway (Figure 1F). This may be because of target genes being regulated by a balance of ALK-1 versus ALK-5 pathways, and the lack of endoglin increases the activity of the ALK-5 pathway, disrupting this balance.
Induction of CA–ALK-1 resulted in increased phosphorylation of Smad1/5/8, but had no effect on pSmad2/3, while induction of CA–ALK-5 led to phosphorylation of pSmad2/3, and no changes in pSmad1/5/8. Similar absence of cross-talk between CA–ALK-1 and CA–ALK-5, and their respective Smads has been reported in endothelial cells.25 Our studies revealed a stimulatory effect for ALK-1 on hemangioblast and primitive hematopoietic development while ALK-5 dramatically inhibited these processes. Loss-of-function studies targeting different molecules of the TGF-β family, including TGF-β1 (ligand), TβRII, endoglin, ALK-1, or ALK-5 reveal a similar phenotype, characterized by embryonic lethality and impaired vasculature,37-40 however only TGF-β1, TβRII, and endoglin have been indicated to display defective primitive hematopoiesis.30,37,38 Hematopoietic activity in ALK-5−/− embryos has been shown to be intact or stimulated, as yolk sacs from these embryos contain a higher frequency of erythroid and macrophage colonies compared with control littermates.40,41 Although no similar study has been applied to the other TGF-β knockouts, these findings are in agreement with our results, supporting a negative role for TGF-β/ALK-5 in early hematopoiesis. TGF-β1 has been thought to be a negative regulator of hematopoiesis42 however TGF-β1 null embryos present impaired hematopoiesis.37 It may be the case that a fine tuning between ligand dosage and TβR binding (ALK-1 vs ALK-5, and endoglin) dictates the TGF-β response. In this respect, ALK-1 expression during development correlates with TGF-β1 and endoglin, as these molecules can be detected as early as 7.5 dpc in the extraembryonic mesoderm,22,34,36,43 suggesting that this pathway is active, and potentially critical at these early stages of hematopoietic and endothelial development.
Our findings in early EBs point to a disrupted ALK-1/Smad1 signaling pathway in the absence of endoglin (Figure 1), which most likely leads to defective hemangioblast and primitive hematopoietic development in this knockout, as overexpression of WT-ALK-1 clearly rescues the defective hemangioblast/hematopoiesis phenotype (Figure 6). Despite the negative effect observed for CA–ALK-5 in hemangioblast and hematopoietic development (Figures 4 and 5), we have no direct evidence that this signaling pathway is increased in endoglin-deficient EBs, although we did observe up-regulation of ALK-5/Smad2 target genes in these cells (Figure 1F). Nevertheless, induction of ALK-1 in early Eng−/− EBs is accompanied by increased phosphorylation of Smad1/5/8, proteins that were found reduced in the endoglin knockout. These results are in agreement with those of Zafonte and colleagues, who previously demonstrated that Smad1 induction during EB development stimulates hemangioblast as well as hematopoietic development.44 On the other hand, loss of Smad5 has been shown to enhance the frequency of hemangioblast precursors, while inhibiting primitive erythropoiesis.45 Based on the similarity between Smad1 and Smad5, one would expect that lack of Smad5 would reduce the frequency of both hemangioblast and hematopoietic precursors. However, it is possible that these proteins play distinct roles at different stages of development. It has been shown that a critical threshold level for Smad1 and Smad5 needs to be maintained for proper mesoderm formation and patterning.46
It is important to emphasize that BMPs also phosphorylate Smad1/5/8 on forming a heteromeric complex with BMPRII or ActRII, and one of their type I receptors (ALK-2, ALK-3, or ALK-6).17,47 Among the BMPs, BMP-4 is well known to be required for mesoderm formation48 as well as for the commitment of mesodermal cells to the hematopoietic lineage,48,49 and as so embryos lacking BMP-4 display impaired primitive hematopoiesis.50 In this regard, endoglin has also been shown to interact with BMPs, in particular to BMP-7 and BMP-2, in association with their respective ligand binding receptor kinases.20 Although up to now an interaction between BMP-4 and endoglin has not been documented, our microarray analyses showed down-regulation of BMP-4 in the absence of endoglin (Figure 1 and supplemental Figure 4), which suggests a link between this ligand and receptor. More recent studies have demonstrated that besides TGF-β, BMP-9 and BMP-10 also bind to ALK-1 and endoglin, leading to phosphorylation of Smad1/5/8.51,52 Nevertheless, regardless of the ligand, there is strong evidence to conclude that endoglin is necessary for proper ALK-1 signaling in the endothelial lineage,27,53 and our findings show for the first time that this signaling pathway is required for proper hemangioblast and primitive hematopoietic development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of R.C.R.P.'s laboratory for helpful discussions, in particular Radbod Darabi for detailed protocols and advice, and Yi Ren for support with FACS sorting experiments.
This project was supported by National Institutes of Health grant R01 HL085840-01 to R.C.R.P and HL061186 to M.K.
National Institutes of Health
Authorship
Contribution: L.Z. designed and performed research, analyzed data, and wrote the manuscript; a.m. designed and performed research and analyzed data; J.C. performed research and analyzed data; Z.X. and M.K. contributed essential reagents; and R.C.R.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rita C. R. Perlingeiro, PhD, Lillehei Heart Institute, University of Minnesota, 4-124 Nils Hasselmo Hall, 312 Church St SE, Minneapolis, MN 55455; e-mail: perli032@umn.edu.