The heterogeneous clinical course in follicular lymphoma (FL) is poorly understood. However, the supporting role of the microenvironment in malignant B-cell survival is clearly observed in this disease,1 prompting many to consider a “seed-versus-soil” hypothesis.
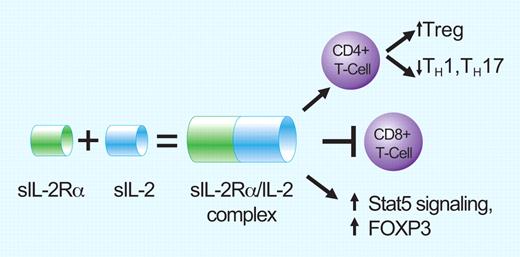
The alpha subunit of the IL-2 receptor (called sIL-2Ra) is released from the surface of T cells and is a poor prognostic marker in follicular lymphoma. Instead of blocking IL-2 activity, binding of sIL-2Ra to IL-2 is agonistic and enhances IL-2 effects on the lymphoma milieu. Effects include promotion of T-reg differentiation, blocking CD8 antitumor activity, and increasing T reg activity via Stat5 signaling and increased FOXP3. Professional illustration by Paulette Dennis.
The alpha subunit of the IL-2 receptor (called sIL-2Ra) is released from the surface of T cells and is a poor prognostic marker in follicular lymphoma. Instead of blocking IL-2 activity, binding of sIL-2Ra to IL-2 is agonistic and enhances IL-2 effects on the lymphoma milieu. Effects include promotion of T-reg differentiation, blocking CD8 antitumor activity, and increasing T reg activity via Stat5 signaling and increased FOXP3. Professional illustration by Paulette Dennis.
In this model, the malignant B-cell “seed” requires and promotes a nurturing T-cell “soil.“ Exactly how this occurs remains uncertain and likely underlies, to some degree, the drug resistance and incurability associated with FL. In this issue of Blood, Yang et al find that soluble IL-2Rα (sIL-2Rα) binds soluble sIL-2 and supercharges it into an agonist that promotes tumor-friendly Treg cells rather than antitumor T cells.2 The consequence of the shift to Treg proliferation (as opposed to T-helper proliferation) is a microenvironment that fosters malignant B-cell survival.
Many groups have shown that tumor-infiltrating T cells are not merely innocent bystanders but that the nature of the background non-malignant cells affects clinical outcome and/or clinical behavior of FL.1,3,4 However, not all T cells are created equal, and there has been controversy over which T-cell subsets help and, conversely, which ones antagonize malignant B-cell growth. Recently, several lines of evidence recently support that Treg cells (CD4+CD25+FOXP3+ or CD4+CD25+GITR+), are the ones promoting tumor survival, in part by attenuating the typical immune responses of either CD4+ helper T cells or CD8+ cytotoxic T cells.5,6
IL-2 is a cytokine that exerts control over many aspects of Treg growth, development, and function (reviewed in Campbell and Koch7 ) and thus has a potentially important role over background T cells in the FL microenvironment. IL-2 has been shown to skew T-cell differentiation toward Treg cells rather than T-helper cells such as TH1 and TH17. Without IL-2, both Treg numbers and activity are diminished. IL-2 works by binding to its receptor, IL-2R, which is on the surface of T cells. The IL-2R itself is composed of 3 subunits: IL-2Rα (CD25), IL-2Rβ (CD122), and γc (CD132), all of which are required for IL-2 interaction.8 Circulating sIL-2R portends a poor prognosis with shorter progression-free and overall survival in several aggressive lymphoma subtypes independent of the International Prognostic Index (IPI) and of rituximab use.9-12 By itself, the α subunit (IL-2Rα) does not appear to influence IL-2 activity. However, it can be cleaved off the surface of activated T cells and circulate as sIL-2Rα. Once in the circulation, does it block IL-2 activity by mopping up sIL-2? Or does it prolong IL-2 survival in the circulation and thus promote IL-2 activity?
With this in mind, Yang and colleagues sought to determine the role of sIL-2Rα in FL. Using primarily patient-derived tumor samples, the authors first showed that exogenous IL-2 preferentially increased Treg proliferation as opposed to TH1 or TH17 cells, and that this could be reversed by blocking antibodies against either IL-2, IL-2Rα, or IL-2Rβ. Second, instead of blocking IL-2 activity, sIL-2Rα augmented IL-2 effects on IL-2R signaling as reflected by Stat5 phosphorylation and FOXP3 expression. In addition, it appears that the source of sIL-2Rα is the intratumoral T cell itself, thus providing a self-promoting environment. Finally, sIL-2Rα enhanced IL-2's suppressive effects on both CD8+ T-cell proliferation and CD8+ T-cell cytolytic granule perforin and granzyme-B production. Collectively, these sets of data support that circulating sIL-2Rα aids and abets IL-2 in promoting an antitumor microenvironment via effects on T-cell populations and function.
At a clinical level, Yang et al found that sIL-2Rα levels were significantly higher in 30 treatment-naive patients with FL enrolled in a trial of single-agent rituximab compared with 24 healthy control patients. Despite the small sample size, they found that the time to progression was only 12 months for FL patients with high sIL-2Rα levels versus 40 months for FL patients with low sIL-2Rα levels (P = .008). Multivariate analysis that included the IPI showed that the sIL-2Rα retained independent prognostic significance for progression-free survival prediction in the treatment-naive patients.
While these results are of strong interest, it is important to remember that the factors influencing outcome in FL are complex, and that the sIL-2Rα/IL-2 complex is likely only one piece of a larger picture controlling the tumor microenvironment. Several groups have found that the architectural location (perifollicular vs intrafollicular compartment) of T cells is also important, and this may affect the significance of Yang et al's findings.4,13 Despite being performed in patient-derived samples, these were all in vitro studies, and other components of the antitumor immune response to lymphoma are necessarily absent. In addition, other cytokines (ie, IL-15) and growth signals clearly play a role in T-cell proliferation and function.7 Finally, in its effort to answer a very specific question regarding the role of sIL-2Rα, the present article is not able to address the malignant lymphoma B-cell population itself and how it interacts with intratumoral T cells.
Nevertheless, this report by Yang and colleagues is the first to describe the adverse prognostic relevance of sIL-2Rα in patients with FL. The authors provide valuable insights into the intratumoral T-cell milieu and how CD4+ T cells are lulled into a tolerant Treg phenotype while the CD8+ cytotoxic T cells are made less effective by the sIL-2Rα/IL-2 complex. In contrast to blocking IL-2 activity, circulating sIL-2Rα binds to IL-2 and significantly enhances its signaling. Although it remains unknown as to why sIL-2Rα is increased, it is derived directly from activated T cells themselves, and is part of the toxic fertilizing mix permitting malignant B cells to grow.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■