Abstract
Cytokines released from innate immune cells play key roles in the regulation of the immune response. These intercellular messengers are the source of soluble regulatory signals that initiate and constrain inflammatory responses to pathogens and injury. Although numerous studies describe detailed signaling pathways induced by cytokines and their specific receptors, there is little information on the mechanisms that control the release of cytokines from different cell types. Indeed, the pathways, molecules, and mechanisms of cytokine release remain a “black box” in immunology. Here, we review research findings and new approaches that have begun to generate information on cytokine trafficking and release by innate immune cells in response to inflammatory or infectious stimuli. Surprisingly complex machinery, multiple organelles, and specialized membrane domains exist in these cells to ensure the selective, temporal, and often polarized release of cytokines in innate immunity.
Introduction
The production and release of cytokines from innate immune cells are critical responses to inflammation and infection in the body. Innate immune cells comprise populations of white blood cells such as circulating dendritic cells (DCs), neutrophils, natural killer (NK) cells, monocytes, eosinophils, and basophils, along with tissue-resident mast cells and macrophages.1 Residing at the frontline of defense in immunity, these cells control opportunistic invasion by a wide range of viral, fungal, bacterial, and parasitic pathogens, in part by releasing a plethora of cytokines and chemokines to communicate with other cells and thereby to orchestrate immune responses. This array of soluble mediators secreted by different innate immune cells includes TNF, IFNγ, interleukins IL-1β, IL-4, IL-6, IL-10, IL-12, IL-18, CCL4/RANTES, and TGFβ. Cytokine release can be directly evoked by immunoglobulin- or complement receptor-mediated signaling or by pathogens through a diverse array of cellular receptors, including pattern recognition receptors such as TLRs.1,2 The Gram-negative bacterial coat component lipopolysaccharide (LPS), the main culprit behind toxic shock syndrome and sepsis, is a highly potent trigger of cytokine secretion through TLR4.
For the immune system to function appropriately, the synthesis and release of cytokines must be highly regulated and sequentially and temporally orchestrated. Thus, cascades of cytokines released by innate immune cells initially mount inflammatory or allergic responses and then later ensure that the responses subside in a timely fashion.3 Proinflammatory cytokines also serve to recruit and active T lymphocytes and other cells to mount adaptive immune responses.1 However, even with the overwhelming and detailed literature on cytokine actions, just how cytokines are released or secreted by innate immune cells remains a significant “black box” in immunology. Most diagrams in textbooks and reviews show a simple arrow indicating cytokine release from a given cell type after activation of a signaling cascade in response to receptor stimulation. Perhaps surprisingly, the precise mechanisms of cytokine trafficking and release remain obscure in most cell types.
Our knowledge of cytokine secretion is superimposed on the field of intracellular trafficking, and both have advanced, thanks to new technologies that allow newly synthesized proteins to be visualized and tracked through cells as fluorescently tagged proteins (eg, with green fluorescent protein [GFP] and its many derivatives) with the use of imaging in live and fixed cells.4,5 The functional components of the trafficking machinery can now be studied in vitro with RNA interference and expression of mutated proteins, as well as through the use of knockout and transgenic mice.
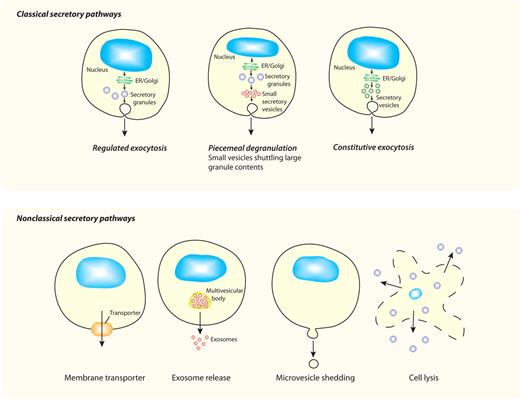
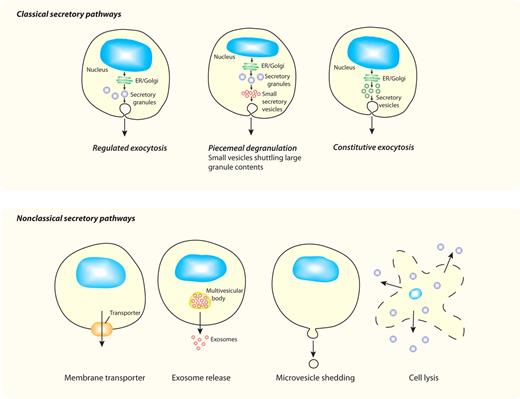
We discuss here new findings showing that cytokine secretion is a complex and tightly controlled process, and we show that the intracellular pathways available for release are often uniquely tailored to each cytokine and cell type. In classical secretory pathways (Figure 1), cytokines with signal peptides are cotranslationally inserted into the endoplasmic reticulum (ER) for synthesis as either soluble or transmembrane precursors. They are then trafficked in vesicles to the Golgi complex for further processing, and at the trans-Golgi network (TGN) they are loaded into vesicles or carriers for constitutive delivery to the cell surface or other organelles. In specialized cell types, additional modes of secretion are proffered by loading cytokines and other cargo into granules for storage and later release. Herein, studies on the release of CCL5/RANTES from eosinophils and the secretion of TNF by macrophages are described as examples of classical secretory pathways via granule-mediated and constitutive routes, respectively. Finally, we consider the possible mechanisms for nonclassical release (Figure 1) of cytoplasm-derived cytokines such as IL-1β and IL-18.
Different secretory pathways offer variable modes of release for cytokines. All cells have ≥ 1 variations on the classic secretory pathways depicted in the top panel, whereby proteins (eg, cytokines) synthesized in the ER and Golgi complex are transported in membrane-bound vesicles, granules, or both to the cell surface for release. Although all cells have constitutive pathways, specialized cell types additionally have regulated (granule-mediated) pathways, and some cells have a variation of this process (piecemeal degranulation) in which membrane-bound vesicles are used to selectively transport cytokines from secretory granules to the cell surface. All of these pathways have multiple transport steps, each requiring sets of trafficking machinery molecules to execute carrier budding, movement, and membrane fusion. The bottom panel shows nonclassical secretory pathways, which involve movement of proteins (eg, cytokines) directly from their point of synthesis in the cytoplasm to the external milieu. Various mechanisms proposed for crossing the plasma membrane, as the single transport step required, include the use of membrane transporters, exosome release, microvesicle shedding, or cell lysis for cytokine release.
Different secretory pathways offer variable modes of release for cytokines. All cells have ≥ 1 variations on the classic secretory pathways depicted in the top panel, whereby proteins (eg, cytokines) synthesized in the ER and Golgi complex are transported in membrane-bound vesicles, granules, or both to the cell surface for release. Although all cells have constitutive pathways, specialized cell types additionally have regulated (granule-mediated) pathways, and some cells have a variation of this process (piecemeal degranulation) in which membrane-bound vesicles are used to selectively transport cytokines from secretory granules to the cell surface. All of these pathways have multiple transport steps, each requiring sets of trafficking machinery molecules to execute carrier budding, movement, and membrane fusion. The bottom panel shows nonclassical secretory pathways, which involve movement of proteins (eg, cytokines) directly from their point of synthesis in the cytoplasm to the external milieu. Various mechanisms proposed for crossing the plasma membrane, as the single transport step required, include the use of membrane transporters, exosome release, microvesicle shedding, or cell lysis for cytokine release.
Trafficking machinery
Like all newly synthesized proteins, most cytokines rely on a host of membrane-bound and cytoplasmic cellular proteins, the so-called trafficking machinery, to mediate their transport through the cell. Trafficking machinery molecules are needed to shepherd newly synthesized proteins into secretory carriers, then for the budding, movement, and fusion of the membrane-bound carriers at each transport step (Figure 1).6-8 This complex machinery includes large families of proteins from which individual family members operate in distinct combinations to provide specificity for each transport step (Table 1). Much of this machinery resides in the membrane and is assembled at specific sites on organelle or carrier membranes to facilitate the transport of newly synthesized proteins.
For bidirectional transport between the ER and Golgi, members of the ADP ribosylation factor (ARF)/Sar family of small guanosine triphosphatase (GTPases) and their accessory proteins help to sort and load secretory cargo into vesicles coated with coating protein I or II.23 At the TGN, ADP ribosylation factor proteins and adaptor complexes sort some proteins into clathrin-coated vesicles for transport to late endosomes, whereas secretory proteins are commonly loaded into uncoated tubulovesicular carriers for constitutive transport to endosomes or directly to the cell surface.7 Vesicle budding at each step requires membrane curvature to form “buds” or tubules and fission to release them as carriers. These actions involve series of proteins, lipid kinases, and phosphatases, along with the GTPase dynamin, as well as other fission proteins.7 Both actin- and microtubule-based motors assist in the movement of carriers through the cell.24,25 The docking and fusion of carriers at target membranes then involve specific combinations of tethering complexes, Rab GTPases8 and SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein [SNAP] receptors) fusion proteins.26 Small GTPases of the 60-member Rab family associate with and demarcate the membranes of different organelles8 ; for instance, Rabs 5, 7, and 11 associate with early, late, and recycling endosomes, respectively. Rabs operate through multiple effectors to bring about vesicle formation, movement, and prefusion docking.8
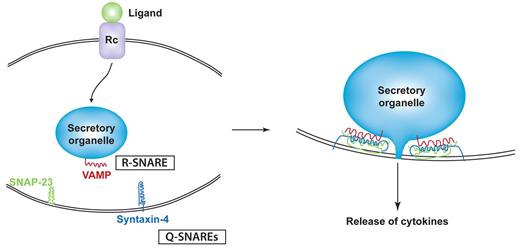
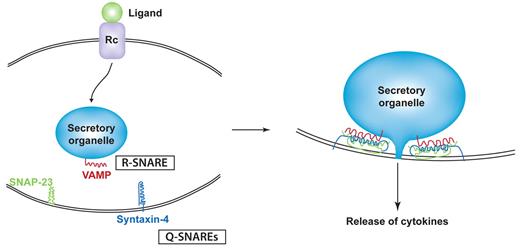
Among the best studied of the trafficking machinery proteins in cytokine release are the membrane fusion proteins known as SNAREs.26 The SNAREs include subfamilies of vesicle-associated membrane proteins (VAMPs) and syntaxins which are classified by the amino acid composition of their core SNARE domains as R-SNAREs and Q-SNAREs, respectively. Typically, Q- and R-SNAREs on opposing target and vesicle membranes unite as a 4-helix coiled-coil bundle that winches tethered membranes together for fusion (Figure 2).27 SNARE-mediated fusion has to occur for each intracellular transport step, but the fusion of granules, vesicles, or endosomes with the plasma membrane is the final event necessary for the surface delivery and release of cytokines. A Q-SNARE complex consisting of syntaxin-4 and synaptosomal-associated protein 23 (SNAP-23) on the plasma membrane of many innate immune cells commonly performs the final fusion step by pairing with different VAMPs on approaching granules or vesicles (Table 1).28 Identifying the specific cognate pairs of R- and Q-SNAREs for intracellular transport steps has been a useful experimental approach to help chart the trafficking routes of cytokines through cells.28 SNARE-binding proteins, such as Munc proteins, can regulate Q-SNARE complexes, and Rab GTPases work with SNAREs to align docking and fusion of vesicles at the target membranes for IFNγ and TNF trafficking.29
Binding model for SNAREs in cytokine secretion. Ligand/receptor binding elicits signaling that initiates trafficking in classic secretory pathways. At each step of transport (eg, a secretory granule fusing with the plasma membrane), a specific R-SNARE (VAMP) in the secretory organelle membrane partners with a Q-SNARE complex (eg, composed of SNAP-23 and syntaxin-4) in the target membrane. SNARE binding involves the formation of a 4-helix coiled-coil structure that is modeled to winch membranes together to allow membrane fusion and extrusion of cytokines from the granule interior.
Binding model for SNAREs in cytokine secretion. Ligand/receptor binding elicits signaling that initiates trafficking in classic secretory pathways. At each step of transport (eg, a secretory granule fusing with the plasma membrane), a specific R-SNARE (VAMP) in the secretory organelle membrane partners with a Q-SNARE complex (eg, composed of SNAP-23 and syntaxin-4) in the target membrane. SNARE binding involves the formation of a 4-helix coiled-coil structure that is modeled to winch membranes together to allow membrane fusion and extrusion of cytokines from the granule interior.
Whereas the trafficking machinery in cells was previously thought of as having a passive housekeeping role, in fact, many trafficking molecules are tightly coupled to cell activation and respond to the need for cytokine secretion through being recruited to membranes or through up-regulation of their expression. Thus, in macrophages, LPS and IFNγ up-regulate the expression of specific SNAREs such as VAMP-3 and induce recruitment of regulators such as PI3Kδ to TGN membranes.19,28 LPS also acutely increases the budding of a subpopulation of TGN carriers. Taken together, these LPS-induced changes enhance the activated cell's ability to rapidly traffic and secrete proinflammatory cytokines.
Regulated and constitutive exocytosis of cytokines in granule-containing cells
Although all innate immune cells have the capacity for constitutive exocytosis, in some cell types, notably granulocytes, cytokines or other proteins may be loaded into secretory granules, lysosome-related organelles, or secretory lysosomes for storage and later release at the cell surface via ≥ 1 of several mechanisms. Such pathways are the “regulated” or “granule-mediated” pathways for exocytosis or secretion which can use a combination of granules and smaller secretory vesicles to actually release stored cytokines. The storage of preformed cytokines in granules allows cells to orchestrate their immediate release into the local microenvironment within minutes of receptor stimulation. This rapid response to injury or infection provides granule-containing cells with a unique ability to spatiotemporally modulate the inflammatory response.
Neutrophils are a prominent granulocyte at the first line of defense in innate immunity and possess ≥ 4 different types of secretory organelles: azurophilic granules, specific granules, tertiary granules, and secretory vesicles.30 However, surprisingly little is known about the way in which these organelles contribute to cytokine trafficking and release. Neutrophils produce a wide range of cytokines and chemokines, and their precise intracellular locations are not known. Immunohistologic examination of mature peripheral blood neutrophils suggests that TGFα, TNF, IL-6, IL-12, and CXCL2/IL-8 are stored within peroxidase-negative organelles,31-33 which may be secondary or tertiary granules, or endosomal secretory vesicles. SNAREs, including VAMP-2, VAMP-7, syntaxin-4, syntaxin-6, and SNAP-23, are expressed in neutrophils and regulate the trafficking and release of a number of these secretory organelles.34-37 However, the dependency of neutrophil cytokine release on Rab GTPases, SNAREs, and other trafficking machinery has not been characterized. Thus, evidence for neutrophil cytokine production and trafficking is scant, and much more work is needed to understand the contribution of each of the neutrophil secretory organelles to cytokine release. The basophil is another granulocyte that is important in the generation of cytokines, particularly IL-4 in allergic inflammation,38 although the trafficking pathway for release of this cytokine is also not known.
Mast cells, which are important innate immune cells in allergy and inflammatory diseases that reside in tissues, secrete numerous cytokines and chemokines, many of which are stored as preformed mediators in their secretory granules.39 Classical degranulation of secretory granules from mast cells involves simultaneous fusion of a large number of granules with the cell surface for bulk release of histamine and other mediators, determined by capacitance measurements and electron microscopic analysis.40,41 Cytokines are released during classical degranulation, shown by the rapid secretion of IL-4 and TNF during receptor-mediated exocytosis by cross-linking cell surface complexes of IgE and Ag.42-45 However, cytokine release from mast cells may involve trafficking of a distinct population of secretory vesicles independently of the granules, in a process similar to piecemeal degranulation or constitutive exocytosis (Figure 1). Piecemeal degranulation is a type of regulated exocytosis that is considered to be a general secretory pathway used by immune cells and other cell types, including enteroendocrine cells of the gastrointestinal tract, adrenal chromaffin cells, and chief cells of the parathyroid gland.46 This form of vesicular trafficking is characterized by small vesicles budding from large secretory granules, which then perform the final step of transporting cytokines to the cell surface for release.47-49
Piecemeal degranulation or constitutive exocytosis may occur in cytokine secretion from mast cells, whereby IL-1–induced IL-6 trafficking to the cell surface occurs in the absence of secretory granule release.50 Cytokine trafficking that takes place independently of secretory granule exocytosis is further supported by studies on the SNARE protein VAMP-8 in granule release with the use of BM mast cells from VAMP-8 knockout mice.51 Degranulation measured as β-hexosaminidase and histamine release in response to FcϵRI cross-linking was defective in VAMP-8 knockout mast cells, whereas the release of cytokines TNF, IL-6, and CCL3 occurred normally in these cells.51 Similar findings were found in another study showing that VAMP-8 was necessary for granule protein release in response to FcϵRI-mediated secretion but not for TNF release.52 Thus, despite mast cells having a prominent, characteristic capacity for VAMP-8–mediated degranulation, cytokine secretion largely occurs through a distinct and probable constitutive pathway. Similarly, stimulation by distinct TLR2 activators in mast cells leads to differential cytokine release of GM-CSF and IL-1β independently of degranulation.53 These studies highlight the presence of multiple classic secretory pathways for granule proteins and cytokines in mast cells.
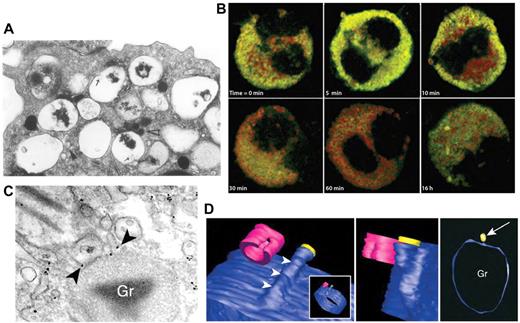
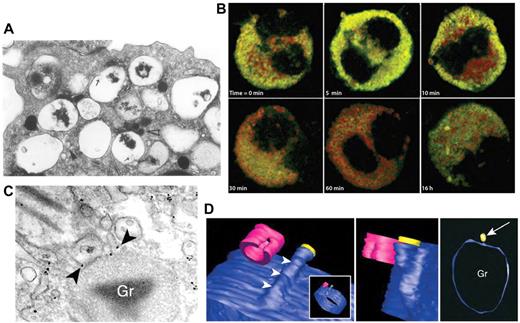
Another major effector cell in allergy and asthma is the eosinophil, which can release ≤ 35 different cytokines, chemokines, and growth factors.54,55 Many of these cytokines are stored as preformed mediators in the eosinophil's unique crystalloid granules for later release in response to receptor stimulation (Figure 3).54 Eosinophils have been shown to use piecemeal degranulation as a trafficking mechanism for cytokines. Piecemeal degranulation in eosinophils is characterized by “hollowed out” crystalloid granules along with the appearance of numerous tubulovesicular vesicles (carriers) (Figure 3A). A structure that closely resembles piecemeal degranulation is frequently observed by electron microscopy in tissue eosinophils from allergic subjects, with ≤ 67% of all nasal polyp eosinophils from subjects with allergic rhinitis exhibiting this type of exocytosis (Figure 3A).60
Piecemeal degranulation from eosinophils. (A) Piecemeal release of crystalloid granules with a “hollowed out” appearance, shown by electron microscopic analysis of human cultured eosinophils. Arrows show lucent vesicles budding into granule interior, suggestive of vesicular trafficking to and from crystalloid granules. Reprinted from Am J Pathol. 1991;138:69-82 with permission from the American Society for Investigative Pathology.56 (B) Time course of IFNγ-induced CCL5/RANTES release (green) by piecemeal degranulation from major basic protein-positive crystalloid granules (red) in eosinophils. This research was originally published in Blood.57 Copyright The American Society of Hematology. (C) Immunogold labeling of IL-4 (arrowheads) present in small vesicles budding from a crystalloid granule (Gr), indicating piecemeal degranulation as a pathway for cytokine release. Reproduced with permission from Spencer et al.58 (Cytokine receptor-mediated trafficking performed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103(9):3333-3338. Copyright 2006 National Academy of Sciences, USA.) (D) Meshed model of an eosinophil granule with small vesicles budding from it, using contours generated by automated electron tomography. A protrusion can be seen progressively emerging from the granule, in blue (arrowheads). In the center panel, a vesicle is shown in yellow to indicate the way in which vesicles dissociate from the granule. In the right panel, the arrow indicates the same vesicle from a different perspective near the granule (Gr). This model was generated by meshes of contours arising from serial sections of the same granule in a single cell. Cells were fixed and processed for conventional transmission electron microscopy after stimulation with eotaxin. Images were reproduced from Melo et al.59 Reprinted with permission from Traffic. 2005;6:1047-1057, published by John Wiley and Sons.
Piecemeal degranulation from eosinophils. (A) Piecemeal release of crystalloid granules with a “hollowed out” appearance, shown by electron microscopic analysis of human cultured eosinophils. Arrows show lucent vesicles budding into granule interior, suggestive of vesicular trafficking to and from crystalloid granules. Reprinted from Am J Pathol. 1991;138:69-82 with permission from the American Society for Investigative Pathology.56 (B) Time course of IFNγ-induced CCL5/RANTES release (green) by piecemeal degranulation from major basic protein-positive crystalloid granules (red) in eosinophils. This research was originally published in Blood.57 Copyright The American Society of Hematology. (C) Immunogold labeling of IL-4 (arrowheads) present in small vesicles budding from a crystalloid granule (Gr), indicating piecemeal degranulation as a pathway for cytokine release. Reproduced with permission from Spencer et al.58 (Cytokine receptor-mediated trafficking performed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103(9):3333-3338. Copyright 2006 National Academy of Sciences, USA.) (D) Meshed model of an eosinophil granule with small vesicles budding from it, using contours generated by automated electron tomography. A protrusion can be seen progressively emerging from the granule, in blue (arrowheads). In the center panel, a vesicle is shown in yellow to indicate the way in which vesicles dissociate from the granule. In the right panel, the arrow indicates the same vesicle from a different perspective near the granule (Gr). This model was generated by meshes of contours arising from serial sections of the same granule in a single cell. Cells were fixed and processed for conventional transmission electron microscopy after stimulation with eotaxin. Images were reproduced from Melo et al.59 Reprinted with permission from Traffic. 2005;6:1047-1057, published by John Wiley and Sons.
For example, eosinophils synthesize and store CCL5/RANTES, a CC chemokine that acts as a potent chemoattractant, in their crystalloid granules (Figure 3B).57,61 On stimulation by IFNγ, eosinophils release CCL5/RANTES in a piecemeal manner by shuttling this chemokine through a pool of small secretory vesicles from the crystalloid granule to the cell surface (Figure 3B).57 These small secretory vesicles are selectively released and located quite apart from main basic protein-containing crystalloid granules, and their presence was confirmed by subcellular fractionation of homogenized eosinophils separated by linear density gradients.57,58
Eosinophils were shown to traffic Th1 (IFNγ, IL-12) and Th2 cytokines (IL-4, IL-13), as well as TNF, IL-6, and IL-10, through a tubulovesicular system and small secretory vesicles that bud from the crystalloid granules, and that serve to shuttle cytokines from the granules to the cell membrane.62,63 Immunoelectron microscopy confirms that IL-4 is synthesized and stored in crystalloid granules.58,59,63 After stimulation by CCL11/eotaxin, a potent chemokine that triggers chemotaxis in eosinophils, IL-4 is transported through the tubulovesicular system and small secretory vesicles (Figure 3C).58 These secretory vesicles bud off the crystalloid granules to form the extensive tubulovesicular network found in the cytoplasm of eosinophils (Figure 3D). Intriguingly, the IL-4 receptor actually participates in trafficking and release of the cytokine. This led to the proposal that IL-4 is linked to its receptor on the luminal side of the secretory vesicle membrane to allow its selective packaging, trafficking, and release.58
The use of cytokine receptors to transport cytokines presages what may be a mechanism used more widely for the release of cytokines in other pathways and different cell types. Indeed, other innate immune cells also express cytokine receptors in their intracellular granules. In particular, neutrophils secrete IL-1064 and express IL-10 receptor in association with their specific granules.65 Moreover, mast cell granules contain chemokine receptors CCR3 and CXCR2,66,67 and human mast cells secrete the ligands for these receptors: eotaxin, CCL5/RANTES, and IL-8. Finally, cytokine receptor-mediated trafficking has also been described for constitutive trafficking and secretion of the proinflammatory cytokine IL-15 from DCs. In these cells, IL-15 is colocalized with the IL-15 receptor α chain as preassembled complexes in the ER and Golgi complex.68
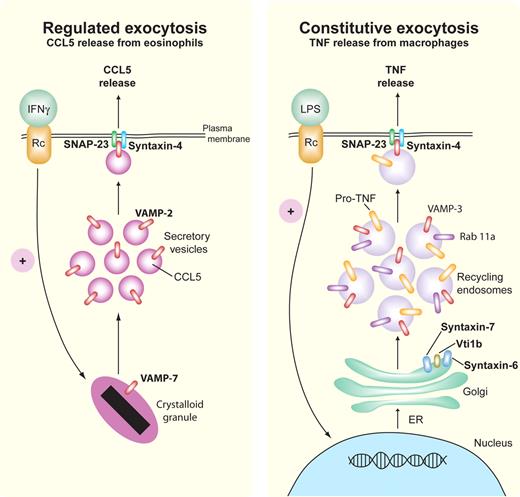
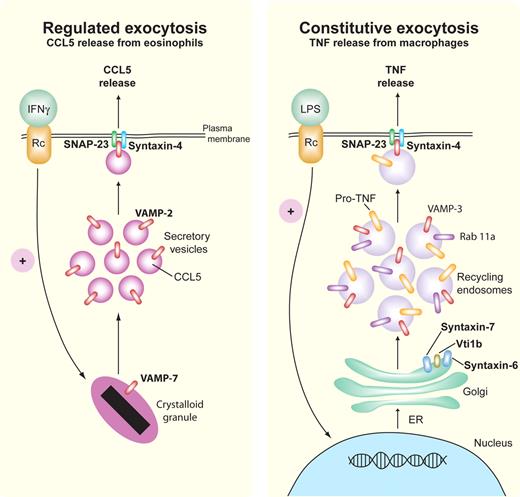
Some of the trafficking machinery governing piecemeal degranulation of CCL5/RANTES in eosinophils has been characterized, using a pathway that depends on the membrane fusion R-SNARE protein, VAMP-2 (synaptobrevin-2), and the Q-SNAREs syntaxin-4 and SNAP-23 (Figure 4).9,15 Human eosinophils express VAMP-2 in small secretory vesicles containing CCL5/RANTES, which translocate to the cell surface on stimulation by IFNγ (Figure 4). Syntaxin-4 and SNAP-23 localize to the cell surface where they function as putative cognate intracellular receptors for VAMP-2, allowing the vesicles to fuse with the cell surface. Eosinophils also express VAMP-7 and VAMP-8 on their crystalloid granules, where VAMP-7 is required for membrane fusion during crystalloid granule exocytosis (Figure 4).37
Regulated and constitutive exocytosis of cytokines. Representative pathways are shown for SNARE-mediated regulated (eosinophil, left) and constitutive (macrophage, right) release of CCL5/RANTES and TNF, respectively. In regulated exocytosis, IFNγ-stimulated eosinophils release preformed CCL5/RANTES by piecemeal degranulation from their crystalloid granules via small secretory vesicles that express the Q-SNARE VAMP-2 (red) for binding to cognate R-SNAREs, SNAP-23 (green), and syntaxin-4 (blue). Constitutive exocytosis occurs during stimulation by LPS, which leads to continuous trafficking of newly synthesized pro-TNF from the ER through the Golgi to recycling endosomes, which use the SNARE complex Vti1b/syntaxin-6/syntaxin-7 to transport pro-TNF to recycling endosomes. From the recycling endosome to the cell membrane, the R-SNARE VAMP-3 (red) on recycling endosomes binds to Q-SNAREs SNAP-23 (green) and syntaxin-4 (blue) in the cell membrane to fuse the recycling endosomes for TNF cleavage and release at the cell exterior. Rab11a (purple) is involved in directing SNARE binding at the cell membrane.
Regulated and constitutive exocytosis of cytokines. Representative pathways are shown for SNARE-mediated regulated (eosinophil, left) and constitutive (macrophage, right) release of CCL5/RANTES and TNF, respectively. In regulated exocytosis, IFNγ-stimulated eosinophils release preformed CCL5/RANTES by piecemeal degranulation from their crystalloid granules via small secretory vesicles that express the Q-SNARE VAMP-2 (red) for binding to cognate R-SNAREs, SNAP-23 (green), and syntaxin-4 (blue). Constitutive exocytosis occurs during stimulation by LPS, which leads to continuous trafficking of newly synthesized pro-TNF from the ER through the Golgi to recycling endosomes, which use the SNARE complex Vti1b/syntaxin-6/syntaxin-7 to transport pro-TNF to recycling endosomes. From the recycling endosome to the cell membrane, the R-SNARE VAMP-3 (red) on recycling endosomes binds to Q-SNAREs SNAP-23 (green) and syntaxin-4 (blue) in the cell membrane to fuse the recycling endosomes for TNF cleavage and release at the cell exterior. Rab11a (purple) is involved in directing SNARE binding at the cell membrane.
NK cells are cytotoxic cells of the innate immune system. NK cells are armed with lytic granules that release proteins such as perforin and granzyme to kill virally infected or tumorigenic target cells.69 Similar to mast cells and eosinophils, cytokine release in NK cells is mediated independently of lytic granules. However, the carriers for NK cell–generated cytokines differ from granulocytes. TNF and IFNγ produced in response to NK-cell activation are sorted away from the contents of lytic granules at the TGN and are instead packaged together into dynamic carriers for constitutive transport and release.12 Although lytic granules are targeted to the immunologic synapse for docking and release, cytokine carriers are released from all points of the NK-cell surface for nonpolarized release of cytokines. TNF is delivered both to the immunologic synapse and to the rest of the cell surface for dissemination.12 Thus, despite having secretory granules for the release of specialized contents, often in a polarized fashion, NK cells also maintain the ability to widely broadcast cytokines by releasing them via separate carriers and constitutive pathways.
Taken together, a major route for cytokine release from mast cells and NK cells is through separate pathways from secretory granules. In the case of eosinophils, cytokine trafficking occurs through piecemeal degranulation relying on a tubulovesicular network that buds from crystalloid granules and provides a readily available pool of cytokines for immunoregulatory processes in inflammation and infection. This allows cells to modulate or fine-tune the immune response through separate intracellular machinery apart from granule exocytosis.
Constitutive exocytosis of inflammatory cytokines in macrophages
Constitutive exocytosis is the predominant mechanism for cytokine release from macrophages and DCs, which do not have typical secretory granules.70 The trafficking pathway and molecular machinery for TNF in activated macrophages is currently one of the most comprehensively studied pathways for cytokine release. TNF is a potent proinflammatory cytokine secreted by many innate immune cells, particularly activated macrophages, but also neutrophils, mast cells, eosinophils, DCs, and NK cells.31,32,43,71,72 The exocytic pathways responsible for TNF release vary between these cell types, and in macrophages TNF is released by constitutive exocytosis only after being synthesized in response to inflammatory stimuli.71 Macrophages also produce and secrete a cascade of other proinflammatory and anti-inflammatory cytokines, such as IL-6, IL-10, IL-12, and a host of chemokines that are trafficked and secreted by constitutive exocytosis, moving, sometimes simultaneously, through the ER and Golgi complex.70,71,73
Macrophages have a pool of constitutively transcribed TNF mRNA, which, on TLR4 signaling, is stabilized and rapidly translated, giving rise to a transmembrane TNF precursor,74 which is trafficked from the ER to the Golgi complex, where it can be detected by immunostaining within 20 minutes of LPS activation.71 By imaging live cells, GFP-tagged TNF can be seen exiting the TGN in highly dynamic, tubular carriers that stretch out and break off, undergoing fission, to release TNF-loaded carriers.10,18 Fluorescently tagged IL-6 can also be visualized in live cells being loaded, as a soluble cargo, into tubules at the TGN. The extension of TGN membranes into tubules helps to load and sort the cargo proteins. Members of the coiled-coil GRIP golgin family of tethering proteins, golgin97 and golgin-245 (p230), label separate TGN tubular carriers,75 and live cell imaging shows that TNF is specifically transported in p230-labeled tubules. LPS up-regulates the number of p230 tubules forming at the Golgi in activated macrophages, generating a greater capacity for cytokine trafficking. RNAi knockdown of p230 in macrophage cell lines or in macrophages from retrogenic p230-deficient mice inhibits the formation of TNF carriers, halting TNF at the Golgi and blocking its secretion.18
Membrane lipids also have a major role in the formation and function of these tubules and carriers, undergoing rearrangements to allow curvature, extension, and fission of membranes and also binding the cytoplasmic proteins needed to mediate these processes.76 Choline cytidylyltransferase mediates synthesis of phosphatidylcholine at the TGN in macrophages to allow the exit and subsequent secretion of TNF and IL-6.77 Although PI3Ks were previously thought to regulate trafficking predominantly at endosomes and at the cell surface, recent studies show that one isoform, PI3Kδ, functions at the TGN to produce carriers for TNF exit and secretion. The p110 subunit of PI3Kδ is recruited to Golgi membranes in LPS-activated macrophages, where it, in turn, recruits dynamin II for fission of the tubules.19 Pharmacologic or genetic inactivation of PI3Kδ reduces TNF secretion from macrophages as a result of impaired fission of p230 Golgi-derived carriers for TNF.19 PI3Kδ probably variably regulates the secretion of different cytokines, depending on which tubules they use for exit from the TGN. The differential exit of cytokines from the TGN is emerging as a definitive point in the constitutive secretory pathway, and one that may be amenable to pharmacologic intervention.
Previous dogma held that constitutively secreted proteins were trafficked directly from the TGN to the cell surface for release. However, with the advent of live cell imaging, it became apparent that many newly synthesized proteins leaving the TGN are next delivered to intervening endosomes, before being transported in a subsequent step to the plasma membrane for release (Figure 4). These are tubulovesicular “recycling endosomes,” based on the presence of markers such as Rab11 and VAMP-3 and their recycling cargo of transferrin,78 and in many cell types they are now recognized as compartments in both endocytic and exocytic pathways.79,80 Most cell types, both immune and nonimmune cells, contain recycling endosomes and, although these organelles have been studied in NK cells, mast cells, and macrophages, they are yet to be characterized in neutrophils or eosinophils. Cytokine trafficking through recycling endosomes is not unique to macrophages. Microglial cells represent yet another innate immune cell type in which TNF secretion occurs by recycling endosomes.81 In NK cells, the constitutive, nonpolarized release of TNF and IFNγ also requires involvement of recycling endosomes.12
SNARE complexes and SNARE-interacting proteins mediate membrane fusion at successive transport steps from the Golgi to the cell membrane to facilitate the secretion of cytokines in macrophages.28 Carriers exiting the Golgi with TNF as cargo display a distinct Q-SNARE complex composed of syntaxin-6/syntaxin-7/Vti1b.14 This complex engages with its cognate R-SNARE, VAMP-3, on recycling endosomes11 to deliver TNF to these endosomal/exocytic compartments in the cell periphery. VAMP-3, or in some cells VAMP-2, is the principal R-SNARE typically found on recycling endosomes and on the cell surface, where it mediates constitutive exocytosis at the cell surface (Figure 4). By high-resolution imaging, TNF colocalizes with VAMP-3 in the membrane of recycling endosomes, and these 2 proteins migrate together to the cell surface where VAMP-3 engages with the Q-SNARE complex of syntaxin-4 and SNAP-23 for fusion, finally delivering TNF to the cell surface.10,11,17
Additional machinery interacts with the SNAREs either directly or indirectly to sort and load cargo and affect its movement. For example, the sec/munc protein Munc-18c also participates in this SNARE-mediated fusion at the macrophage cell surface (Table 1),17 although its potential role remains controversial. Secretory carrier membrane proteins are transmembrane proteins associated with secretory organelles, and in macrophages secretory carrier membrane protein (SCAMP) 5 on recycling endosomes interacts with the relevant SNARE proteins to regulate calcium-triggered traffic and secretion of TNF and CCL5/RANTES in response to ionomycin or TLR4 signaling.21
Thus, although many individual proteins of the macrophage trafficking machinery have been implicated in cytokine secretion, as yet we do not have a unified picture of how they all coordinately work to transport newly synthesized cytokines step-wise through the cell and regulate their release during immune responses.
Polarized release of cytokines
Polarized cytokine delivery has been functionally associated with microtubule organizing center polarization at the immunologic synapse in T cells and NK cells.69 A recent study has shown that DCs also secrete the Th1 cytokine IL-12 in a polarized manner toward the immunologic synapse and that this polarization of IL-12 release depends on Cdc42 GTPase.82 Recycling endosomes have a key role in the selective delivery of cytokines to polarized domains of the cell surface. In macrophages, the transmembrane precursor of TNF moves in a polarized fashion from recycling endosomes to the filopodia and nascent phagocytic cups at the cell surface.11 In the recycling endosomes, TNF destined for phagocytic cups is sorted away from other cargo, including recycling transferrin or soluble cytokine IL-6, which are delivered to other points on the cell surface.10 The clustering and sorting of TNF at the recycling endosome is reportedly mediated by the adaptor complex AP-1 in combination with clathrin, after which cleaved subunits of AP-1 shepherd the TNF toward the phagosome.20,83 Recycling endosomes labeled for GFP-VAMP-3 and Rab11a contribute extra membrane needed to form phagocytic cups as they extend out from the cell surface during the initial stages of phagocytosis.83-89 By comigrating with this recycling endosome membrane TNF is conjointly, and efficiently, delivered to the cell surface.11,20,22,83 TNF is delivered to raft domains in the cholesterol-rich membranes of phagocytic cups and filopodia. Finally, the TNF cleaving enzyme (TACE, or ADAM17) is also concentrated at the phagocytic cup, ready to cleave the transmembrane TNF for extracellular release before the phagocytic cup closes over and internalizes.11,22
Thus, cytokine trafficking through classic secretory pathways, whether through regulated or constitutive exocytic routes, is regulated both temporally and spatially to orchestrate immune responses. The mode of release is often customized to enhance the rapidity or efficiency of cytokine release, to direct cytokines to a target, or to maximize their dispersal.
Nonclassical secretory pathways
Cytokines such as IL-1β, IL-15, IL-18, and macrophage inhibitory factor lack the N-terminal signal sequence required for ER entry and are thus synthesized in the cytoplasm and thereafter released from cells by so called “nonclassical” pathways.90 A number of possible, and quite different, routes have been proposed for these cytokines to cross the plasma membrane and achieve exit from the cell.
The proinflammatory cytokine IL-1β is one of the most crucial mediators of inflammation and host responses to infection,91 although its mechanism of release remains among the least understood of all cytokines. IL-1β is first generated as pro-IL-1β, which is biologically inactive, and is synthesized directly in the cell cytoplasm.91 This precursor is processed into mature, biologically active IL-1β by caspase-1and then released directly into the extracellular milieu. How it exits the cell, by crossing the plasma membrane directly or by entering or associating with intracellular membranes, is still controversial. Several different mechanisms have been proposed for IL-1β release, and these have been reviewed in detail previously.91 Purported nonclassical mechanisms for release include (1) export of IL-1β through ABC transporters in the plasma membrane,92 (2) release of IL-1β–containing exosomes from multivesicular bodies, and (3) shedding of plasma membrane microvesicles.91 There is also evidence that a significant proportion of IL-1β production may occur through necrosis and cell lysis (Figure 1).
Similarly, IL-18, a potent inducer of IFNγ,93 is made in the cytoplasm from where it is translocated, by unknown mechanisms, into secretory lysosomes for classic secretion in DCs.94 These secretory lysosomes are restricted to the immunologic synapse in a calcium-dependent manner to release IL-18 at this site.95 The polarization and constrained release of IL-18 allows activation of NK cells to up-regulate cytotoxicity without spreading this cytokine nonspecifically to collateral tissues.
In addition, a cytolytic pathway of nonclassical granule release has been described in eosinophils that generate “cell-free” granules that can be detected in tissues of persons with allergies.60 Cytolysis is responsible for ∼ 33% of the degranulation events seen in vivo during allergic reactions, aside from the predominant form of release through piecemeal degranulation. Although controversial, cell-free granules were shown to function as extracellular secretory organelles for IL-4 and IL-6 release in response to IFNγ.96 This intriguing observation deserves further exploration to understand how cell-free granules may function in immunomodulation.
Looking ahead: future directions
Cytokines are currently at the forefront of medicine, both as culprits in the pathogenesis of a large number of diseases associated with inflammation and as increasingly popular targets for therapeutic antibodies.97,98 Both issues compel us to develop a comprehensive understanding of cytokine trafficking and release, first to explain how individual cytokines are differentially handled and regulated in different cell types and second to identify the trafficking machinery involved. Such knowledge may then help us to refine the use of therapeutic antibodies, or it may show new targets or new strategies for selectively reducing or controlling cytokine release in disease.
A plethora of cytokines are also released by cells of the adaptive immune system. Thanks to the use of approaches such as live-cell imaging, some of the pathways and molecules regulating cytokine release in helper and cytotoxic T-cell populations have been elucidated, and these are described elsewhere.99 Eventually, the combined knowledge of cytokine release in both innate and adaptive immune systems will instruct our understanding of cell communication and effectors across the spectrum of immunity and disease.
Finally, studies into cytokine trafficking will benefit greatly from systems biology100 and “omic” approaches to trafficking and from sophisticated bioinformatics being used to predict, test, and visualize molecular networks.101-103 With high-throughput imaging and RNAi approaches it is now eminently possible to study and compare all members of molecular families, such as Rabs, SNAREs, adaptors, lipid kinases, and phosphatases, in trafficking pathways. Such family-wide studies have yet to be applied in earnest to studies of trafficking in cells of the innate immune system. Cytokine secretion from single cells can be measured with great sensitivity,104 and the behavior of cytokine-secreting cells can be visualized with increasing sensitivity with the use of in vitro live-cell imaging, alongside 2-photon imaging in vivo. Finally, super-resolution fluorescence imaging and ultrastructural approaches are anticipated to define the membrane microdomains and molecular interactions that underpin secretion in innate immune cells.105
Acknowledgments
We thank Amanda Stanley and Adam Wall for many helpful suggestions in the preparation of this manuscript.
This work was supported by grants from the Alberta Heritage Foundation for Medical Research (Visiting Speaker from Alberta Award), the Australian Research Council International Discovery Grant, and the National Health and Medical Research Council of Australia.
Authorship
Contribution: P.L. and J.L.S. contributed equally to this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paige Lacy, Pulmonary Research Group, 559 HMRC, Department of Medicine, University of Alberta, Edmonton, AB T6G 2S2, Canada; e-mail: paige.lacy@ualberta.ca.