Abstract
In a previous study, we demonstrated unique secretory dynamics of tissue plasminogen activator (tPA) in which tPA was retained on the cell surface in a heavy chain–dependent manner after exocytosis from secretory granules in vascular endothelial cells. Here, we examined how retained tPA expresses its enzymatic activity. Retained tPA effectively increased the lysine binding site–dependent binding of plasminogen on the cell surface and pericellular area; this was abolished by inhibition of enzymatic activity of either tPA or plasmin, which suggests that de novo generation of carboxyl-terminal lysine as a consequence of degradation of surface/pericellular proteins by plasmin is essential. Retained tPA initiated zonal clot lysis of a fibrin network that had been formed on vascular endothelial cells, which was preceded by the binding of plasminogen to the lysis front. Our results provide evidence that secreted and retained tPA is essential for maintaining both high fibrinolytic activity and effective clot lysis on the vascular endothelial cell surface.
Introduction
Vascular endothelial cell (VEC) surface–associated plasminogen activation is essential not only in maintaining high fibrinolytic activity in the vasculature but also in a variety of physiologic events, including vascular remodeling, angiogenesis, inflammation, and tumor invasion.1-3 Plasminogen activators (PAs) cleave a single peptide bond in plasminogen to generate plasmin. Tissue-type PA (tPA) is primarily involved in intravascular fibrinolysis, whereas urokinase-type PA (uPA) is largely involved in pericellular proteolysis in the extravascular space, together with its specific receptor, uPAR. We previously demonstrated unique secretory dynamics of tPA in which tPA was retained on the cell surface in a heavy chain–dependent manner after exocytosis from VECs.4 Here, we demonstrate that prolonged retention of tPA is essential for effective expression of cell surface–associated plasmin activity and its amplification on VECs.
Methods
Materials and cells
Human Glu-plasminogen purified from fresh-frozen human plasma and human fibrinogen (Enzyme Research Laboratories) was labeled with Alexa Fluor 568 (plg-568) and Alexa Fluor 647 (fbg-647), respectively. Human recombinant PA inhibitor-1 was purified as described previously.5 Aprotinin, human α2-antiplasmin, carboxypeptidase B, and human thrombin were purchased from Bayer Schering Pharma, Calbiochem, Sigma, and Benesis, respectively. The human VEC line EA.hy926, which was established to retain endothelial cell–specific functions, including fibrinolytic characteristics, was kindly provided by Dr C.J. Edgell.6,7 Cells were cultured as described previously.4
Microscopic analyses
The total internal reflection fluorescence (TIRF) microscopy system with an emission splitter used in the present study was described previously.4,8 We used a confocal laser scanning microscope (CLSM; FV1000, Olympus) equipped with a 60× oil-immersion objective lens and a temperature control system.
Plasminogen-binding analysis
Cells were grown to confluence in 35-mm glass-bottom dishes and transfected with plasmids (tPA–green fluorescent protein [GFP], tPA-S478A-GFP, and tPA-CD-GFP, a deletion mutant tPA-GFP, which were described in our previous study4 ). After overnight incubation, plasmid-transfected cells were treated with HEPES-buffered solution (140mM NaCl, 5mM KCl, 1mM MgCl2, 2.5mM CaCl2, 10mM glucose, and 10mM HEPES-NaOH, pH 7.3) that contained 480nM nonlabeled human plasminogen (NL-plg) plus 20nM plg-568. To quantify the amount of plg-568 accumulation on or around the cell surface, we measured the fluorescence intensity at a 570- to 670-nm wavelength in each cell, including the pericelluar area.
Fibrin clot lysis imaging
A fibrin clot overlaid on plasmid-transfected cells was prepared by mixing HEPES-buffered solution that contained 480nM NL-plg, 20nM plg-568, 2 U/mL thrombin, and 1 mg/mL nonlabeled human fibrinogen together with 10 μg/mL fibrinogen-647. We calculated the lysis area that originated from the spot of exocytosed tPA-GFPs using FV10-ASW software (Olympus).
Statistical analysis
Statistical significance was evaluated with the Student t test.
Results and discussion
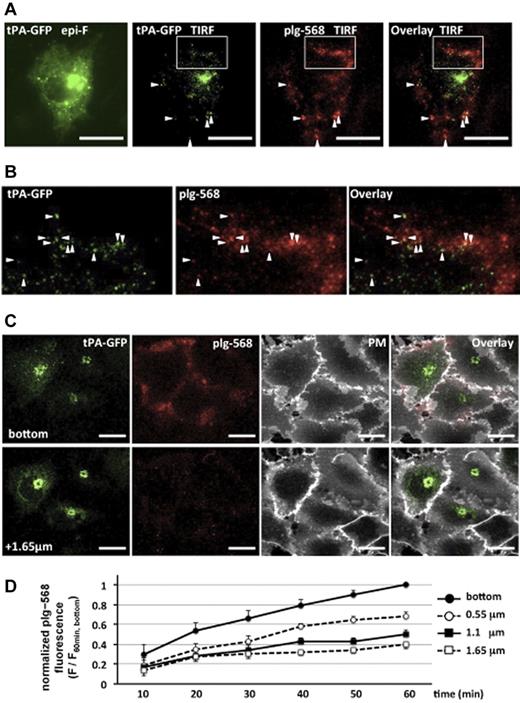
We first analyzed the localization of plg-568 bound on tPA-GFP–expressing EA.hy926 cells. Images captured with the total internal reflection fluorescence system clearly showed the existence of plg-568 on the cell surface at spots where tPA-GFP was retained (Figure 1A-B). Using the CLSM system, we took focal images along the z axis at a 550-nm optical slice thickness every 10 minutes after application of plg-568 in the recording medium. The fluorescence of plg-568 gradually increased on the tPA-GFP–expressing cell surface, predominantly in the adhesion side (bottom area), in a time-dependent manner (Figure 1D; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Labeling of the plasma membrane with deep red fluorophores with Cell Mask Deep Red plasma membrane stain (Molecular Probes) after the images were captured at 60 minutes suggested that plg-568 predominantly accumulated in intercellular spaces, as well as in the pericellular/matrix adhesive area (Figure 1C).
Binding and accumulation of plg-568 on tPA-GFP–expressing EA.hy926 cells. (A) After tPA-GFP–transfected cells were incubated with 480nM NL-plg plus 20nM plg-568 at 37°C for 30 minutes, localization of tPA-GFP and plg-568 was determined by total internal reflection fluorescence (TIRF) microscopy. Both fluorophores were excited at 488 nm, and the light emitted was collected through an emission splitter (W-View system; Hamamatsu Photonics) equipped with a 550-nm dichroic mirror and 2 emission filters, a 510/23-nm band-pass filter for GFP and a 600-nm long-pass filter for Alexa Fluor 568. Representative images are shown. Left panel shows epifluorescence image obtained only by GFP channel; others are TIRF images of tPA-GFP and/or plg-568. In addition to the high fluorescence intensity of tPA-GFP in possible Golgi complex, TIRF images demonstrated a number of clear spots, some of which colocalized with plg-567 (arrowheads). Bars represent 10 μm. (B) Expanded images of the area indicated by a white box in panel A. (C) Representative images captured by the CLSM system are shown. The plasma membrane was labeled with Cell Mask Deep Red plasma membrane stain in tPA-GFP–expressing cells, which were incubated with 480nM NL-plg plus 20nM plg-568 for 60 minutes. Top panels are the most basal focal images (the “bottom”), and bottom panels are optical images taken along the z axis on a 1.65 μm focal plane from the bottom. PM indicates plasma membrane stain. plg-568 (red) accumulated not only in the plasma membrane area (white) but also in the pericellular/matrix adhesive area in tPA-GFP–expressing cells. Bars represent 10 μm. (D) Time-dependent accumulation of plg-568 in each focal plane. The values of fluorescence intensity at every 10 minutes were normalized to that at the bottom at 60 minutes; relative fluorescence changes are referred to as F/F60 min, bottom. The plots are indicated in each focal plane as mean ± SE of 7 independent experiments.
Binding and accumulation of plg-568 on tPA-GFP–expressing EA.hy926 cells. (A) After tPA-GFP–transfected cells were incubated with 480nM NL-plg plus 20nM plg-568 at 37°C for 30 minutes, localization of tPA-GFP and plg-568 was determined by total internal reflection fluorescence (TIRF) microscopy. Both fluorophores were excited at 488 nm, and the light emitted was collected through an emission splitter (W-View system; Hamamatsu Photonics) equipped with a 550-nm dichroic mirror and 2 emission filters, a 510/23-nm band-pass filter for GFP and a 600-nm long-pass filter for Alexa Fluor 568. Representative images are shown. Left panel shows epifluorescence image obtained only by GFP channel; others are TIRF images of tPA-GFP and/or plg-568. In addition to the high fluorescence intensity of tPA-GFP in possible Golgi complex, TIRF images demonstrated a number of clear spots, some of which colocalized with plg-567 (arrowheads). Bars represent 10 μm. (B) Expanded images of the area indicated by a white box in panel A. (C) Representative images captured by the CLSM system are shown. The plasma membrane was labeled with Cell Mask Deep Red plasma membrane stain in tPA-GFP–expressing cells, which were incubated with 480nM NL-plg plus 20nM plg-568 for 60 minutes. Top panels are the most basal focal images (the “bottom”), and bottom panels are optical images taken along the z axis on a 1.65 μm focal plane from the bottom. PM indicates plasma membrane stain. plg-568 (red) accumulated not only in the plasma membrane area (white) but also in the pericellular/matrix adhesive area in tPA-GFP–expressing cells. Bars represent 10 μm. (D) Time-dependent accumulation of plg-568 in each focal plane. The values of fluorescence intensity at every 10 minutes were normalized to that at the bottom at 60 minutes; relative fluorescence changes are referred to as F/F60 min, bottom. The plots are indicated in each focal plane as mean ± SE of 7 independent experiments.
To evaluate the possible involvement of lysine-binding sites in the plasminogen molecule in the binding and accumulation of plg-568, we used 3 different materials (Figure 2A). Alexa Fluor 568–labeled miniplasminogen, which lacks 4 of 5 kringle domains of native Glu-plasminogen and thus lacks lysine-binding sites, rarely accumulated. Epsilon-aminocaproic acid, a lysine analog, effectively inhibited the binding of plg-568 in a dose-dependent manner. Similarly, preincubation with carboxypeptidase B to remove exposed carboxy-terminal lysine residues of surface proteins attenuated accumulation of plg-568 in a dose-dependent manner. These results suggested that the binding and accumulation of plg-568 on the tPA-GFP–expressing cell surface/pericellular area was lysine-binding-site–dependent, as described previously on the macrophage membrane surface.9
Lysine-binding-site–dependent and plasmin-activity–dependent amplification of cell surface–associated plasminogen activation by surface-retained tPA. (A) The increasing values in plg-568 fluorescence intensity at the most basal focal image in tPA-GFP–expressing cells were calculated from each initial value until that at 30 minutes, which represents the amount of accumulated plg-568 and is referred as dF30 min. Bars show the mean ± SE of dF30 min from 9, 4, 3, and 5 independent experiments in control (C), 480nM nonlabeled miniplasminogen plus 20nM Alexa Fluor 568–labeled miniplasminogen (mini), epsilon-aminocaproic acid (EACA), and carboxypeptidase B (cpB), respectively. All materials examined significantly reduced the accumulation of plg-568 (P < .001 vs control) except for 0.01mM EACA (P = .033 vs control). (B) Bars indicate mean ± SE of dF30 min from 9, 4, 3, 6, 5, and 5 independent experiments in control (C), 1667 U/mL aprotinin (APR), 0.3μM α2-antiplasmin (α2AP), 40nM human recombinant PA inhibitor-1 (PAI-1), S478A, and CD, respectively. Supplementation of PAI-1 or α2AP reduced the accumulation of plg-568 (P = .012 and P = .0028, respectively, vs control), whereas the others significantly reduced the accumulation of plg-568 (P < .001 vs control). S478A indicates tPA-S478A-GFP–expressing cells; CD, tPA-CD-GFP–expressing cells (a heavy chain–deleted mutant of tPA, which is composed of signal peptide and catalytic domain). (C) Representative images during fibrin clot lysis on tPA-GFP–expressing cells (left) or tPA-CD-GFP–expressing cells (right) at 3 μm above and along the z axis at 50 minutes after fibrin clot formation. Green indicates tPA-GFP or tPA-CD-GFP; red, plg-568; and white, fbg-647. (D) Lysis area at 3 μm above the z axis was calculated every 10 minutes after fibrin network formation over a single tPA-GFP–expressing cell ● or tPA-CD-GFP–expressing cell ○. Results of 5 independent experiments are shown as mean ± SE.
Lysine-binding-site–dependent and plasmin-activity–dependent amplification of cell surface–associated plasminogen activation by surface-retained tPA. (A) The increasing values in plg-568 fluorescence intensity at the most basal focal image in tPA-GFP–expressing cells were calculated from each initial value until that at 30 minutes, which represents the amount of accumulated plg-568 and is referred as dF30 min. Bars show the mean ± SE of dF30 min from 9, 4, 3, and 5 independent experiments in control (C), 480nM nonlabeled miniplasminogen plus 20nM Alexa Fluor 568–labeled miniplasminogen (mini), epsilon-aminocaproic acid (EACA), and carboxypeptidase B (cpB), respectively. All materials examined significantly reduced the accumulation of plg-568 (P < .001 vs control) except for 0.01mM EACA (P = .033 vs control). (B) Bars indicate mean ± SE of dF30 min from 9, 4, 3, 6, 5, and 5 independent experiments in control (C), 1667 U/mL aprotinin (APR), 0.3μM α2-antiplasmin (α2AP), 40nM human recombinant PA inhibitor-1 (PAI-1), S478A, and CD, respectively. Supplementation of PAI-1 or α2AP reduced the accumulation of plg-568 (P = .012 and P = .0028, respectively, vs control), whereas the others significantly reduced the accumulation of plg-568 (P < .001 vs control). S478A indicates tPA-S478A-GFP–expressing cells; CD, tPA-CD-GFP–expressing cells (a heavy chain–deleted mutant of tPA, which is composed of signal peptide and catalytic domain). (C) Representative images during fibrin clot lysis on tPA-GFP–expressing cells (left) or tPA-CD-GFP–expressing cells (right) at 3 μm above and along the z axis at 50 minutes after fibrin clot formation. Green indicates tPA-GFP or tPA-CD-GFP; red, plg-568; and white, fbg-647. (D) Lysis area at 3 μm above the z axis was calculated every 10 minutes after fibrin network formation over a single tPA-GFP–expressing cell ● or tPA-CD-GFP–expressing cell ○. Results of 5 independent experiments are shown as mean ± SE.
Because de novo generation of carboxy-terminal lysine as a consequence of plasmin catalysis plays an essential role in fibrin-dependent enhancement of plasminogen activation by tPA,10,11 we next examined the relevance of enzymatic activity of plasmin generated by secreted and retained active tPA-GFP in the accumulation of plg-568 on the cell surface/pericellular area (Figure 2B). Inhibition of plasmin activity by either aprotinin or α2-antiplasmin effectively attenuated the accumulation of plg-568. Similarly, supplementation with 40nM human recombinant PA inhibitor-1, which facilitates the dissociation of exocytosed tPA from the cell surface by forming high-molecular-weight complexes,4 partially reduced the accumulation of plg-568. When a catalytically inactive mutant of tPA-GFP, tPA-S478A-GFP, was used, we completely failed to detect the binding and accumulation of plg-568 (supplemental Figure 2C). Collectively, plasmin generated by active tPA-GFP on the cell surface effectively facilitated further accumulation of plasminogen through de novo generation of carboxyl-terminal lysine as a consequence of degradation of surface/pericellular proteins.
Finally, we evaluated how plasminogen activation on the cell surface by retained tPA contributed to the dissolution of fibrin clots that formed on the tPA-GFP–expressing cells. Lysis of the fibrin network triggered by wild-type tPA-GFP gradually expanded and coincided with the appearance of a narrow zone where plg-568 clearly accumulated, the so-called lysis front (narrower than 10 μm; Figure 2C-D; supplemental Figure 3B-D). This is in agreement with a previous cell-free study in which strikingly enhanced accumulations of fibrinolytic components were demonstrated at the lysis front from the surface of the preformed fibrin clot after superimposition by tPA.12 Although limited accumulation of plg-568 was clearly demonstrated only at the lysis front where plg-568 coincided with fbg-647 fiber, accumulation of tPA-GFP was not detected in this layer, most likely because its concentration was much lower than that of plasminogen and fibrinogen. We here suggest that retained tPA, which was de novo synthesized and secreted but not supplemented externally, is essential for maintenance of high fibrinolytic activity on VECs and effectively initiates clot lysis once a clot has formed on the surface of VECs.
In the case of tPA-CD-GFP, which possesses only the catalytic domain without the heavy chain and shows very quick secretory kinetics without being retained on the cell surface,4 lysis of fibrin developed only scarcely, and only a small amount of plg-568 accumulated on fibrin fibers around the cells (Figure 2C-D; supplemental Figure 4A-B). Retention of tPA on the cell surface for a certain period of time, as well as its binding to fibrin, appeared essential for efficient activation of plasminogen on both the cell surface/pericellular area and the lysis front of the fibrin network, which then accelerated both further accumulation of plasminogen and efficient fibrin clot lysis on VECs.
In conclusion, we propose a positive-feedback loop in endothelial cell–associated plasmin generation triggered by cell surface–retained tPA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant-in-aid for scientific research (C:22590826 [Y.S.] and C:21590230 [T.U.]) from the Japan Society for the Promotion of Science, a grant from Takeda Science Foundation (Y.S.), and a grant from the Smoking Research Foundation (T.U.).
Authorship
Contribution: Y.S. designed the study, performed the experiments, and wrote the paper; H.Y., T.B., and H.M. performed experiments; and T.U. designed the study and wrote the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Yuko Suzuki, 1-20-1, Handa-yama, Higashi-ku, Hamamatsu, 431-3192, Japan; e-mail address: seigan@hama-med.ac.jp.