Abstract
Stimulation via the T-cell receptor (TCR) activates p38α and p38β by phosphorylation of p38 Tyr-323 (p38Y323). Here we characterize knockin mice in which p38α and/or β Tyr-323 has been replaced with Phe. We find that p38α accounts for two-thirds and p38β the remainder of TCR-induced p38 activation. T cells from double knockin mice (p38αβY323F) had defects in TCR-mediated proliferation and Th1 and Th17 skewing, the former corresponding with an inability to sustain T-bet expression. Introduction of p38αY323F into Gadd45α-deficient mice, in which the alternative p38 pathway is constitutively active, reversed T-cell hyperproliferation and autoimmunity. Furthermore, p38αβY323F mice had delayed onset and reduced severity of the inflammatory autoimmune diseases collagen-induced arthritis and experimental autoimmune encephalomyelitis. Thus, T cell-specific alternative activation of p38 is an important pathway in T-cell proliferation, Th skewing, and inflammatory autoimmunity, and may be an attractive tissue-specific target for intervention in these processes.
Introduction
p38, a member of mitogen activated protein kinase (MAPK) family, is a key signaling intermediate downstream of proinflammatory cytokine receptors and environmental stress.1 The p38 MAPK family has 4 separately encoded members: α, β, γ, and δ. p38α, β, and δ are expressed in T cells, whereas p38γ is largely restricted to skeletal muscle.2 p38α (the major T-cell isoform) and β are the most highly related, sharing 74% homology at the amino acid level.3 All p38 isoforms are activated via a series of sequential phosphorylation steps. The most stimulus-proximal kinase is a MAP kinase kinase kinase (MAPKKK), which phosphorylates dual-specificity MAP kinase kinases (MAPKKs). Two MAPKKs, MKK3 and MKK6, then phosphorylate Tyr180 and Thr182 in the p38 activation loop, causing conformational changes that result in better access to substrate and increased catalytic activity.4-6
In contrast to the MAPK cascade, which is present in all cells, we have described an alternative signaling pathway downstream of the T-cell receptor (TCR) that leads to p38 activation. Ligation of the TCR results in Lck-dependent activation of ZAP70, which in turn phosphorylates p38α and p38β on Tyr-323 (p38δ does not possess a tyrosine at this position).7 Once phosphorylated, p38 autophosphorylates residue T180 (but not Tyr-182) in the activation loop, and enzymatic activity is increased.6 It is noteworthy that p38 phosphorylated at only T180 has a different substrate specificity than dual-phosphorylated p38, raising the interesting possibility that the alternative pathway may have arisen to support biologic responses unique to T cells. To understand the biologic significance of the alternative p38 activation pathway in vivo, we created knockin mice in which a Tyr-to-Phe substitution was introduced at p38α residue 323 (p38αY323F).8 This mutation abolished p38α activation via TCR signaling without affecting canonical MAPK cascade-induced activation. Lack of TCR-induced p38α activity led to a modest but reproducible delay in the onset of T-cell proliferation and decreased production of inflammatory cytokines, such as IFN-γ and TNF-α. p38αY323F CD4+ T cells could be skewed to Th1 cells in vivo, but these effector cells produced less IFN-γ than wild-type (WT) Th1 cells when stimulated via the TCR.8
In resting T cells, p38 activity is inhibited by Gadd45α, genetic disruption of which results in constitutive up-regulation of the T cell alternative p38 activation pathway, with hyperproliferation in response to TCR-mediated signals and spontaneous development of lupus-like autoimmunity.9 Gadd45α binds p38 and inhibits kinase activity induced by Tyr-323 phosphorylation.10 Interestingly, Gadd45α also binds and activates MEKK4, an MAPKKK upstream of MKK3 and MKK6,11 which explains the paradox that in non-T cells Gadd45α is a positive regulator of p38 kinase activity, and its absence results in decreased p38-dependent responses, such as IL-12 and CD40 expression in activated dendritic cells and reduced UV-induced apoptosis of keratinocytes.12,13 The autoimmunity seen in Gadd45α−/− mice was presumed to be secondary to elevated T-cell p38 activity and hyperproliferation, but it was not possible to rule out other, uncharacterized, activities of Gadd45α in its pathogenesis.
Whereas impaired production of T-cell cytokines negatively affects immune responses to pathogens,14,15 excessive production of proinflammatory cytokines contributes to chronic inflammation and autoimmune diseases.16 For example, increased levels of TNF-α were found in serum of patients with pulmonary obstructive disease and in synovial fluids of rheumatoid arthritis patients.17,18 In experimental autoimmune encephalomyelitis (EAE), CD4+ T cells infiltrate the central nervous system before the development of clinical symptoms, secrete IFN-γ and IL-17, and activate CD11b+ microglia to produce TNF-α.19,20 p38 phosphorylation (P-p38) was found to be elevated in spinal cord tissue from rats, both in the peak and recovery phases of EAE, and high levels of P-p38 were detected in T cells in EAE lesions.21 In rheumatoid arthritis, IL-1 and TNF-α are major cytokines in initiating inflammatory and destructive processes in affected joints.18 Collagen-induced arthritis (CIA), a murine model of rheumatoid arthritis, can be substantially inhibited by pharmacologic inhibition of p38α and p38β.22,23
Given the data demonstrating roles for p38 in autoimmune and inflammatory processes, we asked whether there was a specific contribution made by T-cell p38 activated via TCR occupancy and the alternative pathway. To completely abrogate this pathway, we created p38βY323F knockin mice, which were crossed to p38αY323F mice to generate p38αβY323F double knockins. We found that, in the absence of TCR-induced p38αβ activity, there is a large reduction in T-cell proliferation, proinflammatory cytokine production, and reduced susceptibility to EAE and CIA, establishing alternatively activated T-cell p38α/β as a major factor in regulating T-cell activation and the development of autoimmunity and inflammatory diseases.
Methods
Mice
Gadd45α−/− mice24 were backcrossed onto the C57BL/6 (B6) background for 8 generations, and mice heterozygous for Gadd45α were intercrossed to obtain Gadd45α−/− animals used in these studies. p38αY323F8 and p38βY323F mice were backcrossed onto B6 for at least 6 generations. B10.RIII mice were obtained from The Jackson Laboratory and were crossed with p38αβY323F mice to generate the p38αβY323F.H-2r animals. Mice were maintained in an National Cancer Institute pathogen-free animal facility, and all animal experiments were performed under a National Institutes of Health-approved animal study protocol. To generate p38βY323F mice, BAC-DNA (clone RP23–269F24) containing the mouse p38β gene was obtained from BACPAC Resource Center. A SacI digest yielding a 5.1-kb region starting upstream of exon 11 and extending downstream of exon 12 was used to construct a targeting vector consisting of a neo-resistance cassette flanked by 2 loxP sites, into which the Y323F mutation was inserted using the QuickChange PCR mutagenesis system (Stratagene). Introduction of the Y323F mutation deleted a BanII-specific restriction site, which was later used for detection of the p38βY323F mutation. Embryonic stem cells [(129 × C57BL/6)F1] were transfected with the targeting vector and injected into C57BL/6 blastocysts. For genotyping, polymerase chain reaction was used to amplify part of p38β exon 11 (primers 5′ ACA TACATCCAGTCTCTGCCTCCC 3′ and 5′ TGCTCTCTTTCCCATCCTCACG 3′) yielding a 375-bp product that was digested with BanII.
Reagents
Antibodies against p38α, phospho-p38, and phospho-ATF-2 were purchased from Cell Signaling; anti-p38β, anti–β-actin, anti–T-bet and anti–GATA-3 from Santa Cruz Biotechnology; and CD4, CD8, IL-10, TNF-α, IL-2, IL-17, IFN-γ, IL-4, and phospho-p38-PE antibodies from BD Biosciences. Anti–rabbit and anti–mouse antibodies conjugated to AlexaFluor680 or JRDye800 were obtained from Invitrogen or Rockland Chemicals, respectively. Phorbol myristate acetate (PMA), ionomycin, dsDNA, histone, and poly-L-lysine were purchased from Sigma-Aldrich.
T-cell isolation, activation, and Th differentiation
Cell suspensions from lymph nodes or spleen were incubated with BioMag goat antimouse IgG and IgM beads (Polysciences) at room temperature for 40 minutes, and B cells were depleted using magnetic separation, yielding 95%-97% pure populations of T cells. Cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 250 μg/mL gentamicin, 100 U/mL penicillin, 4mM glutamine, and 50μM 2-mercaptoethanol (complete medium). Unless indicated otherwise, T cells were stimulated with 5 μg/mL plate-bound α-CD3 (145-2C11; BD Biosciences PharMingen) and 3 μg/mL α-CD28 (37.51; BD Biosciences PharMingen), or PMA (20 ng/mL) and ionomycin (1 μg/mL). To induce Th polarization, T cells were cultured for 4 to 6 days in the presence of plate-bound α-CD3 and α-CD28 in medium supplemented with IL-12 (2 ng/mL) and anti–IL-4 (10 μg/mL) for Th1 polarization, with anti–IL-12 (10 μg/mL) and IL-4 (10 ng/mL) for Th2 polarization, and with anti–IL-4 (10 μg/mL), IL-6 (30 ng/mL), and TGF-β (10 ng/mL) for Th17 differentiation.
Proliferation assay
The 96-well flat-bottom plates were coated with α-CD28 and various amounts of α-CD3. Cells were pulsed with 1 μCi [3H]thymidine 14 hours before harvesting, and [3H]thymidine uptake was determined with a Wallac 1450 MicroBeta Liquid Scintillation Counter.
Immunoblotting and kinase assays
Protein extracts were prepared from equivalent numbers of cells and protein concentrations measured using Bio-Rad protein assay and equalized. Lysates were separated by SDS-PAGE, transferred to nitrocellulose membranes (Invitrogen), and immunoblotted with the indicated antibodies. For kinase assays, p38α or p38β was immunoprecipitated from cell lysates using isoform-specific antibodies and Protein G–Sepharose 4B Fast Flow Beads (Sigma-Aldrich). Beads were washed twice with lysis buffer (PBS with 0.5% Triton X-100) and once in kinase buffer (25mM Tris, 5mM β-glycerophosphate, 1mM Na3VO4, 1mM NaF, and 10mM MgCl2) and incubated for 30 minutes at 30°C in 30 μL with 1 μg of glutathione S-transferase-ATF-2 (Cell Signaling) and 100μM adenosine triphosphate, followed by immunoblotting with anti-phospho-ATF-2.
Enzyme-linked immunosorbent assay
Supernatants from T cells stimulated in vitro were analyzed for IFN-γ with regents purchased from BD Biosciences, using mouse recombinant IFN-γ obtained from National Institute of Allergy and Infectious Diseases Reference Reagent Repository as a standard. Serum IFN-α was assayed with an enzyme-linked immunosorbent assay kit obtained from PBL Biomedical Laboratories. For detection of dsDNA and histone antibodies, serially diluted samples were incubated in 96-well plates coated with poly-L-lysine for 1 hour in 37°C and dsDNA (10 μg/mL) or histone (10 μg/mL) overnight at 4°C. The presence of anti-dsDNA or antihistone antibodies was detected using peroxidase-labeled goat anti–mouse IgG(H + L) antibody (Kirkegaard & Perry Laboratories) and TMB Substrate Reagents (BD Biosciences). The antibody titer was calculated according to the method of Frey et al.25
Flow cytometry
To detect intracellular cytokines, cells were treated with monensin (3μM) 3 to 4 hours before harvesting, stained with anti-CD4 antibody, fixed using BD Cytofix/Cytoperm kit (BD Biosciences), and stained in the presence of 2.4G2 antibody to block FcR binding. For detection of P-p38, cells were stimulated for 30 minutes with plate-bound α-CD3 (10 μg/mL) and α-CD28 (3 μg/mL), fixed with 4% paraformaldehyde, and permeabilized in methanol on ice. Flow cytometry was conducted with a FACSCalibur using CellQuest Pro 5.2.1 software (BD Biosciences). Data were analyzed with FlowJo 9.2 software (TreeStar).
Induction of EAE and CIA
EAE was induced by subcutaneous injection of MOG35–55 peptide emulsified in Complete Freund Adjuvant (CFA; Hooke Laboratories) at 2 sites on the lower back and intraperitoneal injection of 100 μL of pertussis toxin, with a second intraperitoneal injection of pertussis toxin the next day. The severity of EAE was scored as follows: 0, no obvious changes; 1, limp tail; 2, limp tail and weakness of hind legs; 3, limp tail and complete paralysis of hind legs; 4, limp tail, complete hind leg and partial front leg paralysis; and 5, complete paralysis. For induction of CIA, 10-week-old H-2r Gadd45α−/− and p38αβY323F mice were injected with 100 μg bovine type II collagen emulsified in CFA at the base of the tail, and 21 days later boosted with 100 μg bovine type II collagen emulsified in Incomplete Freund Adjuvant. Each paw was scored as follows: 0, no evidence of erythema; 1, erythema and mild swelling confined to tarsals; 2, erythema and mild swelling extending from the ankle to midfoot; 3, swelling extending from the ankle to metatarsal joints; and 4, severe swelling/ankylosis of the whole foot.
Results
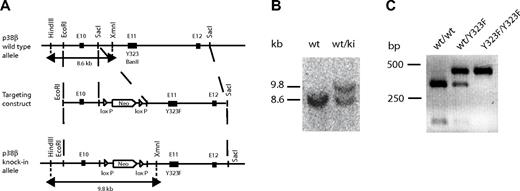
Generation of p38βY323F mice
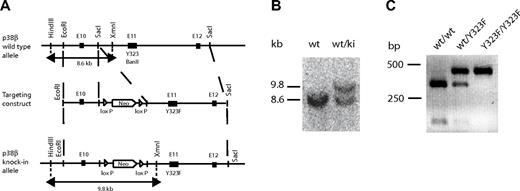
To assess the contribution of p38β to T-cell activation, we generated knockin mice in which p38β Tyr-323 was replaced with Phe (p38βY323F). A homologous recombinant targeting vector containing a neo-resistance cassette and the Y323F mutation was constructed. Introduction of the Y323F mutation caused the deletion of a BanII-specific restriction site, which was used to test for the presence of the mutation (Figure 1A). Embryonic stem cells [(129 × C57BL/6)F1] were transfected with the targeting vector, and recombinant embryonic stem clones were injected into C57BL/6 blastocysts. The presence of the p38βY323F allele was determined by Southern blot using HindIII and XmnI restriction enzymes. This digestion yielded a sequence containing both parts of the construction vector and the endogenous p38β gene. The WT band detected by Southern blot was 8.6 kb, and the knockin band was 9.8 kb because of the presence of the neo-cassette (Figure 1B). Insertion of the Y323F mutation was confirmed by BanII digestion, which failed to cleave the p38β PCR product (Figure 1C). Homozygous p38βY323F mice were viable, fertile, and of normal size and weight.
Generation of p38βY323F mice. (A) A 5.1-kb genomic fragment of the mouse p38β locus (top) was cloned using SacI specific restriction sites and used to create the targeting vector (middle), into which a Y323F mutation and LoxP-flanked neomycin resistance gene were inserted. Insertion of the Y323F mutation deleted a BanII restriction site in the knockin allele. XmnI and HindIII specific sites used for Southern blot screening for the knockin allele. (B) Genotyping of p38βY323F mice was performed by Southern blot, yielding a 8.6-kb band that indicates the presence of WT allele (wt) and a 9.8-kb band representing the knockin (ki) allele. (C) Digestion of the WT p38β PCR product with BanII yielded a 274- and 101-bp band, and digestion of the p38βY323F mutant yielded an uncut 375-bp band lacking the BanII restriction site.
Generation of p38βY323F mice. (A) A 5.1-kb genomic fragment of the mouse p38β locus (top) was cloned using SacI specific restriction sites and used to create the targeting vector (middle), into which a Y323F mutation and LoxP-flanked neomycin resistance gene were inserted. Insertion of the Y323F mutation deleted a BanII restriction site in the knockin allele. XmnI and HindIII specific sites used for Southern blot screening for the knockin allele. (B) Genotyping of p38βY323F mice was performed by Southern blot, yielding a 8.6-kb band that indicates the presence of WT allele (wt) and a 9.8-kb band representing the knockin (ki) allele. (C) Digestion of the WT p38β PCR product with BanII yielded a 274- and 101-bp band, and digestion of the p38βY323F mutant yielded an uncut 375-bp band lacking the BanII restriction site.
Quantitating relative contributions of p38α and p38β in TCR-stimulated T cells
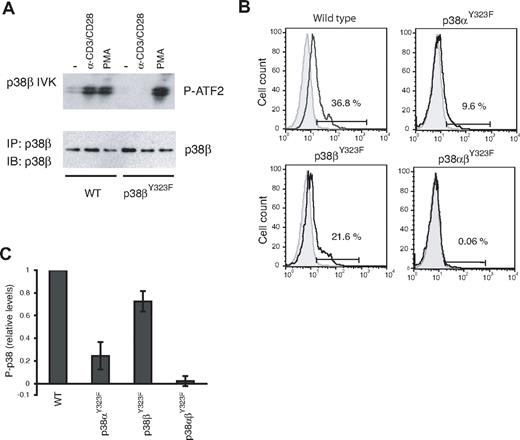
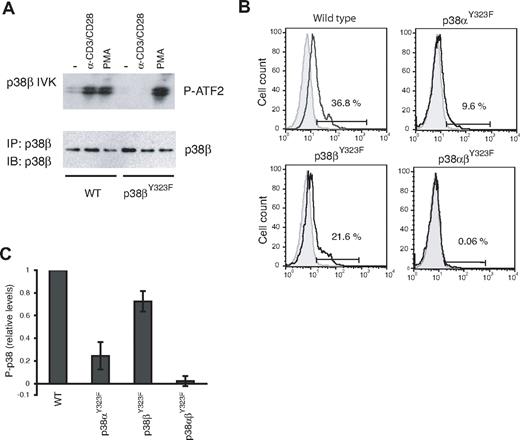
T cells from p38βY323F mice were examined for induction of p38β activity in response to TCR signaling using an in vitro kinase assay. Stimulation with anti-CD3/anti-CD28 resulted in increased p38β activity in WT but not p38βY323F T cells (Figure 2A). This defect was specific to the alternative activation pathway because p38β activity was induced to similar levels in T cells of both genotypes by PMA, which activates the classic MAPK cascade. TCR-induced p38α activity was normal (data not shown). These results are the reciprocal of those obtained with p38αY323F T cells, in which p38β but not p38α was activated via the TCR.8
p38 isoform activity and the relative contribution of p38α and p38β in TCR-stimulated T cells. (A) T cells purified from lymph nodes of WT or p38βY323F mice were stimulated with plate-bound anti-α-CD3/α-CD28 antibodies for 30 minutes or with PMA for 10 minutes. p38β was specifically immunoprecipitated from whole cell lysates, and in vitro kinase (IVK) assays were performed with ATF-2 as substrate. (B) Unstimulated (gray filled histograms) or α-CD3/α-CD28-stimulated (solid line) T cells from mice of the indicated genotypes were stained for intracellular phospho-p38 at 30 minutes. (C) Summary of the fraction of T cells expressing phospho-p38 after stimulation with α-CD3/α-CD28, relative to WT (n = 5 mice per group).
p38 isoform activity and the relative contribution of p38α and p38β in TCR-stimulated T cells. (A) T cells purified from lymph nodes of WT or p38βY323F mice were stimulated with plate-bound anti-α-CD3/α-CD28 antibodies for 30 minutes or with PMA for 10 minutes. p38β was specifically immunoprecipitated from whole cell lysates, and in vitro kinase (IVK) assays were performed with ATF-2 as substrate. (B) Unstimulated (gray filled histograms) or α-CD3/α-CD28-stimulated (solid line) T cells from mice of the indicated genotypes were stained for intracellular phospho-p38 at 30 minutes. (C) Summary of the fraction of T cells expressing phospho-p38 after stimulation with α-CD3/α-CD28, relative to WT (n = 5 mice per group).
Activated p38 can be quantitated by flow cytometry using antibodies that detect p38 Thr180 phosphorylation. It has not been possible to determine the relative contributions of p38α versus p38β in cells because antibodies that detect the phosphorylated and activated species cannot distinguish between these isoforms. However, because the Y323F substitution prevents activation loop phosphorylation of only the mutated isoform, the p38α and p38β knockin mice allowed us to quantitate p38 isoform activation in vivo. T cells from WT, p38αY323F, p38βY323F, and p38αβY323F mice were stimulated with plate-bound anti-CD3 and anti-CD28 and stained with phospho-specific p38 antibodies. A representative experiment is shown in Figure 2B. Activation-induced total P-p38 was reduced in T cells from both knockin mice, but clearly to a greater extent in p38αY323F than p38βY323F T cells. Little, if any, P-p38 was detected in double knockin T cells, demonstrating that the alternative pathway accounts for the vast majority of p38 activation subsequent to stimulation via the TCR. Results from 5 independent experiments revealed that the relative contribution of p38α to total p38 activity after TCR stimulation is ∼ 70%, with p38β accounting for the remainder (Figure 2C). It is noteworthy that the contribution of p38β to total T-cell p38 activity is substantial and higher than one would expect from the relative levels of p38 isoform mRNA in CD4 T cells, where it was judged that p38β was present at 2%-5% of p38α levels.26
Alternative p38 activation is a positive regulator of T-cell proliferation
Alternative activation of both p38 isoforms is abrogated in p38αβY323F mice, which allowed us to ask what biologic functions are downstream of TCR-activated p38. Previous studies of p38αY323F T cells showed a modest delay in the onset and extent of proliferation.8 Given that p38β makes a substantial contribution to total T-cell p38 activity (Figure 2C), we asked whether p38β could partially compensate for the lack of p38α. WT, p38αY323F, p38βY323F, and p38αβY323F T cells were stimulated with various concentrations of anti-CD3 plus a constant amount of anti-CD28 and proliferation measured by [3H]thymidine incorporation after 48 hours. Whereas the p38αY323F and p38βY323F T cells proliferated slightly less well than WT T cells, T cells in which neither p38 isoform could be activated proliferated much less well, having a 3- to 5-fold shift in the dose-response curve to the right compared with WT T cells (Figure 3A). Previous results with p38αY323F T cells found no differences in induction of IL-2 expression compared with WT cells,8 which was true for TCR-stimulated p38αβY323F T cells as well (data not shown; L.J. and J.D.A., unpublished observations, 2010). Therefore, p38α and p38β play partially redundant and positive roles in T-cell proliferation.
T-cell proliferationand Th skewingareimpaired in p38αβY323F T cells. (A) T cells from mice of the indicated genotypes were stimulated with various concentrations of plate-bound α-CD3 and α-CD28 (2 μg/mL) for 48 hours and proliferation assessed by [3H]thymidine incorporation. The figure represents the mean ± SEM from 3 independent experiments. (B) WT (solid line) and p38αβY323F (dotted line) T cells were skewed toward Th1 for 6 days, rested overnight, and restimulated with α-CD3 or PMA and ionomycin (P + I) for 6 hours. IFN-γ expression was detected by intracellular staining. The gray filled histogram indicates unstimulated WT T cells. (C) Percentage of WT (black bar) and p38αβY323F (white bar) Th1 IFN-γ–positive cells from 3 independent experiments. (D) Th2- and (E) Th17-differentiated WT and p38αβY323F CD4+ T cells were stimulated as indicated and after 6 hours were intracellularly stained for IL-10 and IL-4 (D) or IL-17 and IFN-γ (E). The numbers indicate the percentage of cells in the corresponding quadrant. (F-G) Summary of IL-4– and IL-10–positive (F) or IL-17–positive (G) WT (black bars) and p38αβY323F (white bars) effectors from 3 independent experiments. Data are mean ± SEM. N.S. indicates not significant.
T-cell proliferationand Th skewingareimpaired in p38αβY323F T cells. (A) T cells from mice of the indicated genotypes were stimulated with various concentrations of plate-bound α-CD3 and α-CD28 (2 μg/mL) for 48 hours and proliferation assessed by [3H]thymidine incorporation. The figure represents the mean ± SEM from 3 independent experiments. (B) WT (solid line) and p38αβY323F (dotted line) T cells were skewed toward Th1 for 6 days, rested overnight, and restimulated with α-CD3 or PMA and ionomycin (P + I) for 6 hours. IFN-γ expression was detected by intracellular staining. The gray filled histogram indicates unstimulated WT T cells. (C) Percentage of WT (black bar) and p38αβY323F (white bar) Th1 IFN-γ–positive cells from 3 independent experiments. (D) Th2- and (E) Th17-differentiated WT and p38αβY323F CD4+ T cells were stimulated as indicated and after 6 hours were intracellularly stained for IL-10 and IL-4 (D) or IL-17 and IFN-γ (E). The numbers indicate the percentage of cells in the corresponding quadrant. (F-G) Summary of IL-4– and IL-10–positive (F) or IL-17–positive (G) WT (black bars) and p38αβY323F (white bars) effectors from 3 independent experiments. Data are mean ± SEM. N.S. indicates not significant.
Alternative p38 activation, cytokine production, and Th skewing
Production of the proinflammatory cytokines IFN-γ and TNF-α in TCR-stimulated unskewed primary p38αY323F T cells was reduced to a small degree.8 Intracellular staining of IFN-γ and TNF-α in TCR-activated p38βY323F T cells found that production of these cytokines was also reduced, but to a lesser extent than in p38αY323F cells, and that the p38αβY323F double-knockin T cells had reductions only slightly greater than p38αY323F T cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, unlike T-cell proliferation, the contribution of p38 activity to the production of IFN-γ and TNF-α by unskewed cells is largely mediated by p38α.
Previous results with T cells from p38αY323F mice found normal Th1 skewing in vitro, as judged by IFN-γ production in response to stimulation with PMA and ionomycin (P + I).8 The question of what role p38 might have in Th skewing was revisited with mice completely lacking the alternative pathway by stimulating purified WT and p38αβY323F T cells with plate-bound anti-CD3/anti-CD28 antibodies in the presence of Th1-, Th2-, or Th17-skewing cytokines. After 6 days, the cells were washed, rested overnight, and restimulated with either with plate-bound anti-CD3 or P + I for 6 hours and stained for intracellular cytokines (Figure 3B-G). WT cells cultured under Th1-skewing conditions had ∼ 48% and 59% IFN-γ–positive cells after TCR and P + I stimulation, respectively (Figure 3B-C). The number of IFN-γ+ cells was reduced ∼ 40% in p38αβY323F Th1 cells in response to either stimulus. Moreover, the amount of IFN-γ produced per cell, as judged by mean florescence intensity, was reduced approximately 3-fold (mean florescence intensity of TCR stimulated cells: 74 [WT] vs 22 [p38αβY323F]; of P + I stimulated cells: 67 [WT] vs 27 [p38αβY323F]; Figure 3B). The finding that the IFN-γ response to P + I, a stimulus that bypasses the TCR and activates the classic MAPK cascade, is reduced was unexpected given the previous results with the p38αY323F knockin mice in which the response was normal.8 Given that there was no effect on Th1 skewing in p38βY323F T cells (data not shown), these results indicate that p38α and p38β have an important and redundant role in this process. The lack of alternatively activated p38 had a very different effect on Th2 skewing. The number of Th2-skewed cells producing IL-4 was similar between WT and p38αβY323F cells (Figure 3D,F). There was a small but reproducible increase in the number of p38αβY323F Th2 cells that produced IL-10 compared with WT cells. Th17 differentiation was induced in vitro with TGF-β and IL-6 for 6 days, and on restimulation with anti-CD3 or P + I both WT and p38αβY323F cells produced IL-17 but not IFN-γ. However, the percentage of IL-17–producing cells was decreased in p38αβY323F Th17-skewed cells by 20%-40% (Figure 3E,G). Th1 and Th17 skewing was impaired in the absence of TCR-induced p38 activation.
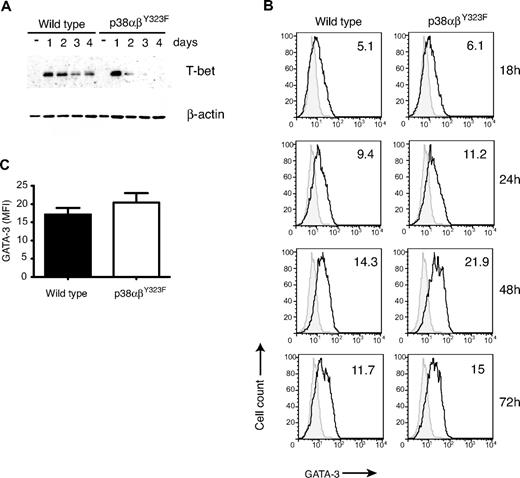
T helper cell differentiation is driven by transcription factors T-bet (toward a Th1 phenotype) and GATA-3 (toward Th2).27 Because we observed decreased production of Th1 cytokines by skewed p38αβY323F effectors, we investigated the levels of T-bet and GATA-3 achieved in these cells. WT and p38αβY323F cells were cultured under Th1- or Th2-skewing conditions, and on days 1 through 4 the cells were harvested and either lysed and assayed for T-bet expression by Western blot or fixed and analyzed for GATA-3 expression by flow cytometry. WT T cells expressed high levels of T-bet on day 1 and day 2; and although declining thereafter, the protein was still easily detectable on day 4 (Figure 4A). p38αβY323F T cells also expressed T-bet on day 1, but the level rapidly declined by day 2, and the signal was low to undetectable by day 4. On the other hand, p38αβY323F cells cultured under Th2 conditions expressed slightly more GATA-3 than WT cells at later time points (Figure 4B-C). Diminished expression of IFN-γ, TNF-α, and T-bet in p38αβY323F Th1 cells and a small but reproducible prolongation of GATA-3 elevation and production of IL-10 in p38αβY323F Th2 cells point to a role for TCR-induced p38 activity during Th skewing as well as in effector cell function.
T-bet and GATA-3 in skewed p38αβY323F Th cells. (A) WT and p38αβY323F T cells were harvested each day during Th1 skewing, lysed, and T-bet expression detected by immunoblotting, followed by reblotting with anti–β-actin. One of 3 experiments with similar results is shown. (B) WT and p38αβY323F T cells were cultured under Th2-skewing conditions, fixed at the indicated times, and GATA-3 expression detected by intracellular staining. Gray filled histograms represent staining with secondary antibody only. The numbers in the graph indicate mean fluorescence intensity. (C) Summary of GATA-3 expression in cells differentiated under Th2-skewing conditions for 48 hours (mean ± SEM; n = 4).
T-bet and GATA-3 in skewed p38αβY323F Th cells. (A) WT and p38αβY323F T cells were harvested each day during Th1 skewing, lysed, and T-bet expression detected by immunoblotting, followed by reblotting with anti–β-actin. One of 3 experiments with similar results is shown. (B) WT and p38αβY323F T cells were cultured under Th2-skewing conditions, fixed at the indicated times, and GATA-3 expression detected by intracellular staining. Gray filled histograms represent staining with secondary antibody only. The numbers in the graph indicate mean fluorescence intensity. (C) Summary of GATA-3 expression in cells differentiated under Th2-skewing conditions for 48 hours (mean ± SEM; n = 4).
T-cell hyperproliferation and spontaneous autoimmunity in Gadd45α−/− mice is ablated by introducing p38αY323F
Unchecked production of inflammatory cytokines is implicated in development of chronic autoimmune conditions, such as multiple sclerosis and rheumatoid arthritis.18,19,28,29 In resting T cells, Gadd45α inhibits the spontaneous activity of Tyr-323-phosphorylated p38, and Gadd45α-deficient mice have constitutively active T-cell p38 and, as they age, develop a systemic autoimmune disease characterized by high titers of anti-dsDNA and antihistone antibodies, glomerulonephritis, and proteinuria.9,10 We had originally hypothesized that the T-cell hyperproliferation and autoimmunity in Gadd45α-deficient mice were the result of spontaneous activation of p38,10 but at that time there was no means to directly test this. This can be tested now, however, by crossing p38αY323F with Gadd45α−/− mice to generate Gadd45α−/− (GKO)/p38αY323F animals. Spontaneous p38α activity was reduced in T cells from GKO/p38αY323F mice compared with Gadd45α-deficient animals and was not up-regulated in response to TCR signaling (supplemental Figure 2). As previously shown,10 Gadd45α-deficient T cells proliferated better than WT T cells in response to TCR-mediated stimulation. TCR-mediated proliferation was reduced by introduction of p38αY323F mutation to that of, or even a bit less than, WT levels (Figure 5A). The proliferation of GKO/p38αY323F T cells was still greater than that of p38αY323F T cells, consistent with a role for the residual alternatively activated p38β in these cells. This was indeed the case because GKO/p38αβY323F T cells proliferated less well than WT cells (Figure 5B). In this case, the proliferation of GKO/p38αβY323F T cells was reduced to the level of p38αβY323F T cells, confirming that the hyperproliferation of Gadd45α-deficient T cells is the result of their spontaneously elevated p38 activity.
Introduction of p38αY323F mutation into Gadd45αKO mice abolishes the spontaneous development of autoimmunity. (A) T cells from mice of the indicated genotypes were stimulated with plate-bound α-CD3 (5 μg/mL) and α-CD28 (2 μg/mL), and [3H]thymidine was added to the cultures 16 hours before the cells were harvested at the indicated time points (n = 3). (B) T cells were stimulated with α-CD28 (2 μg/mL) and various amounts of α-CD3 for 48 hours, and proliferation was assessed by [3H]thymidine incorporation (n = 2). (C) WT, Gadd45αKO (GKO), and GKO/p38αY323F mice were bled at 6, 9, 12, and 15 months of age, and the serum was assayed for antihistone (left panel) or anti-dsDNA (right panel) antibodies. Each point represents mean ± SEM; n = 15. (D) Serum obtained from mice at 12 months of age was assayed for the presence of IFN-α by enzyme-linked immunosorbent assay. P values were calculated by t test; n = 15. (E) Twelve-month-old mice were screened for the presence of proteinuria. The data represent the mean ± SEM; n = 15. P value was determined by t test. N.S. indicates not significant.
Introduction of p38αY323F mutation into Gadd45αKO mice abolishes the spontaneous development of autoimmunity. (A) T cells from mice of the indicated genotypes were stimulated with plate-bound α-CD3 (5 μg/mL) and α-CD28 (2 μg/mL), and [3H]thymidine was added to the cultures 16 hours before the cells were harvested at the indicated time points (n = 3). (B) T cells were stimulated with α-CD28 (2 μg/mL) and various amounts of α-CD3 for 48 hours, and proliferation was assessed by [3H]thymidine incorporation (n = 2). (C) WT, Gadd45αKO (GKO), and GKO/p38αY323F mice were bled at 6, 9, 12, and 15 months of age, and the serum was assayed for antihistone (left panel) or anti-dsDNA (right panel) antibodies. Each point represents mean ± SEM; n = 15. (D) Serum obtained from mice at 12 months of age was assayed for the presence of IFN-α by enzyme-linked immunosorbent assay. P values were calculated by t test; n = 15. (E) Twelve-month-old mice were screened for the presence of proteinuria. The data represent the mean ± SEM; n = 15. P value was determined by t test. N.S. indicates not significant.
Gadd45α-deficient mice develop mild systemic autoimmunity, especially female animals, and begin to die at approximately 16 months of age.9 The animals in that study were littermates that were intercrossed from the (129 × C57BL/6)F1 stage. We subsequently backcrossed those animals to C57BL/6 for 8 generations and found that they no longer died prematurely, and that although still present, signs of autoimmunity were less pronounced. This is consistent with other reports that the 129 background is more prone to autoimmunity than C57BL/6.30 Evidence of autoimmunity was quantitated by determining the titers of dsDNA and histone antibodies in the sera of aging GKO, p38αY323F, and GKO/p38αY323F female mice (n = 15 per group). GKO mice had elevated antibodies against histone and dsDNA compared with WT by 6 (antihistone) or 9 (anti-dsDNA) months of age (Figure 5C). Notably, introduction of p38αY323F resulted in titers that were similar to WT levels. Elevated levels of autoantibodies can trigger TLR-7 and TLR-9 signaling, leading to increased production of IFN-α by plasmacytoid dendritic cells.31 In agreement, we found increased levels of circulating IFN-α in GKO mice that were reduced to normal levels by the introduction of p38αY323F (Figure 5D). Autoantibodies can lead to glomerulonephritis, which was detected in the GKO mice on the 129 × C57BL/6 background.9 Consistent with the moderating influence of the C57BL/6 background on autoimmunity, the B6 GKO mice had no histologic evidence of glomerulonephritis (data not shown; L.J. and J.D.A., unpublished observations, 2010), but they did have elevated levels of protein in the urine (Figure 5E). This too was reversed by introduction of p38αY323F. Hence, introduction of the p38αY323F mutation into mice lacking Gadd45α abolished signs of autoimmunity in aging mice, indicating that activation of alternatively activated p38α in T cells was an essential etiologic factor in the development of disease.
Disruption of alternative T-cell p38 activation reduces the severity of autoimmunity and inflammation in CIA and EAE
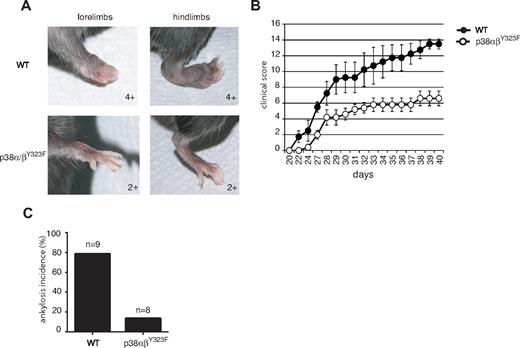
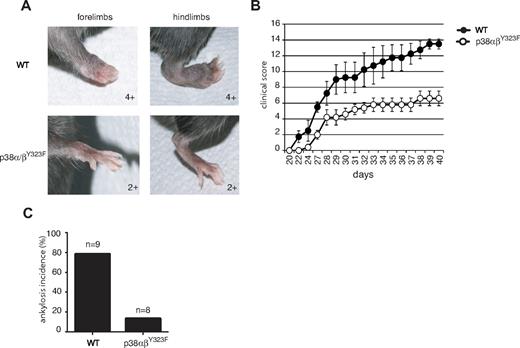
Rheumatoid arthritis is an inflammatory autoimmune disorder characterized by high levels of TNF-α, IL-1, and other inflammatory cytokines in rheumatoid joints.18 The importance of p38 in the production of TNF-α23,32,33 prompted us to test the role of TCR-mediated p38 activation in CIA, a mouse model of rheumatoid arthritis. WT and p38αβY323F C57BL/6 mice were backcrossed onto B10.RIII to obtain animals homozygous for H-2r, the MHC haplotype that provides susceptibility to CIA.34 Ten-week-old mice were immunized with type II collagen in CFA at the base of the tail and boosted with type II collagen in Incomplete Freund Adjuvant 21 days later. WT animals showed the first signs of disease one day after the second injection, whereas no signs were detected in p38αβY323F animals until 6 days had passed (Figure 6B). Moreover, arthritis progressed in the WT animals to a much greater degree of severity than in p38αβY323F mice. This was exemplified by that fact that most WT animals developed ankylosis and loss of function involving 2 or more limbs, compared with only mild swelling (score of 2+) and rare ankylosis in p38αβY323F mice (Figure 6A,C).
p38αβY323F mice are less susceptible to CIA than WT mice. WT (n = 4) and p38αβY323F (n = 5) mice were immunized with bovine type II collagen in CFA and 3 weeks later boosted with collagen in Incomplete Freund Adjuvant. (A) Representative images of limbs of WT animals that developed ankylosis, and p38αβY323F mice that developed only mild swelling. (B) The clinical scores are shown over time. These data are representative of 2 independent experiments. (C) The percentage of mice that developed ankylosis in 2 independent experiments: WT, n = 9; p38αβY323F, n = 8.
p38αβY323F mice are less susceptible to CIA than WT mice. WT (n = 4) and p38αβY323F (n = 5) mice were immunized with bovine type II collagen in CFA and 3 weeks later boosted with collagen in Incomplete Freund Adjuvant. (A) Representative images of limbs of WT animals that developed ankylosis, and p38αβY323F mice that developed only mild swelling. (B) The clinical scores are shown over time. These data are representative of 2 independent experiments. (C) The percentage of mice that developed ankylosis in 2 independent experiments: WT, n = 9; p38αβY323F, n = 8.
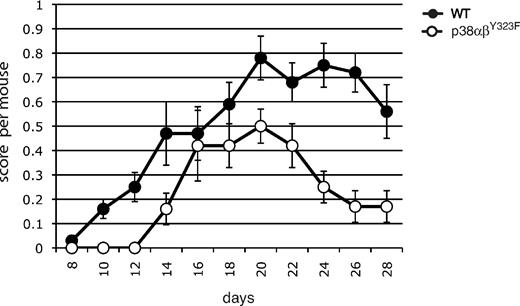
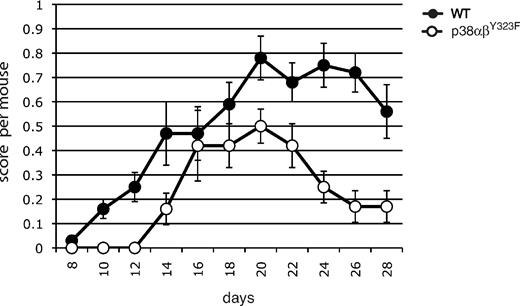
To ask whether a role for alternatively activated p38 might be extended to other examples of autoimmunity, we characterized the development and progression of EAE, a mouse model of the inflammatory autoimmune disease multiple sclerosis. Elevated phosphorylated (active) p38 activity has been found in T cells in EAE lesions,21 and recent findings suggest that infiltration of the central nervous system with IL-17– and IFN-γ–producing T cells mediates inflammation leading to clinical signs of EAE.19 EAE was induced in WT and p38αβY323F mice by immunization with MOG35–55 (Figure 7). The onset of EAE was delayed in p38αβY323F mice compared with WT animals. The clinical severity was lower throughout the course of the experiment, and the p38αβY323F animals recovered much earlier than their WT counterparts. Taken together, these results demonstrate that T-cell p38 activation downstream of the TCR contributes to autoimmune inflammatory disease.
p38 αβY323F mice have reduced susceptibility to EAE. WT or p38αβY323F mice were injected with MOG35-55 in CFA. EAE clinical scores were determined between days 8 and 28 after injection, and each point represents the mean per animal ± SEM collected from 3 independent experiments (WT, n = 13; p38αβY323F, n = 8).
p38 αβY323F mice have reduced susceptibility to EAE. WT or p38αβY323F mice were injected with MOG35-55 in CFA. EAE clinical scores were determined between days 8 and 28 after injection, and each point represents the mean per animal ± SEM collected from 3 independent experiments (WT, n = 13; p38αβY323F, n = 8).
Discussion
The physiologic function of the T cell-specific alternative p38 activation pathway, as opposed to the MAPK-dependent pathway, is an open question. Most of our understanding of the biologic functions of p38 comes from studies using inhibitors of p38 catalytic activity (in particular p38α and p38β), the interpretations of which can be confounded by off-target effects. Knockout approaches have been problematic because loss of p38α, the major isoform in most cells, results in midgestational lethality because of defects in angiogenesis, placental insufficiency, and anemia.35 When such mice were allowed to come to term by using tetraploid rescue, no gross abnormalities were observed. Furthermore, p38α-deficient T cells in chimeric mice produced by RAG-deficient blastocyst complementation developed normally and proliferated well to mitogenic stimuli.36 Mice deficient in p38β had no obvious phenotype.37 Because loss of p38α results in embryonic death, possible in vivo redundancy between the α- and β-isoforms has not been explored. We have approached the question of how the alternative p38 activation regulates T-cell and immune responses by generating knockin mice in which p38 Tyr-323 has been replaced with a Phe and therefore cannot be phosphorylated by ZAP70. Because p38 catalytic function is intact and the classic MAPK activation pathway is undisturbed, these mice develop normally. The various p38 mutations allowed us to directly measure activation of each isoform in response to TCR-mediated stimulation. Surprisingly, we found that p38β makes a substantial contribution: ∼ 30% of the total p38 phosphorylated on the activation loop. Notably, little, if any, p38 phosphorylation was detected in p38αβY323F T cells, demonstrating that the alternative pathway is the sole mediator of p38α and p38β activation in response to TCR signaling in primary cells. Consistent with its substantial contribution to total p38 activation, p38β did indeed have functions that are redundant with p38α. Whereas single p38αY323F and p38βY323F cells had only a modest decrease in TCR-induced proliferation8 (Figure 3A), p38αβY323F T cells had a much more pronounced defect, with up to a 10-fold shift in the dose-response curve. We also observed previously unappreciated changes in Th1 differentiation with p38αβY323F T cells. Like p38α-deficient CD4+ T cells,36 p38αY323F CD4+ T cells were able to differentiate into Th1 type cells and secrete normal amounts of IFN-γ in response to PMA plus ionomycin but reduced amounts in response to TCR signaling, indicating that Th1 skewing had occurred but expression of IFN-γ required p38α signaling.8 In contrast, ablation of all p38 alternative activation in p38αβY323F mice had an adverse effect on Th1 differentiation, as evidenced by decreased IFN-γ production in response to PMA plus ionomycin. Reciprocal data were obtained with Gadd45α-deficient T cells, in which case T cells are hyperproliferative9 and produce more IFN-γ and IL-17 on activation (supplemental Figure 3). Together, these results demonstrate that TCR-activated p38α and p38β are important and redundant positive regulators of T-cell proliferation and Th skewing.
Uncontrolled production of inflammatory cytokines is a major contributing factor in the chronic inflammation typical of autoimmune diseases.38 Although there is a great deal of information about the connection between p38 and inflammation, relatively little is known about the specific role of T-cell p38, or in particular about the role of p38 activated in response to TCR signaling, issues that could be addressed with the p38 knockin mice. Several models of autoimmunity and inflammation were examined. Gadd45α-deficient elevated alternatively activated T-cell p38, T-cell hyperproliferation and develop autoimmunity.9,10 Introduction of p38αY323F into Gadd45α knockout mice (GKO/p38αY323F) not only prevented T-cell hyperproliferation, it reduced antihistone and anti-dsDNA antibodies and proteinuria to WT levels, despite the presence of WT p38β. Therefore, T-cell p38α activated by the alternative pathway is necessary for the development of autoimmunity in Gadd45α knockout mice.
CIA and EAE are widely used murine models for rheumatoid arthritis and multiple sclerosis, respectively. In both cases, lack of TCR-signaled p38 activation resulted in substantial reductions in the severity of disease. It is noteworthy that Gadd45α mice, in which T-cell p38 is constitutively active, had more severe CIA and EAE than WT counterparts (L.J. and J.D.A., unpublished observations, 2010). Characterization of T-cell responses to activation suggests several mechanisms that may underlie the resistance of p38αβY323F mice. First, these T cells do not proliferate as well as WT cells in response to TCR-mediated stimulation, so the T-cell response to the disease-initiating antigens would be blunted. Second, IFN-γ and IL-17 production was decreased in p38αβY323F and increased in Gadd45αKO, Th-skewed cells. These cytokines are implicated in the development of EAE20 and multiple sclerosis,29 and suppression of Th1/Th17 cytokine production is one of the therapeutic approaches being applied to multiple sclerosis and rheumatoid arthritis.38,39 The positive role of p38 in the production of inflammatory cytokines has been a rationale for the use of p38 inhibitors to alleviate chronic inflammation. However, despite some positive results,40 global inhibition of p38 activity is associated with side effects, such as infection and gastrointestinal disturbances,41-43 possibly because of the ubiquitous expression of p38 and its large variety of functions.44,45 Our data provide direct genetic evidence that T-cell p38 activated in response to TCR signaling contributes to inflammatory autoimmune responses. Because the TCR activates p38 by a mechanism distinct from other receptors and stresses, these results raise the possibility that specific interference with the alternative p38 activation pathway might be beneficial in inflammatory autoimmune conditions. Furthermore, the preservation of normal p38 activation in response to non–TCR-mediated stimuli and in non-T cells might mitigate unwanted side effects. Gadd45α, which inhibits p38 activated by the alternative pathway but not the MAPK cascade,10 provides evidence that pathway-specific inhibition is possible.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lino Tessarollo and Eilleen Southon (Gene Targeting Facility, National Cancer Institute-Frederick) for their assistance during generation of p38βY323F mice and Ehydel Castro for help with mouse disease models. The p38αβY323F double-mutant mice will be made available at The Jackson Laboratory Repository (JAX stock no. 012566; http://jaxmice.jax.org/query or http://jaxmice.jax.org/query).
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: P.R.M. and N.D.S. designed and made the mutant p38 constructs used to make the knockin mice; L.J. and M.L.G.T. performed experiments, analyzed results, and made the figures; and L.J. and J.D.A. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan D. Ashwell, Laboratory of Immune Cell Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bldg 37, Rm 3002, Bethesda, MD 20892; e-mail: jda@pop.nci.nih.gov.

![Figure 3. T-cell proliferation and Th skewing are impaired in p38αβY323F T cells. (A) T cells from mice of the indicated genotypes were stimulated with various concentrations of plate-bound α-CD3 and α-CD28 (2 μg/mL) for 48 hours and proliferation assessed by [3H]thymidine incorporation. The figure represents the mean ± SEM from 3 independent experiments. (B) WT (solid line) and p38αβY323F (dotted line) T cells were skewed toward Th1 for 6 days, rested overnight, and restimulated with α-CD3 or PMA and ionomycin (P + I) for 6 hours. IFN-γ expression was detected by intracellular staining. The gray filled histogram indicates unstimulated WT T cells. (C) Percentage of WT (black bar) and p38αβY323F (white bar) Th1 IFN-γ–positive cells from 3 independent experiments. (D) Th2- and (E) Th17-differentiated WT and p38αβY323F CD4+ T cells were stimulated as indicated and after 6 hours were intracellularly stained for IL-10 and IL-4 (D) or IL-17 and IFN-γ (E). The numbers indicate the percentage of cells in the corresponding quadrant. (F-G) Summary of IL-4– and IL-10–positive (F) or IL-17–positive (G) WT (black bars) and p38αβY323F (white bars) effectors from 3 independent experiments. Data are mean ± SEM. N.S. indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/12/10.1182_blood-2011-01-333039/4/m_zh89991176660003.jpeg?Expires=1766088020&Signature=3xEpteQm1PEmblbmHa3emzyuqiajDel5lJc8IV4vdnu17XH1vXYv4H9-8ppSsphb9uMUDjBJRXyHXlKiFbjvu9TV9znJeBlfyoNIQjW78KQnmwiaEoCpj3WiKV~Q-ajoi1fDlFK9IG-gXLryeAMt8hzOEKMTogiEWyv~19LoP8sgiQ8gmyEckrhR40t~trX9LDggtHdglnSDgl2GlachA4B4z0AOP9zTH4jALCB~fbju3v~blzjwiO~QH~X1w3sdsN5uamhRNGVusvO4KmTW3WpAlN~VcdAfiuEheUaa1eUk0k0RFIEO6RZkAxblKwD~HoobxivWRTwkh3oyqM8R7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Introduction of p38αY323F mutation into Gadd45αKO mice abolishes the spontaneous development of autoimmunity. (A) T cells from mice of the indicated genotypes were stimulated with plate-bound α-CD3 (5 μg/mL) and α-CD28 (2 μg/mL), and [3H]thymidine was added to the cultures 16 hours before the cells were harvested at the indicated time points (n = 3). (B) T cells were stimulated with α-CD28 (2 μg/mL) and various amounts of α-CD3 for 48 hours, and proliferation was assessed by [3H]thymidine incorporation (n = 2). (C) WT, Gadd45αKO (GKO), and GKO/p38αY323F mice were bled at 6, 9, 12, and 15 months of age, and the serum was assayed for antihistone (left panel) or anti-dsDNA (right panel) antibodies. Each point represents mean ± SEM; n = 15. (D) Serum obtained from mice at 12 months of age was assayed for the presence of IFN-α by enzyme-linked immunosorbent assay. P values were calculated by t test; n = 15. (E) Twelve-month-old mice were screened for the presence of proteinuria. The data represent the mean ± SEM; n = 15. P value was determined by t test. N.S. indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/12/10.1182_blood-2011-01-333039/4/m_zh89991176660005.jpeg?Expires=1766088020&Signature=l0i4C0DeG8qw8m-MxjXdPlHvvJbx0onCWgYn~0ZNG306amAFyGj4P2DDSi8BoFP-yxLCjqRjq8wZ~N7qobiLE3TGCEwvT-PvVnHTcars6XDXSqQUbpR22oLBVXMV3E~lJIrLB8ZsfwhEs4Zjjxefo6pLrRiOPBFdYvEesfL1HQUm9ZYic-wb9PfXg0EIt4ezfSIogOhYmhgKwwIkE-x7XtDCeUjAKVgnpsExhS2~RJBUung2F7rS2QwaHz6WaNwnqq0MIbNjrpJc4gp8k-vfjCa5OjdYGZYSV6U4X4ltcY6UivMJruDrxMEfK~xy5cDyc-ywpOmgQNuxX9zlSRkVTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. T-cell proliferation and Th skewing are impaired in p38αβY323F T cells. (A) T cells from mice of the indicated genotypes were stimulated with various concentrations of plate-bound α-CD3 and α-CD28 (2 μg/mL) for 48 hours and proliferation assessed by [3H]thymidine incorporation. The figure represents the mean ± SEM from 3 independent experiments. (B) WT (solid line) and p38αβY323F (dotted line) T cells were skewed toward Th1 for 6 days, rested overnight, and restimulated with α-CD3 or PMA and ionomycin (P + I) for 6 hours. IFN-γ expression was detected by intracellular staining. The gray filled histogram indicates unstimulated WT T cells. (C) Percentage of WT (black bar) and p38αβY323F (white bar) Th1 IFN-γ–positive cells from 3 independent experiments. (D) Th2- and (E) Th17-differentiated WT and p38αβY323F CD4+ T cells were stimulated as indicated and after 6 hours were intracellularly stained for IL-10 and IL-4 (D) or IL-17 and IFN-γ (E). The numbers indicate the percentage of cells in the corresponding quadrant. (F-G) Summary of IL-4– and IL-10–positive (F) or IL-17–positive (G) WT (black bars) and p38αβY323F (white bars) effectors from 3 independent experiments. Data are mean ± SEM. N.S. indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/12/10.1182_blood-2011-01-333039/4/m_zh89991176660003.jpeg?Expires=1766503986&Signature=WHSW5ApU7L2dd6LStIx5JGERW71cKuZwb4zIj03u4r45h19ppjj6DSgfY3~fLe82DMj6DtScHwXsHntf1WAUSyJ4mjCmy4vFBu47h4dSoYefM5zLwyoWvUwodarAAagQJ8VHFuxgIVfFySJUvugPkriC5-Xgsf4Me~8uuCCwRk4fwjOzM8A1D0lCjdyLYqcokL15RDkaOSvG35KtSyx2ltFrrwGykmMCQ91nQIcRUWSJNhPSosubIRxvSe5pxWxlv7uEVsnHiiTtuVyMdLiVgIxRGMAlrMUFnQZVlIjqxAUToKN139tY3alKxi0MdkKEB82hengzw3qE6H4zOvMBJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Introduction of p38αY323F mutation into Gadd45αKO mice abolishes the spontaneous development of autoimmunity. (A) T cells from mice of the indicated genotypes were stimulated with plate-bound α-CD3 (5 μg/mL) and α-CD28 (2 μg/mL), and [3H]thymidine was added to the cultures 16 hours before the cells were harvested at the indicated time points (n = 3). (B) T cells were stimulated with α-CD28 (2 μg/mL) and various amounts of α-CD3 for 48 hours, and proliferation was assessed by [3H]thymidine incorporation (n = 2). (C) WT, Gadd45αKO (GKO), and GKO/p38αY323F mice were bled at 6, 9, 12, and 15 months of age, and the serum was assayed for antihistone (left panel) or anti-dsDNA (right panel) antibodies. Each point represents mean ± SEM; n = 15. (D) Serum obtained from mice at 12 months of age was assayed for the presence of IFN-α by enzyme-linked immunosorbent assay. P values were calculated by t test; n = 15. (E) Twelve-month-old mice were screened for the presence of proteinuria. The data represent the mean ± SEM; n = 15. P value was determined by t test. N.S. indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/12/10.1182_blood-2011-01-333039/4/m_zh89991176660005.jpeg?Expires=1766503986&Signature=yG-Ua9hwgWpgBUYqzWnBR~zci~ppSIQIN0GTIq~1NO6PuOHxO5Hsjsa19yhYX7ySJzoJYulS2wIlDpqJY0gdmE7rHxwE9cnkcidkI47bLVzW5Ac1BX7vtdJqBijvZZI2KRVWtb8ReRHLXm~mWog9h~cKwu9Enb29tgCgxxUtOK6g62rRPRVLFHxg64XWycxovxGVqpY4G0dVo8osQyzVBh-01BfR8SXjPQjfAqTh9JnxBPZWpfiKFWBggntxHgEHbYb7AlFVM7-xCm8shqDWacb0oY5K8TecuKiRXAV6WF~b~G9-FQ-rrovLvyrxYInSfk-ba0T-Cs17gaCr9QUaQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)