Abstract
Notch is a critical regulator of angiogenesis, vascular differentiation, and vascular integrity. We investigated whether Notch signaling affects macrophage function during retinal angiogenesis in mice. Retinal macrophage recruitment and localization in mice with myeloid-specific loss of Notch1 was altered, as these macrophages failed to localize at the leading edge of the vascular plexus and at vascular branchpoints. Furthermore, these retinas were characterized by elongated endothelial cell sprouts that failed to anastomose with neighboring sprouts. Using Notch reporter mice, we demonstrate that retinal macrophages localize between Dll4-positive tip cells and at vascular branchpoints, and that these macrophages had activated Notch signaling. Taken together, these data demonstrate that Notch signaling in macrophages is important for their localization and interaction with endothelial cells during sprouting angiogenesis.
Introduction
Previous studies have shown that during retinal angiogenesis, macrophages are closely associated with sprouting endothelial cells1 and facilitate anastomosis by forming bridges between neighboring sprouts.2 The Notch signaling pathway has been extensively studied in endothelial cells, where tip cells express high levels of the Notch ligand Delta-like 4 (Dll4), which signals to Notch receptors on neighboring cells to maintain their fate as nonsprouting stalk cells.3,4 The role of Notch signaling in macrophages in the developing retina has not previously been assessed.
In this study, we show that Notch signaling is important for macrophage recruitment and localization in the developing retina. We also found an increased frequency of elongated sprouts that did not anastomose with neighboring sprouts in retinas in mice with myeloid-specific loss of Notch1. Furthermore, we show Notch signal activation in macrophages that interact with Dll4-positive tip cells, and that macrophages with Notch signaling are found predominately at the vascular front and in association with vascular branchpoints. These data suggest a novel way that Notch signaling regulates retinal angiogenesis.
Methods
Notch1 mutant mice have been described.5 Mice with a conditional allele of Notch1 (Notch1flox)6 and the myeloid-specific Cre recombinase driver line (LysMCre)7 were obtained from The Jackson Laboratory. Transgenic Notch reporter mice (TNR), harboring an enhanced GFP sequence under the control of 4 tandem copies of the CBF1 binding site consensus sequence8 were also obtained from The Jackson Laboratory. All procedures were carried out according to approved protocols and guidelines established by the Columbia University Institutional Animal Care and Use Committee. Eyes from postnatal day 5 mice were fixed for 2 hours in 4% paraformaldehyde. Retinas were permeabilized in 1% BSA and 0.5% Triton X-100 overnight at 4°C and washed in PBLEC buffer (1% Triton X-100, 0.1mM MgCl2, 0.1mM CaCl2, 0.1mM MnCl2 in PBS pH 6.8), then incubated overnight in PBLEC plus isolectin-B4 (Sigma-Aldrich), anti-F4/80 (Abcam), anti-GFP (Invitrogen), or anti-Dll4 (R&D Systems). After washing, retinas were incubated with Alexa Fluor–conjugated secondary antibodies (Invitrogen), washed, and mounted on slides with Vectashield (Dako) mounting medium for visualization using a LSM Meta 510 or Nikon A1R MP Multiphoton confocal microscope.
Results/discussion
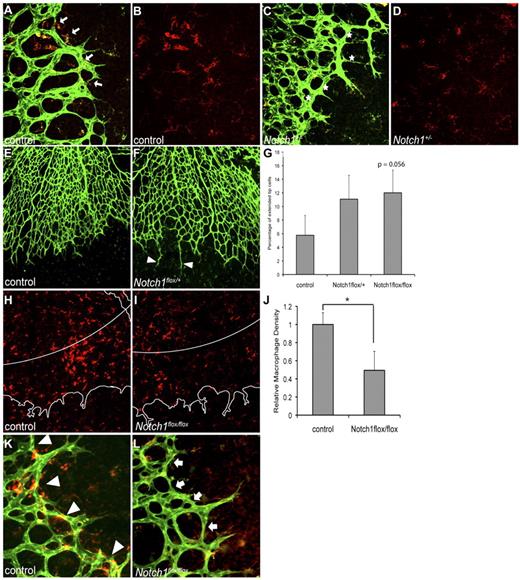
Previous studies have shown that loss of Dll4 in mice leads to excessive sprouting during retinal angiogenesis.3,4 We first investigated whether decreased expression of Notch1 would lead to a similar defect. We found increased vascular density in retinas from Notch1+/− mice compared with control littermates (Figure 1A,C and data not shown), supporting the model where Notch1 and Dll4 in endothelial cells regulate sprouting. Because macrophages are present in the retina and are known to express Notch1,9,10 we assessed macrophage recruitment and localization in retinas from Notch1+/− mice. Macrophage localization at the vascular front was altered in Notch1+/− retinas compared with control (Figure 1A-D). Macrophages in control retinas were found in close proximity to tip cells and newly formed branchpoints at the vascular front (Figure 1A-B). In contrast, macrophages were noticeably absent from many branchpoints at the vascular front in Notch1+/− retinas (Figure 1C-D). However, given the known role of Notch1 in vascular patterning, it is likely that loss of Notch1 in endothelial cells, rather than macrophages, plays a dominant role in the vascular phenotype in these retinas.
Notch1 in macrophages is important for macrophage localization during retinal angiogenesis. Whole mount P5 retinas were stained by immunohistochemistry for F4/80 or isolectin-B4 to visualize macrophages or endothelial cells, respectively. (A) Macrophages (red) in control retinas were found in close proximity to endothelial cells (green) and at vascular branchpoints (arrows). (B) F4/80 staining of image shown in panel A. Macrophages in control retinas were densely localized to the vascular front, at the leading edge of vessel migration and anastomosis. (C) Macrophages (red) in retinas from Notch1+/− mice were often not associated with vascular branchpoints (indicated with stars). (D) F4/80 staining of image shown in panel C. Macrophages in Notch1+/− retinas were scattered at the vascular front where vessel anastomosis occurs. (E-G) Retinal angiogenesis in mice with myeloid-specific reduction of Notch1 was characterized by an increased frequency of elongated sprouts that failed to anastomose with neighboring sprouts. For quantification of elongated sprouts, 10× images of retinas were used to assess the frequency of sprouts longer than 100 μm, normalized to the total number of sprouts in that field. Sprouts were defined as endothelial protrusions from the vascular front that did not branch. Results were averaged for each genotype. Error bars represent SEM of n = 4 control, n = 6 LysMCre; Notch1flox/+, and n = 4 LysMCre;Notch1flox/flox retinas. (H-L) Decreased macrophages at the retinal vascular front in mice with myeloid-specific loss of Notch1. For quantification, paneled 30× images of the entire retina were taken. To determine F4/80 staining density (shown in red), the number of red pixels within the outer 20% of the retina (indicated by white line in panels H and I, outline of vascular plexus shown in white) was counted and compared with the total area covered by vasculature within that region. The leading edge was defined as the distal 20% of vasculature measured from the center of the optic nerve to the edge of the vascular front. Error bars represent SEM of n = 4 control and n = 4 LysMCre;Notch1flox/flox retinas. *P ≤ .05. (K-L) Macrophages in control retinas were found at the vascular front and localized to vascular branchpoints (arrowheads), whereas there was decreased macrophage density at the vascular front in retinas from mice with loss of Notch1 in the myeloid lineage (arrows). Images shown at 40× (A-D,K,L), or 10× (E,F,H,I), original magnification. Images are representative of at least 3 independent experiments.
Notch1 in macrophages is important for macrophage localization during retinal angiogenesis. Whole mount P5 retinas were stained by immunohistochemistry for F4/80 or isolectin-B4 to visualize macrophages or endothelial cells, respectively. (A) Macrophages (red) in control retinas were found in close proximity to endothelial cells (green) and at vascular branchpoints (arrows). (B) F4/80 staining of image shown in panel A. Macrophages in control retinas were densely localized to the vascular front, at the leading edge of vessel migration and anastomosis. (C) Macrophages (red) in retinas from Notch1+/− mice were often not associated with vascular branchpoints (indicated with stars). (D) F4/80 staining of image shown in panel C. Macrophages in Notch1+/− retinas were scattered at the vascular front where vessel anastomosis occurs. (E-G) Retinal angiogenesis in mice with myeloid-specific reduction of Notch1 was characterized by an increased frequency of elongated sprouts that failed to anastomose with neighboring sprouts. For quantification of elongated sprouts, 10× images of retinas were used to assess the frequency of sprouts longer than 100 μm, normalized to the total number of sprouts in that field. Sprouts were defined as endothelial protrusions from the vascular front that did not branch. Results were averaged for each genotype. Error bars represent SEM of n = 4 control, n = 6 LysMCre; Notch1flox/+, and n = 4 LysMCre;Notch1flox/flox retinas. (H-L) Decreased macrophages at the retinal vascular front in mice with myeloid-specific loss of Notch1. For quantification, paneled 30× images of the entire retina were taken. To determine F4/80 staining density (shown in red), the number of red pixels within the outer 20% of the retina (indicated by white line in panels H and I, outline of vascular plexus shown in white) was counted and compared with the total area covered by vasculature within that region. The leading edge was defined as the distal 20% of vasculature measured from the center of the optic nerve to the edge of the vascular front. Error bars represent SEM of n = 4 control and n = 4 LysMCre;Notch1flox/flox retinas. *P ≤ .05. (K-L) Macrophages in control retinas were found at the vascular front and localized to vascular branchpoints (arrowheads), whereas there was decreased macrophage density at the vascular front in retinas from mice with loss of Notch1 in the myeloid lineage (arrows). Images shown at 40× (A-D,K,L), or 10× (E,F,H,I), original magnification. Images are representative of at least 3 independent experiments.
To assess the role of Notch signaling specifically in macrophages during retinal angiogenesis, we analyzed retinas from mice with loss of Notch1 in the myeloid lineage (LysMCre; Notch1flox/+).11 In contrast to mice with global reduction of Notch1 expression (Notch1+/− mice), retinal vascular density and tip cell sprouting in mice with reduction of Notch1 specifically in the myeloid lineage was not altered compared with control (Figure 1E-F and data not shown). However, we observed an increased frequency of elongated endothelial cell sprouts that did not form anastomoses with neighboring sprouts in retinas from LysMCre; Notch1flox/+ and LysMCre; Notch1flox/flox mice compared with control (Figure 1E-G), although this effect only approached statistical significance. When we quantify the frequency of elongated endothelial sprouts that do not form anastamoses in LysMCre; Notch1flox/flox mice compared with litter-matched controls, statistical significance is achieved (P = .029). Macrophage density in the retina as a whole was decreased in mice with loss of Notch1 in the myeloid lineage compared with control (data not shown). The defect in macrophage recruitment was particularly pronounced at the vascular front. We found that the density of macrophages at the leading edge of the retinal vasculature, defined as the distal 20% of the vascular plexus, was decreased 49% in mice with myeloid-specific loss of Notch1 compared with control (Figure 1H-J). The decreased macrophage staining was not because of a change in the expression of the macrophage marker F4/80 in mutant mice, as confirmed by flow cytometry (data not shown). The macrophages at the leading edge of the vascular plexus were often localized to vascular branchpoints in control retinas (Figure 1K), in contrast to LysMCre; Notch1flox/flox mutant retinas (Figure 1L). Because there were decreased macrophages at the leading edge in mice with loss of Notch1 in the myeloid lineage, many branchpoints did not have associated macrophages in mutant retinas (Figure 1L). Thus, Notch1 is important for localization of macrophages to the vascular front where there is active sprouting and endothelial cell anastomosis.
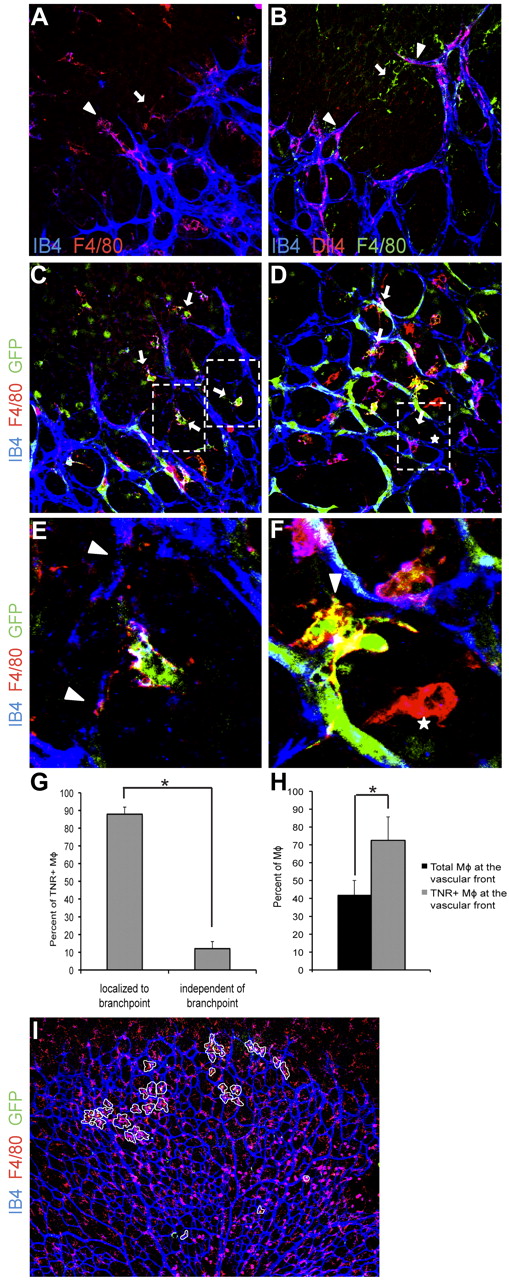
During normal retinal angiogenesis, macrophages consistently localize to tip cells and anastomosing sprouts at the vascular front (Figure 2A). As expected, we found that tip cells that interact with macrophages expressed Dll4, a tip cell marker (Figure 2B). Dll4 was not expressed by macrophages (Figure 2B). Given our results in mice with myeloid-specific reduction of Notch1, we hypothesized that Notch1 activation may be important for the association of macrophages with anastomosing sprouts. To address this, we used Notch reporter mice that express GFP downstream of a Notch-responsive promoter.8 We found that macrophages that localize between anastomosing sprouts have evidence of Notch signal activation, as determined by GFP expression (Figure 2C,E). Proximally in the vascular plexus, we found macrophages with Notch activation in close proximity to endothelial cells at vascular branchpoints (Figure 2D-G). In contrast, macrophages that were not associated with endothelial cells largely did not have Notch signal activation (Figure 2D-G). In assessing all TNR-positive macrophages in retinas, 87% of the TNR-positive macrophages were localized to vascular branchpoints (Figure 2G) whereas 13% were not, demonstrating that Notch signaling in macrophages is activated at vascular branchpoints. Furthermore, we found that roughly 40% of all macrophages are found at the vascular front, the distal fifth of the retinal vascular plexus, whereas > 70% of TNR-positive macrophages are at the vascular front (Figure 2H-I). That is, the frequency of TNR-positive macrophages found at the leading edge of the vasculature was nearly 1.8 times what would be expected by random distribution alone (Figure 2H-I). Thus during retinal angiogenesis, Notch signaling may play a role in macrophage interactions with Dll4-positive sprouts as well as regulate macrophage/endothelial cell interactions at vascular branchpoints.
Macrophages with activated Notch signaling localize to anastomosing sprouts and at vascular branchpoints. Whole mount P5 retinas. (A) F4/80 (red) and isolectin-B4 (blue) staining demonstrates localization of macrophages in close proximity to tip cells (arrowhead) and between neighboring sprouts (arrow). (B) Macrophages (green arrow) bridge between neighboring Dll4-postive (red) tip cells (arrowheads). Isolectin-B4 shown in blue. (C-F) Immunohistochemistry of retinas from Notch reporter mice expressing GFP downstream of a Notch-responsive promoter. Anti-GFP staining shown in green indicates activation of Notch signaling. Vessels were visualized with isolectin-B4 (blue). F4/80 staining shown in red. (C) Macrophages (red) with evidence of Notch signaling (arrows) localize between sprouts at the vascular front. (D) Proximally in the vascular plexus, macrophages with evidence of Notch signaling were found in close association with endothelial cells at vascular branchpoints (arrows). (E-F) Higher magnifications of the boxed areas in panels C and D. Arrowheads indicate anastomosing sprouts (E) or vascular branchpoints (F). Macrophages that were not in association with endothelial cells did not have evidence of Notch signal activation (indicated with stars). (G) The majority of TNR-positive macrophages in retinas were localized to vascular branchpoints (87.9% vs 12.07% of TNR-positive macrophages. (H-I) TNR-positive macrophages are overrepresented in the distal 20% of the retinal vasculature. Forty-two percent of all retinal macrophages were located in the distal 20% of the retinal vasculature. Of TNR-positive macrophages, 72.5% were located in the distal 20% of the retina. TNR-positive macrophages circled in white. Error bars represent data from n = 5 retinas from TNR mice. *P ≤ .05. Images in panels A through D shown at 40× original magnification, panel I shown at 4× original magnification. Data are representative of at least 3 independent experiments.
Macrophages with activated Notch signaling localize to anastomosing sprouts and at vascular branchpoints. Whole mount P5 retinas. (A) F4/80 (red) and isolectin-B4 (blue) staining demonstrates localization of macrophages in close proximity to tip cells (arrowhead) and between neighboring sprouts (arrow). (B) Macrophages (green arrow) bridge between neighboring Dll4-postive (red) tip cells (arrowheads). Isolectin-B4 shown in blue. (C-F) Immunohistochemistry of retinas from Notch reporter mice expressing GFP downstream of a Notch-responsive promoter. Anti-GFP staining shown in green indicates activation of Notch signaling. Vessels were visualized with isolectin-B4 (blue). F4/80 staining shown in red. (C) Macrophages (red) with evidence of Notch signaling (arrows) localize between sprouts at the vascular front. (D) Proximally in the vascular plexus, macrophages with evidence of Notch signaling were found in close association with endothelial cells at vascular branchpoints (arrows). (E-F) Higher magnifications of the boxed areas in panels C and D. Arrowheads indicate anastomosing sprouts (E) or vascular branchpoints (F). Macrophages that were not in association with endothelial cells did not have evidence of Notch signal activation (indicated with stars). (G) The majority of TNR-positive macrophages in retinas were localized to vascular branchpoints (87.9% vs 12.07% of TNR-positive macrophages. (H-I) TNR-positive macrophages are overrepresented in the distal 20% of the retinal vasculature. Forty-two percent of all retinal macrophages were located in the distal 20% of the retinal vasculature. Of TNR-positive macrophages, 72.5% were located in the distal 20% of the retina. TNR-positive macrophages circled in white. Error bars represent data from n = 5 retinas from TNR mice. *P ≤ .05. Images in panels A through D shown at 40× original magnification, panel I shown at 4× original magnification. Data are representative of at least 3 independent experiments.
We propose that interactions between Notch1 and Dll4 in macrophages and endothelial cells, respectively, may define a ‘bridging’ function for macrophages during vessel anastomosis. Previous studies have demonstrated that macrophages act as chaperones that guide endothelial cell anastomoses.2 Our data further elucidate a mechanism for this function, and demonstrate that Notch signaling in macrophages facilitates the steps of angiogenesis that involve fusion between sprouts. Endothelial cells in retinas from mice with myeloid-specific reduction of Notch1 are able to overcome the defect in vascular anastomosis, as the retinal vascular plexus proximal to the vascular front (where vessel remodeling occurs) appeared largely normal. However, we also found macrophages with active Notch signaling in the maturing plexus, where they were in close association with endothelial cells at vascular branchpoints. It is unknown whether Notch signaling in macrophages plays a role in stabilization of the vasculature, such that decreased Notch1 in macrophages would result in a more immature and thus unstable vascular plexus. Further studies to elucidate whether Notch signaling in macrophages affects vascular patterning during pathologic angiogenesis are warranted.
The current model of sprouting angiogenesis dictates that Dll4-positive tip cells activate Notch signaling in neighboring stalk cells to prevent excess sprouting.12 Our data suggest that Dll4-positive endothelial cells also activate Notch signaling in retinal macrophages. It is possible that 2 Dll4-positive tip cells do not effectively interact, and that a Notch1-positive macrophage can bridge and facilitate the connection of these 2 endothelial cells. Proximally in the vascular plexus, where Dll4 is not highly expressed by endothelial cells, other Notch ligands expressed by endothelial or mural cells may activate Notch signaling in macrophages at vascular branchpoints. We have previously found that Notch signaling affects macrophage function during inflammation11 and tumor angiogenesis (V.H.H.D., unpublished data, August 2010). It is therefore likely that activation of Notch signaling in macrophages because of interactions with ligand-expressing endothelial cells affects expression of target genes that mediate both macrophage and endothelial cell function during retinal angiogenesis.
Our data suggest that the current model of sprouting angiogenesis in the developing retina should be expanded. Studies of the effect of Notch inhibition on retinal vascular development or pathologic angiogenesis should take these interactions between macrophages and endothelial cells into account to develop a more comprehensive understanding of Notch as a therapeutic target in angiogenesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health: 5R01CA13667 and 5R01HL062454 (J.K.), F31HL090032 (H.H.O.), and T32DK07328 (N.M.K).
National Institutes of Health
Authorship
Contribution: H.H.O., N.M.K., I.W.T., and N.S. performed experiments; H.H.O and I.W.T. analyzed results; H.H.O. made the figures and wrote the manuscript; and J.K. provided oversight for the research and guidance in preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Kitajewski, PhD, 1130 St Nicholas Ave, Irving Cancer Research Center, New York, NY 10032; e-mail: jkk9@columbia.edu.