Abstract
The antiapoptotic BCL-2 proteins regulate lymphocyte survival and are over-expressed in lymphoid malignancies, including chronic lymphocytic leukemia. The small molecule inhibitor ABT-737 binds with high affinity to BCL-2, BCL-XL, and BCL-W but with low affinity to MCL-1, BFL-1, and BCL-B. The active analog of ABT-737, navitoclax, has shown a high therapeutic index in lymphoid malignancies; developing a predictive marker for it would be clinically valuable for patient selection or choice of drug combinations. Here we used RT-PCR as a highly sensitive and quantitative assay to compare expression of antiapoptotic BCL-2 genes that are known to be targeted by ABT-737. Our findings reveal that the relative ratio of MCL-1 and BFL-1 to BCL-2 expression provides a highly significant linear correlation with ABT-737 sensitivity (r = 0.6, P < .001). In contrast, antiapoptotic transcript levels, used individually or in combination for high or low affinity ABT-737-binding proteins, could not predict ABT-737 sensitivity. The (MCL-1 + BFL-1)/BCL-2 ratio was validated in a panel of leukemic cell lines subjected to genetic and pharmacologic manipulations. Changes after ABT-737 treatment included increased expression of BFL-1 and BCL-B that may contribute to treatment resistance. This study defines a highly significant BCL-2 expression index for predicting the response of CLL to ABT-737.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in the Western world.1 It is characterized by an expansion of small mature B cells in the peripheral blood, lymph nodes, and bone marrow. The standard of care for medically fit patients involves sequential cycles of chemo-immunotherapy with the purine nucleoside analog fludarabine combined with cyclophosphamide and the anti-CD20 monoclonal antibody rituximab.2,3
The BCL-2 family proteins regulate apoptosis primarily at the mitochondria through the intrinsic apoptotic pathway.4,5 These proteins are divided into 3 classes based on sequence homology (Bcl-2 homology domains BH1-BH4) and function.6,7 The antiapoptotic proteins that display sequence homology in all BH1 to BH4 domains are BCL-2, BCL-XL, BCL-W, MCL-1, BCL2A1 (BFL-1, A1), and Bcl-b. The proapoptotic Bcl-2 family members are divided into multidomain effectors (BAX and BAK containing BH1-BH3 domains) and the BH3-only proteins (BIM, PUMA, and NOXA). Increased expression of the antiapoptotic proteins represents the main factor that accounts for blocking the intrinsic apoptosis pathway. This inhibition of apoptosis is accomplished by sequestering proapoptotic proteins and thus preventing mitochondrial outer membrane permeabilization.8-11
Elevated levels of BCL-2, MCL-1, and BCL-XL mRNA and protein are found in many solid tumors and hematologic malignancies, including CLL.12-16 Importantly, BCL-2 and MCL-1 protein expression parallels their mRNA levels in diffuse large B-cell lymphoma and CLL.17,18 In most cases of follicular lymphoma, high BCL-2 levels could be caused by chromosomal translocation t(14;18), which places the BCL-2 gene under the control of the IgH enhancer, leading to overproduction of the BCL-2 transcript and protein.19 In CLL, loss of microRNAs (miR) is involved in regulation of the Bcl-2 family expression levels. 13q14 is the most commonly detected chromosomal deletion in CLL that leads to loss of miR-15a and miR-16, which causes increased BCL-2 levels.20 In addition, MCL-1 can also be regulated by miRs, as miR-29 is known to negatively regulate MCL-1 levels.21 Several reports suggest that Bcl-2 family proteins can be used as a predictive marker for the CLL response to chemotherapy. The MCL-1/BAX ratio was reported to correlate with chemo-resistance to fludarabine and rituximab.22,23 The BCL-2/BAX ratio was also associated with resistance to chlorambucil.24 In addition, BCL-XL levels were found to correlate with resistance to many standard chemotherapeutic agents in the 60-tumor cell line National Cancer Institute (NCI) panel.25
ABT-737 was developed as a rationally designed, small-molecule inhibitor that binds with high affinity to BCL-2, BCL-XL, and Bcl-w but with much lower affinity to MCL-1, BFL-1, and BCL-B.26 The orally available BCL-2–family inhibitor navitoclax (ABT-263) is now in clinical development for lymphoid malignancies, including CLL, both as a single agent and in combination with chemotherapy and/or monoclonal antibodies.26 Navitoclax showed a high therapeutic index in drug-resistant lymphoid malignancies with low incidence of off-target toxic effects in a phase 1 dose-escalation study.27 Moreover, preliminary efficacy data from a phase 1 study with navitoclax (ABT-263) in patients with small cell lung carcinoma (SCLC) and other solid tumors are encouraging as they show that the drug is safe and well tolerated.28 ABT-737 induces apoptosis in primary follicular lymphoma, CLL, acute myeloid leukemia, and solid tumor cells.5,26,29 Interestingly, tumor cell lines tested for ABT-737 response in cell culture have shown similar activity in xenografts derived from the same cell lines in vivo.26,30
Sensitivity to ABT-737 is thought to depend on a BCL-2 complex enriched for BIM.5,6 High levels of MCL-1 were also suggested to account for ABT-737 resistance mostly based on cell line studies.29,31,32 In contrast, other studies report that there is no correlation between MCL-1 expression and the response to ABT-737 in primary CLL and in acute lymphoblastic leukemia xenografts.5,33-35 Moreover, the ratios of BCL-2/BIM, BCL-2/BAX, MCL-1/BIM, or MCL-1/BAX could not predict sensitivity to ABT-737 in primary CLL.34 Interestingly, long-term exposure to ABT-737 resulted in increased levels of both RNA and protein levels of Mcl-1 and Bfl-1 and development of resistance in lymphoma cell lines that were initially sensitive.36
There are currently no satisfactory predictive markers for the wide range of responses to ABT-737 in primary lymphoid cells. Developing a predictive index for ABT-737 response may help in patient selection or choice of drug combinations. In this study, we examined whether quantifying expression of all antiapoptotic Bcl-2 family transcripts whose encoded proteins are known to be targeted by ABT-737 could be used to identify a gene expression index that predicts ABT-737 sensitivity. Here, we used a highly sensitive and quantitative real-time (RT-PCR) assay to examine the expression of 6 antiapoptotic BCL-2 family genes. We defined the relative expression of (MCL-1 + BFL-1)/BCL-2 as the most significant predictive marker for the response of primary CLL and leukemic cell lines to ABT-737 treatment.
Methods
Cell culture
Peripheral blood samples from 57 patients with CLL and 6 healthy donors were obtained with the patients' informed consent according to protocols approved by the Cleveland Clinic Institutional Review Board according to the Declaration of Helsinki. All primary CLL cells were freshly processed without freezing. Lymphocytes were purified by Ficoll-Paque PLUS (GE Healthcare) gradient centrifugation. Cells were washed twice in media and cultured at a density of 1 × 106 cells/mL in RPMI 1640 medium supplemented with 5% FBS (Atlanta Biologicals), l-glutamine (Invitrogen), and 100 units/mL penicillin-streptomycin (Invitrogen). Primary CLL cells were treated for 18 hours with ABT-737 at 50nM, a concentration previously shown to be effective in most primary CLL patients tested.34 Nine leukemic cell lines were used in the study: (1) 3 CLL (Mec-1, Mec-2, and PTA-3920), and (2) 2 Pre-B (Nalm-6 and Reh), 2 B cell–derived (NCI-H929 and IM-9), and 2 T cell–derived (Jurkat and Molt-4). These cell lines were obtained from ATCC, with the exception of Mec-1 and Mec-2, which were a gift from Dr Y. Saunthararajah (Cleveland Clinic). The cell lines, CLL primary cells, and human lymphocytes obtained from healthy donors were all cultured under similar conditions. ABT-737 was obtained from Abbott Laboratories.
Purification of lymphocytes from healthy donors
Highly purified lymphocytes were obtained using the Gambro Elutra Cell Separation System. After the apheresis procedure, cells were washed in a saline/tricitrasol solution before elutriation. Cell composition was verified on Cell Dyn 3700 (Abbott Diagnostics) before elutriation. Cells were separated using a continuous countercurrent buffer flow of Hanks Balanced Salt Solution (Lonza Verviers), with simultaneous centrifugation, using the Caridian Elutra Cell Separation System (CaridianBCT). Fractions were centrifuged at 1425g for 5 minutes at 4°C and pooled to concentrate the product. Purity of the final combined product as determined on the Cell Dyn 3700 was > 97%.
Flow cytometry
Cell death was assessed by phosphatidylserine externalization as we have previously described.37 After 18 hours of culture, primary CLL cells and established cell lines were stained with fluorescein-conjugated annexin V (BD Biosciences) and propidium iodide and analyzed on a BD FACSCalibur flow cytometer. Raw data were analyzed using the CellQuest Version 5.2.1 software. Results were normalized to survival of untreated cells. Spontaneous cell death in freshly derived CLL cells was < 30%.
Flow cytometric immunophenotyping was performed at the Cleveland Clinic as part of the diagnostic evaluation on a FACSCanto instrument (BD Biosciences). Each of these flow cytometric evaluations was performed using fluorescently labeled monoclonal antibodies against CD38 and ZAP-70. Staining protocols were standard lyses/washing protocols, as previously described.37
Fluorescence in situ hybridization
A dual-color probe for CLL panel 1 (ATM/p53) and a tri-color probe for CLL panel 2 (D13S319/13q34/CEP12; Abbott Molecular) were used. Fluorescence in situ hybridization was performed according to the manu-facturer's instructions. The p53 probe corresponds to chromosomal region 17p13.1. The ATM probe corresponds to chromosome region 11q22.3. Chromosomal region 13q34 was detected by the D13S319 probe. Trisomy of chromosome 12 was detected by the CEP12 probe. Abnormalities of 17p present in ≥ 15% of nucleated cells were considered positive. Abnormalities of other probes (13q34, CEP12, and ATM) present in ≥ 10% of nucleated cells were considered positive.
Pharmacologic and genetic manipulation of MCL-1 and BFL-1
Flavopiridol was obtained from Sanofi-Aventis through the NCI Cancer Therapy Evaluation Program. All the ABT-737–resistant cell lines (Mec-1, Mec-2, PTA-3920, NCI-H929, and Nalm-6) were treated with flavopiridol for 4 hours with a 300nM concentration, which was reported to be effective in decreasing MCL-1 levels.36 After the drug was removed, cells were then treated with 100nM of ABT-737 (50% effective concentration [EC50] of sensitive cell lines) for 18 hours.
MCL-1 and BFL-1 knockdown in Mec-1 cells was achieved by shRNAs delivered with the use of lentiviral-mediated transduction. Cell populations with stable expression of SHMCL-1, SHBFL-1, and as control a scrambled shRNA (shControl) were selected by puromycin (Sigma-Aldrich). Lentiviral particles targeting 3 different regions of MCL-1 (sc-35877-V), and BFL-1 (sc-37285-V), and the scrambled shRNA (sc-108080) were obtained from Santa Cruz Biotechnology.
RNA isolation and quantitative RT-PCR
Total RNA was isolated using the Trizol method (Invitrogen); 2 μg of RNA was reverse transcribed using the TaqMan reverse transcription kit and amplified using the SYBR Green Master Mix (Applied Biosystems, N8080234 and 4309155) and examined on a 7500 Real-Time PCR system (Applied Biosystems). Quantitative, real-time RT-PCR was performed as we have described previously,38 using the following specific primers: BCL-B: 5′-GCTGGGATGGCTTTTGTCA-3′ (forward) and 5′- GCCTGGACCAGCTGTTTTCTC-3′ (reverse); Bcl-w: 5′-ACCCCAGGCTCAGCCCAACA-3′ (forward) and 5′-CAGCACACAGTGCAGCCCCA-3′ (reverse); BCL-239 : 5′-GGAGGATTGTGGCCTTCTTT-3′ (forward) and 5′-GGAGGATTGTGGCCTTCTTT-3′ (reverse); BCL-XL40 : 5′-GGATGGC CACTTACCTGA-3′ (forward) and 5′-GCCGTACAGTTCCACAAAGG-3′ (reverse); Bfl-136 : 5′-TTACAGGCTGGCTCAGGACT-3′ (forward) and 5′-AGCACTCTGGACGTTTTGCT-3′ (reverse); MCL-136 : ATGCTTCGGAAACTGGACAT-3′ (forward) and 5′-TCCTGATGCCACCTTCT AGG-3′ (reverse); BIM41 : 5′-TGGCAAAGCAACCTTCTGATG-3′ (forward) and 5′-GCAGGCTGCAATTGTCTACCT-3′ (reverse); NOXA42 : 5′-TGGAAGTCGAGTGTGCTACTCAA-3′ (forward) and 5′-CAGAAGA GTTTGGATATCAGATTCAGA-3′ (reverse); PUMA42 : 5′-GCATGCCTGCCTCACCTT-3′ (forward) and 5′-TCACACGTCGCTCTCTCTAAACC-3′ (reverse); and ACTIN36 : 5′-AGAAAATCTGGCACCACACC-3′ (forward) and 5′-AGAGGCGTACAGGGATAGCA-3′ (reverse). Each experiment was repeated twice. The BCL-B and BCL-W primers were designed using the Primer Express (Applied Biosystems) and Primer 3 (LIST sequence) Version 0.4.0 software, respectively. A lymphocyte sample set was used to establish a baseline comparison between mRNA levels in CLL and healthy donors. The 6 mRNAs were expressed at relatively similar levels in all the healthy donors (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The extent of knockdown of MCL-1 and BFL-1 levels was determined by quantitative RT-PCR using the primers (in this paragraph) as well as those suggested by the manufacturer (Santa Cruz Biotechnology) for MCL-1 (sc-35877-PR) and BFL-1 (sc-37285-PR).
Statistical analysis
Baseline characteristics were grouped by in vitro culture sensitivity to ABT-737 treatment and compared between the resulting 2 groups using Fisher exact test or t test. Spearman correlation was used to assess the strength and direction of association between post-treatment cell viability and each BCL-2 family member individually or in combination. The Cohen interpretation of correlation (r) indicates that when r < 0.3, it represents weak correlation, 0.3 to 0.5 is moderate, and > 0.5 is strong. Three groups were defined based on cell viability. Expression of BCL-2 family members, individually or in combination, were compared among the 3 groups using the Kruskal-Wallis test. Pairwise comparisons between groups were performed using the Wilcoxon rank-sum test. A Bonferroni correction was used for pairwise comparisons. Pretreatment to post-treatment changes in BCL-2 family transcripts were assessed using the Wilcoxon signed rank test. All statistical tests were 2-sided. For pairwise comparisons, P < .017 was used to indicate statistical significance. For all other comparisons, P < .05 was used to indicate statistical significance.
Results
Primary CLL cells display differential sensitivity to ABT-737
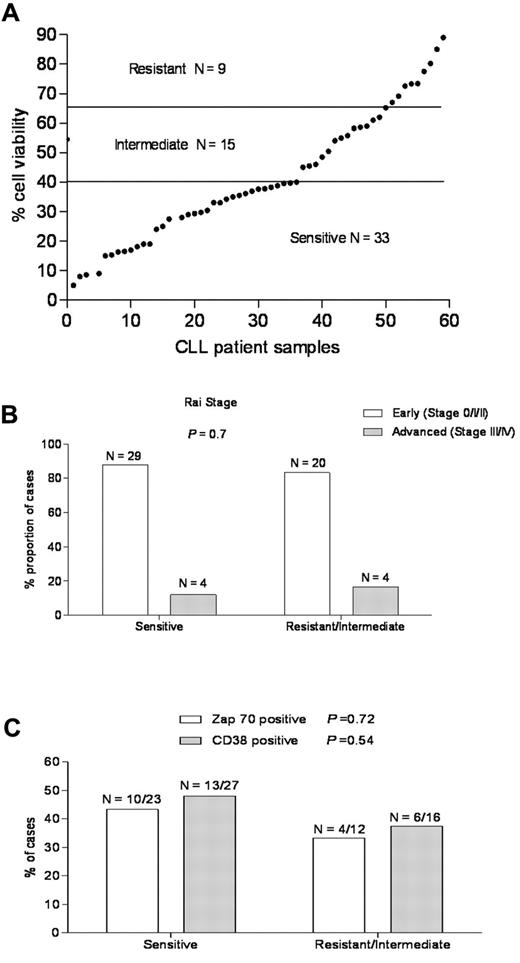
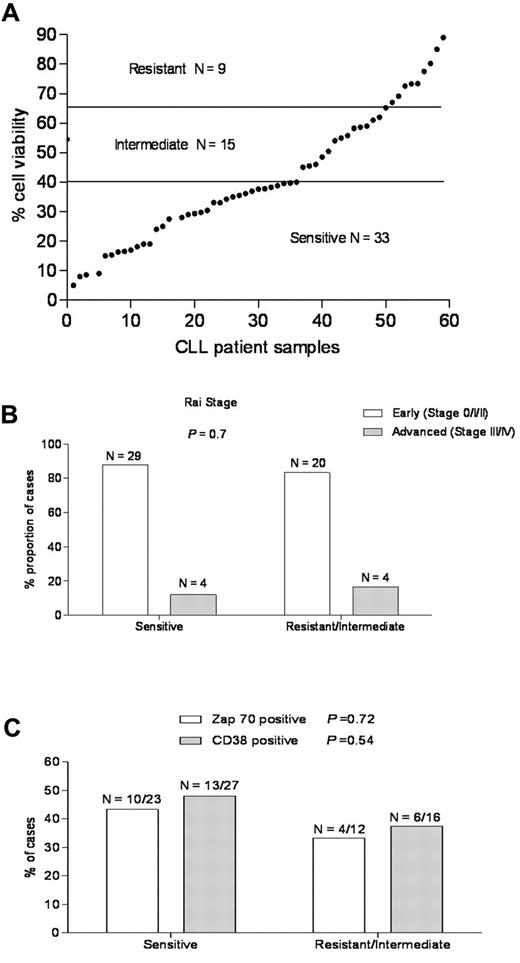
To determine the sensitivity of CLL to ABT-737, freshly isolated primary CLL cells derived from the peripheral blood of patients were examined by flow cytometry for annexin V staining as a cell death assay.37 The majority of primary CLL samples underwent apoptosis after treatment with ABT-737; however, there was a wide range of sensitivity, in agreement with earlier reports (Figure 1A).26,34 Based on their response, primary CLL samples from 57 patients could be classified into 3 groups (Table 2): (1) a sensitive group (33 of 57 cases, 57.8%) with cell viability < 40%, (2) an intermediate group (15 of 57, 26.3%) with viability of 40% to 65%, and (3) a resistant group (9 of 57, 15.7%) with viability > 65%. Increasing the time exposures of ABT-737 from 18 to 36 hours did not change the sensitivity groups for the CLL samples (data not shown).
A wide range of responses to ABT-737 in primary CLL independent of known prognostic markers. (A) Primary CLL cells derived from 57 patients were incubated with ABT-737 (50nM) for 18 hours. Cell survival was assessed by annexin V/fluorescein isothiocyanate binding, as determined by flow cytometric analysis. Values are normalized to survival of untreated cells, and each data point represents one CLL patient. Based on cell viability data, the response to ABT-737 was classified as: resistant (> 65%), intermediate (40%-65%), and sensitive (< 40%). (B-C) The proportion of CLL patients in the resistant/intermediate and sensitive groups, respectively was examined with regard to the common CLL prognostic markers: (B) Rai stage and (C) Zap-70, CD38 expression. P values are shown for comparison of groups.
A wide range of responses to ABT-737 in primary CLL independent of known prognostic markers. (A) Primary CLL cells derived from 57 patients were incubated with ABT-737 (50nM) for 18 hours. Cell survival was assessed by annexin V/fluorescein isothiocyanate binding, as determined by flow cytometric analysis. Values are normalized to survival of untreated cells, and each data point represents one CLL patient. Based on cell viability data, the response to ABT-737 was classified as: resistant (> 65%), intermediate (40%-65%), and sensitive (< 40%). (B-C) The proportion of CLL patients in the resistant/intermediate and sensitive groups, respectively was examined with regard to the common CLL prognostic markers: (B) Rai stage and (C) Zap-70, CD38 expression. P values are shown for comparison of groups.
Baseline characteristics of patients with ABT-737–sensitive and –resistant CLL are similar
Baseline characteristics of CLL patients in this study (Table 1; Figure 1B-C) were grouped by in vitro culture sensitivity to ABT-737 treatment. The median age was similar for both groups. The majority of patients in both the ABT-737–sensitive and –resistant groups had early stage (Rai 0, I, or II) disease and had not been treated for their CLL before ABT-737 testing. The proportion of cases positive for Zap70 and CD38 by flow cytometry and commonly detected chromosomal abnormalities by fluorescence in situ hybridization testing that are indicated do not differ significantly between the ABT-737–sensitive and –resistant groups. ABT-737 sensitivity was similar for patients with early versus advanced stage of the disease (P = .7; Figure 1B). It was also similar for common prognostic markers for CLL, such as Zap-70 (P = .72) or CD38 (P = .54) expression43,44 (Figure 1C), prior treatment, 17p deletion (p53),45,46 or 13q14 deletion (Table 1), consistent with previous findings.5,33,34
Lack of correlation between expression of BCL-2 family members, individually or combined, and their response to ABT-737
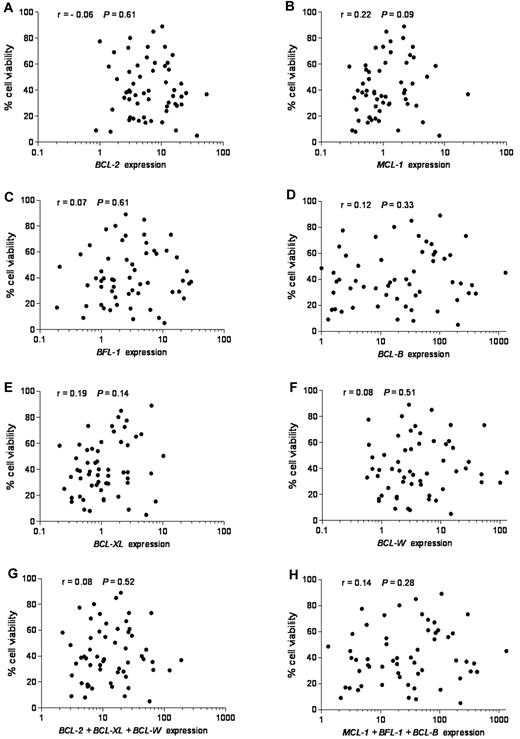
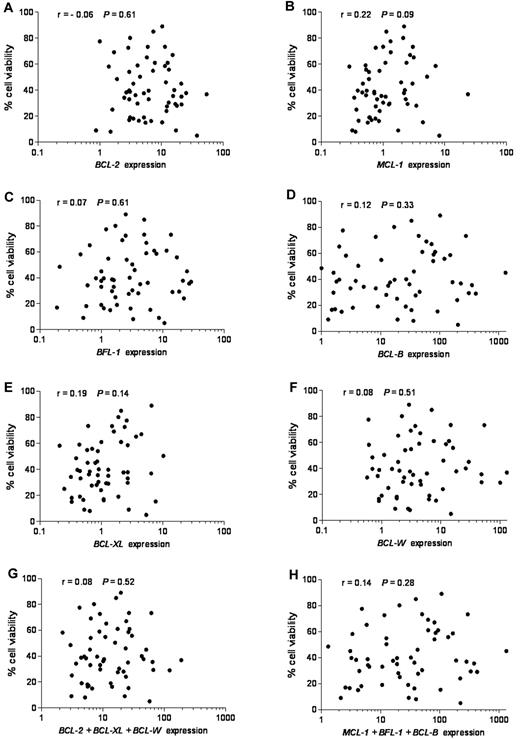
To investigate the possible contribution of BCL-2 family members to ABT-737 response, we determined their expression levels. Of the 9 BCL-2 family members that we have examined, we focused first on the 6 antiapoptotic members because ABT-737 was designed to target antiapoptotic proteins, and we reasoned that their differential expression could define ABT-737 sensitivity. Expression of each individual antiapoptotic transcript or their combinations in untreated CLL was plotted against cell viability after ABT-737 treatment (Figure 2). No linear correlation was found between expression levels of any single antiapoptotic BCL-2 family member (r < 0.3, P > .05) or their combination (r < 0.3, P > .05), considering those with either high affinity (BCL-2, BCL-W, and BCL-XL) or those with low affinity (MCL-1, BFL-1, and MC-1) to ABT-737 (Figure 2). Moreover, MCL-1 levels were more abundant in normal lymphocytes compared with BFL-1, BCL-B, and BCL-W, which were expressed at very low levels (supplemental Figure 1B; note logarithmic scale used to accommodate these large differences). In contrast, BCL-B, BFL-1, and BCL-W were highly up-regulated compared with MCL-1 in primary CLL. Next, we examined the expression of proapoptotic BH3-only BCL-2 family members NOXA, BIM, and PUMA. Their mRNA levels were relatively high in most of the tested CLL (supplemental Figure 2). Nevertheless, no significant correlation was found between expression levels of any of these proapoptotic BCL-2 family members, taken individually (r < 0.3, P > .05) or in combination (r < 0.3, P > .05) and ABT-737 sensitivity.
Lack of correlation between mRNA expression of antiapoptotic BCL-2 family members, individually or combined, and response to ABT-737. Quantitative RT-PCR was used to study antiapoptotic BCL-2 family mRNAs. Expression values of each antiapoptotic transcript or their combinations were plotted against cell viability after ABT-737 treatment. RNA expression for each transcript was normalized to that obtained in lymphocytes isolated from 6 healthy donors. (A) BCL-2, (B) MCL-1, (C) BFL-1 (BCL2A1), (D) BCL-B, (E) BCL-XL, (F) BCL-W, (G) BCL-2 + BCL-XL + BCL-W, and (H) MCL-1 + BFL-1 + BCL-B expression. Spearman correlation (r) and P values are shown.
Lack of correlation between mRNA expression of antiapoptotic BCL-2 family members, individually or combined, and response to ABT-737. Quantitative RT-PCR was used to study antiapoptotic BCL-2 family mRNAs. Expression values of each antiapoptotic transcript or their combinations were plotted against cell viability after ABT-737 treatment. RNA expression for each transcript was normalized to that obtained in lymphocytes isolated from 6 healthy donors. (A) BCL-2, (B) MCL-1, (C) BFL-1 (BCL2A1), (D) BCL-B, (E) BCL-XL, (F) BCL-W, (G) BCL-2 + BCL-XL + BCL-W, and (H) MCL-1 + BFL-1 + BCL-B expression. Spearman correlation (r) and P values are shown.
An antiapoptotic Bcl-2 family index predicts ABT-737 sensitivity
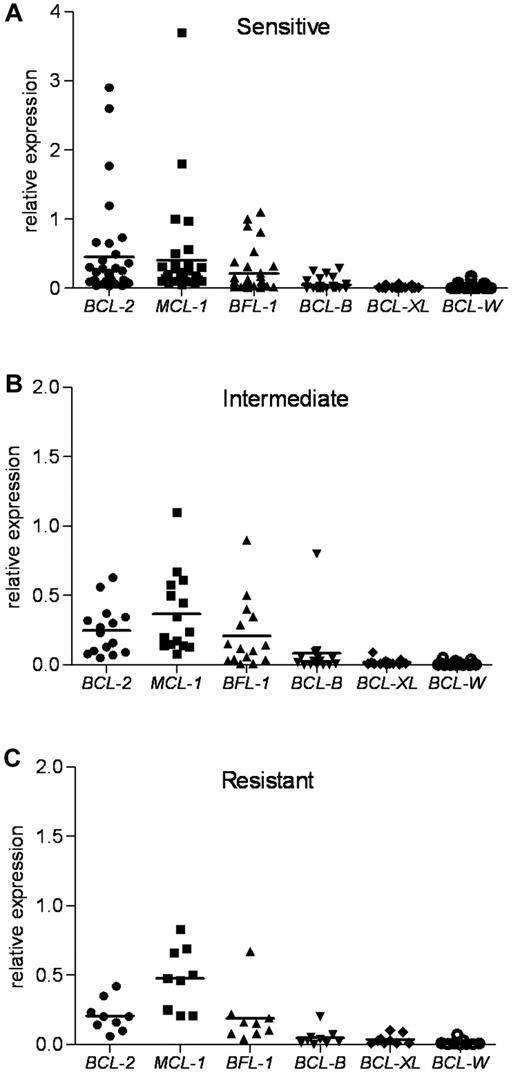
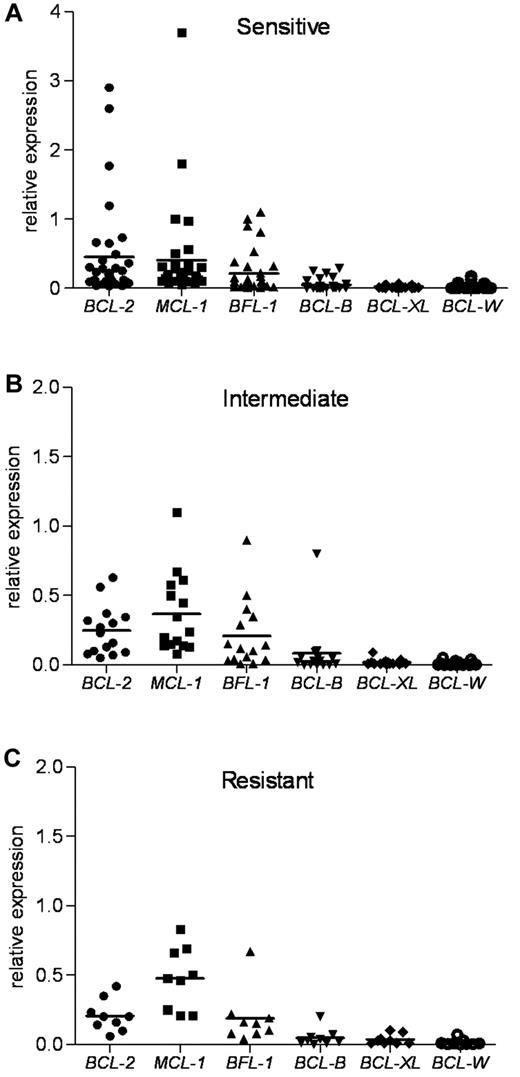
Because each antiapoptotic transcript was expressed at different basal levels in the lymphocytes from healthy donors (supplemental Figure 1B), we next standardized the expression of the 6 mRNAs to one basal level to provide a better method of comparison. Normalization to β-actin revealed the most abundant antiapoptotic determinants in untreated primary CLL (Figure 3). This approach guided us to identify the critical determinants of the ABT-737 sensitivity. The expression levels of MCL-1, BCL-2, and BFL-1 were much higher compared with those of BCL-XL and BCL-W. The sensitive group had the highest levels of BCL-2 compared with expression of MCL-1 and BFL-1. The index mean was 0.45 for BCL-2, 0.40 for MCL-1, and 0.21 for BFL-1. In contrast, compared with BCL-2, the intermediate group had relatively higher levels of MCL-1 and BFL-1, whereas the resistant group had much higher levels of MCL-1 and BFL-1. In the intermediate group, the index mean was 0.25 for BCL-2, 0.37 for MCL-1, and 0.21 for BFL-1. In the resistant group, the index mean was 0.21 for BCL-2, 0.48 for MCL-1, and 0.19 for BFL-1. Next, we examined the expression of proapoptotic BH3-only BCL-2 family members NOXA, BIM, and PUMA. NOXA levels were the highest compared with those of BIM and PUMA in the CLL samples examined (supplemental Figure 3). Thus, NOXA levels were 3.5- and 6-fold higher than those of BIM and PUMA, respectively.
Antiapoptotic BCL-2 family mRNA levels in ABT-737 sensitive, intermediate, and resistant CLL groups. RNA expression of antiapoptotic Bcl-2 family members, as determined by quantitative RT-PCR, was normalized to β-actin for each CLL patient sample. BCL-2, MCL-1, and BCL2A1 (BFL-1) levels were more abundant than those of BCL-B, BCL-XL, and BCL-W in primary CLL. (A) The sensitive group. (B) The intermediate group. (C) The resistant group. Mean values are shown.
Antiapoptotic BCL-2 family mRNA levels in ABT-737 sensitive, intermediate, and resistant CLL groups. RNA expression of antiapoptotic Bcl-2 family members, as determined by quantitative RT-PCR, was normalized to β-actin for each CLL patient sample. BCL-2, MCL-1, and BCL2A1 (BFL-1) levels were more abundant than those of BCL-B, BCL-XL, and BCL-W in primary CLL. (A) The sensitive group. (B) The intermediate group. (C) The resistant group. Mean values are shown.
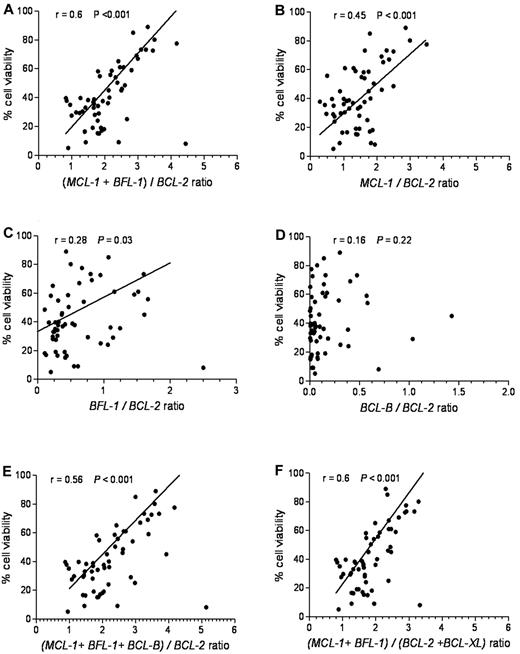
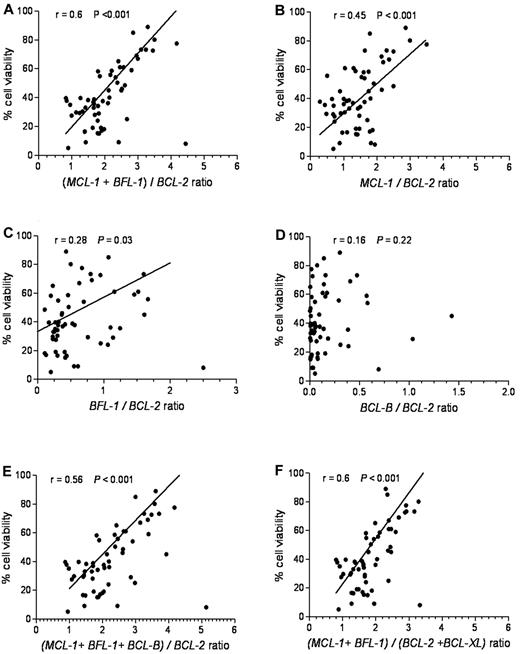
To define an antiapoptotic gene expression index predictive of ABT-737 sensitivity, we plotted several combinations of genes relative to cell viability. The relative ratio of MCL-1 and BFL-1 to BCL-2 expression provided the best linear correlation with ABT-737 sensitivity (r = 0.6, P < .001; Figure 4A). By com-parison, the relative ratios of MCL-1/BCL-2 (Figure 4B) or BFL-1/BCL-2 (Figure 4C) showed a less robust linear correlation: r = 0.45, P < .001 and r = 0.28, P = .030, respectively. The relative ratio of BCL-B/BCL-2 (Figure 4D) showed no significant correlation (r = 0.16, P = .22). Including BCL-B, BCL-XL, (Figure 4E-F), or BCL-W (data not shown) into the (MCL-1 + BFL-1)/BCL-2 model had no significant effect on this correlation.
(MCL-1 + BFL-1)/BCL-2 provides the most significant linear correlation for sensitivity to ABT-737. Cell viability after ABT-737 treatment was plotted against: (A) (MCL-1 + BFL-1)/BCL-2, (B) MCL-1/BCL-2, (C) BFL-1/BCL-2, (D) BCL-B/BCL-2, (E) (MCL-1 + BFL-1 + BCL-B)/BCL-2, and (F) (MCL-1 + BFL-1)/(BCL2 + BCL-XL) ratios. Spearman correlation (r) and P values are shown.
(MCL-1 + BFL-1)/BCL-2 provides the most significant linear correlation for sensitivity to ABT-737. Cell viability after ABT-737 treatment was plotted against: (A) (MCL-1 + BFL-1)/BCL-2, (B) MCL-1/BCL-2, (C) BFL-1/BCL-2, (D) BCL-B/BCL-2, (E) (MCL-1 + BFL-1 + BCL-B)/BCL-2, and (F) (MCL-1 + BFL-1)/(BCL2 + BCL-XL) ratios. Spearman correlation (r) and P values are shown.
The different ratios of antiapoptotic BCL-2 family mRNAs were determined in the resistant, intermediate, and sensitive groups (supplemental Figure 4). Of the different antiapoptotic Bcl-2 family combinations evaluated, (MCL-1 + BFL-1)/BCL-2 provided the highest predictive value for the ABT-737 response in CLL (supplemental Figure 4A), with P < .001 among all the 3 groups. In the sensitive class (average cell viability 26.2%), the average ratio of (MCL-1 + BFL-1)/BCL-2 was 1.67. For the intermediate class (average cell viability 53.5%), the average ratio was 2.3. In the resistant class (average cell viability, 76.3), the average ratio was 3.3 (Table 2).
The (MCL-1 + BFL-1)/Bcl2 ratio is predictive of ABT-737 response in leukemic and lung carcinoma cells
To further address the generality of our predictive model in leukemic cells, the (MCL-1 + BFL-1)/BCL-2 ratio was determined in a panel of leukemic tumor lines (Table 3). Of the 9 cell lines tested, 5 (NCI-H929, Nalm-6, Mec-1, Mec-2, and PTA-3920) were most resistant to ABT-737. They all had a very high (MCL-1 +BFL-1)/BCL-2 ratio, ranging from 6.4-17.5, with EC50 > 1000nM. Two other cell lines, Reh and Molt-4, displayed an intermediate sensitivity to ABT-737 (EC50 = 300 and 400), having a ratio of 2.5 and 2.3, respectively. IM-9 and Jurkat were the most sensitive (EC50 ≤ 100nM) and had ratios of 1.2 and 1.8, respectively (Table 3). These data indicate that the (MCL-1 + BFL-1)/BCL-2 ratio is predictive for the ABT-737 response in a broad range of leukemic tumor cells, both primary and established.
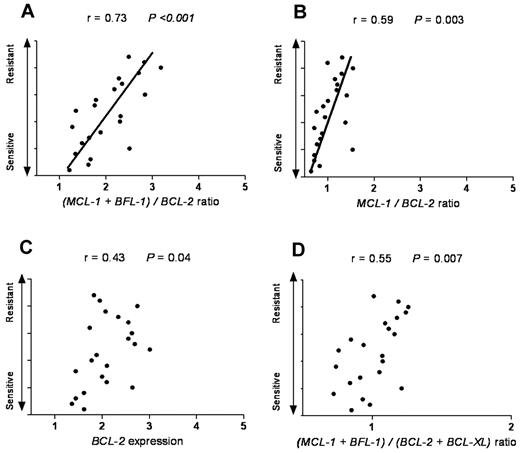
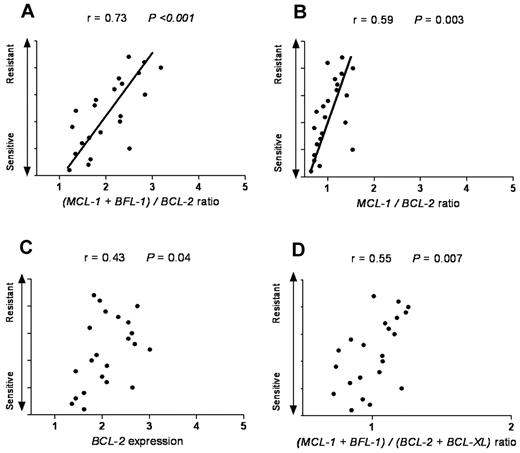
Next, we tested whether our model can also be applied to cancer types other than of hematologic origin. SCLC is a solid tumor that has been reported to have a high expression level of antiapoptotic proteins, such as BCL-2. As navitoclax (ABT-263) is evaluated in clinical trials in SCLC patients, we reasoned that it would be informative to test whether our model can predict ABT-737 response in SCLC cell lines. By using ONCOMINE47 as a cancer microarray database aimed to facilitate discovery from genome-wide expression analyses, we applied our model in SCLC cell lines previously categorized as ABT-737–resistant, –intermediate, and –sensitive groups.48 We found that in the sensitive group the average ratio of MCL-1 and BFL-1 to BCL-2 was 1.7, with the intermediate and resistant groups having a ratio of 2.1 and 2.8, respectively (Table 2). Interestingly, these ratios were very close and consistent with our findings in CLL patients. Of the different antiapoptotic Bcl-2 family combinations evaluated, the (MCL-1 + BFL-1)/BCL-2 ratio provided the highest predictive value for ABT-737 response in SCLC (r = 0.73, P < .001; Figure 5).
The (MCL-1 + BFL-1)/BCL-2 represents the most significant linear correlation for sensitivity to ABT-737 in SCLC. SCLC cell lines with a wide range of response (resistant, intermediate, and sensitive) to ABT-737 were plotted against: (A) (MCL-1 + BFL-1)/BCL-2, (B) MCL-1/BCL-2, (C) BCL-2, and (D) (MCL-1 + BFL-1)/(BCL-2 + BCL-XL) ratios. Spearman correlation (r) and P values are shown. The Olejniczak dataset was used as extracted from the ONCOMINE database.47
The (MCL-1 + BFL-1)/BCL-2 represents the most significant linear correlation for sensitivity to ABT-737 in SCLC. SCLC cell lines with a wide range of response (resistant, intermediate, and sensitive) to ABT-737 were plotted against: (A) (MCL-1 + BFL-1)/BCL-2, (B) MCL-1/BCL-2, (C) BCL-2, and (D) (MCL-1 + BFL-1)/(BCL-2 + BCL-XL) ratios. Spearman correlation (r) and P values are shown. The Olejniczak dataset was used as extracted from the ONCOMINE database.47
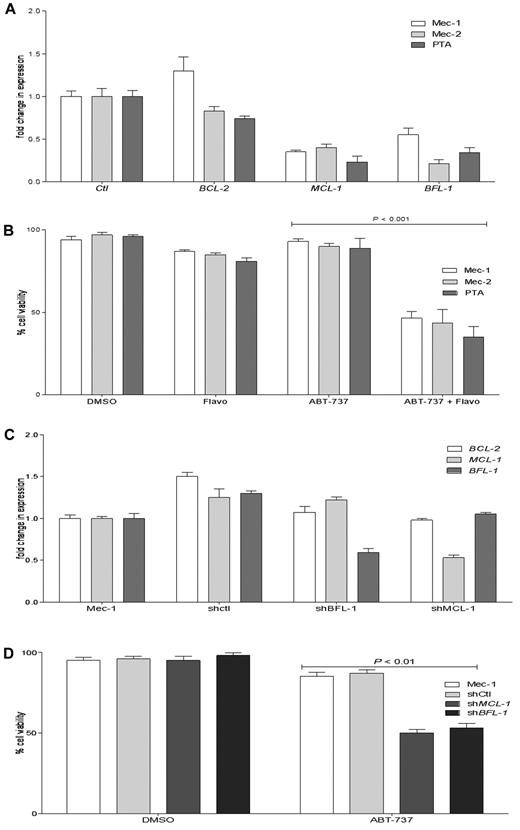
Decreased Mcl-1 and/or Bfl-1 levels enhance ABT-737 response in resistant CLL cell lines
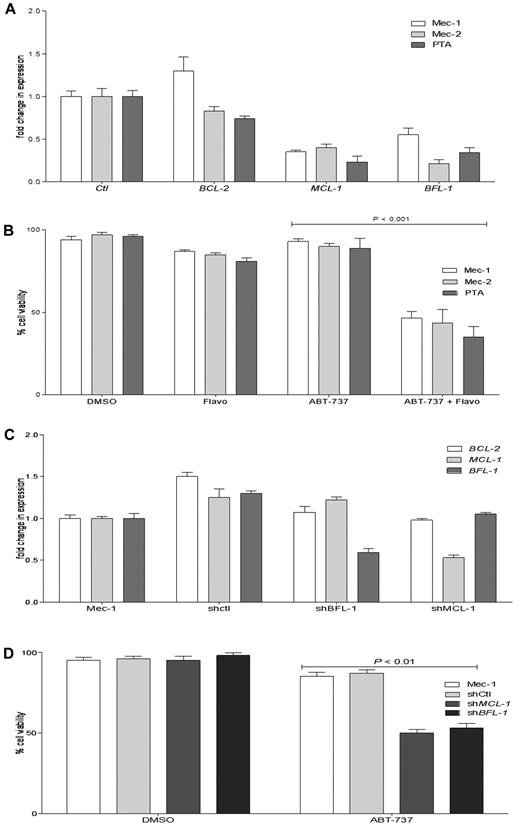
The 5 cell lines that we found to be highly resistant to ABT-737 had also a high ratio of MCL-1 and BFL-1 to BCL-2. We examined whether a decrease in MCL-1 and/or BFL-1 levels by a clinically relevant approach may improve ABT-737 response in resistant cell lines. Flavopiridol, which as a CDK9 inhibitor was shown to decrease MCL-1 at the transcriptional level, also demonstrated a high response rate in a phase 2 clinical trial in relapsed CLL patients.49 All ABT-737–resistant cell lines were treated with flavopridol, and then the BCL-2, MCL-1, and BFL-1 levels were determined by quantitative RT-PCR (Figure 6A; only data for CLL cell lines are shown). A 4-hour flavopiridol treatment decreased MCL-1 and BFL-1 levels, with little effect on BCL-2 levels. After pretreatment with flavopiridol, the (MCL-1 + BFL-1)/BCL-2 predictive index values changed in all 5 ABT-737-resistant cell lines, shifting from highly resistant to an index similar to what we have established for the sensitive CLL group (Table 4). To determine whether this shift of the (MCL-1 + BFL-1)/BCL-2 ratio by flavopiridol can convert the resistant cell lines to the sensitive group, after flavopiridol pretreatment and removal, cells were treated with ABT-737 for 18 hours (Figure 6B). There was a significant increase in cell death when cells were pretreated with flavopiridol before ABT-737 treatment compared with single treatments with ABT-737 or flavopiridol for 18 hours (data for NCI-H929 and Nalm-6 are not shown).
Flavopiridol and shRNA decrease MCL-1 and BFL-1 levels and increase the response to ABT-737 in resistant CLL cell lines. (A) Mec-1, Mec-2, and PTA CLL cells were treated with flavopiridol for 4 hours, and then BCL-2, MCL-1, and BFL-1 levels were assessed by quantitative RT-PCR. (B) Mec-1, Mec-2, and PTA-3920 were treated with flavopiridol for 4 hours; then the drug was removed and cells were cultured without or with ABT-737 (100nM) for an additional 18 hours. (C) Quantitative RT-PCR analyses of BCL-2, MCL-1, and BFL-1 levels in Mec-1 parental, SHMCL-1, SHBFL-1, and as a control, scrambled shRNA (shControl)–expressing cells. (D) Mec-1 parental, shControl, SHMCL-1, and SHBFL-1–expressing cells were treated with ABT-737 (1000nM) for 18 hours. Cell viability was assayed by annexin-V/FITC and propidium iodide staining and analyzed by flow cytometry. Values are normalized to untreated cells, and DMSO treatment served as a negative control. P values were determined by the 2-tailed t test. The graphs presented are representative of 2 independent experiments with data shown as mean ± SD.
Flavopiridol and shRNA decrease MCL-1 and BFL-1 levels and increase the response to ABT-737 in resistant CLL cell lines. (A) Mec-1, Mec-2, and PTA CLL cells were treated with flavopiridol for 4 hours, and then BCL-2, MCL-1, and BFL-1 levels were assessed by quantitative RT-PCR. (B) Mec-1, Mec-2, and PTA-3920 were treated with flavopiridol for 4 hours; then the drug was removed and cells were cultured without or with ABT-737 (100nM) for an additional 18 hours. (C) Quantitative RT-PCR analyses of BCL-2, MCL-1, and BFL-1 levels in Mec-1 parental, SHMCL-1, SHBFL-1, and as a control, scrambled shRNA (shControl)–expressing cells. (D) Mec-1 parental, shControl, SHMCL-1, and SHBFL-1–expressing cells were treated with ABT-737 (1000nM) for 18 hours. Cell viability was assayed by annexin-V/FITC and propidium iodide staining and analyzed by flow cytometry. Values are normalized to untreated cells, and DMSO treatment served as a negative control. P values were determined by the 2-tailed t test. The graphs presented are representative of 2 independent experiments with data shown as mean ± SD.
To further validate the pharmacologic inhibitor data, we applied a targeted genetic approach for reducing MCL-1 or BFL-1, using shRNAs transduced with lentivirus particles. Mec-1 was chosen because it is a highly ABT-737-resistant CLL cell line that has high MCL-1 and BFL-1 levels. Examining knockdown of MCL-1 and BFL-1 by quantitative RT-PCR indicated ∼ 50% and 45% reductions in MCL-1 and BFL-1 levels in SHMCL-1- and SHBFL-1–expressing Mec-1 cells, respectively (Figure 6C). Estimating the index in these 2 cell lines indicated that knockdown of MCL-1 or Bfl-1 by shRNA reduced the index from 9.6 in parental cells to 3.4 in shMCL-1– and 3.8 in shBFL-1-expressing Mec-1 cells, respectively. Treating shMCL-1– and shBFL-1–expressing cells with ABT-737 increased cell death significantly compared with parental Mec-1 cell or those expressing as a control scrambled shRNA (Figure 6D).
Modulation of antiapoptotic Bcl-2 family mRNA expression after ABT-737 treatment
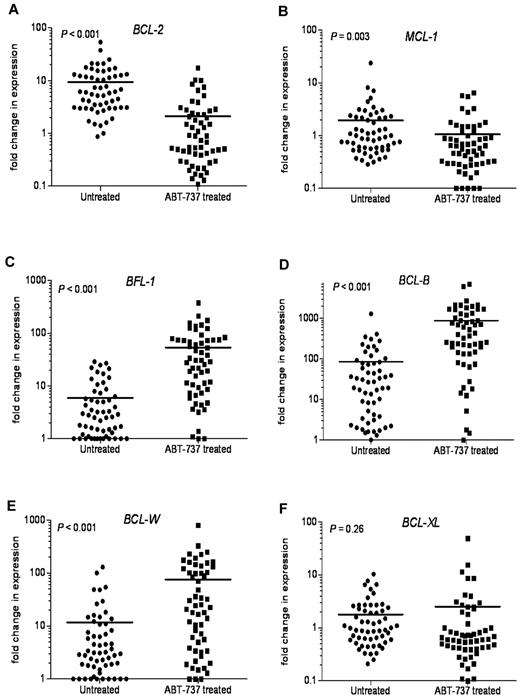
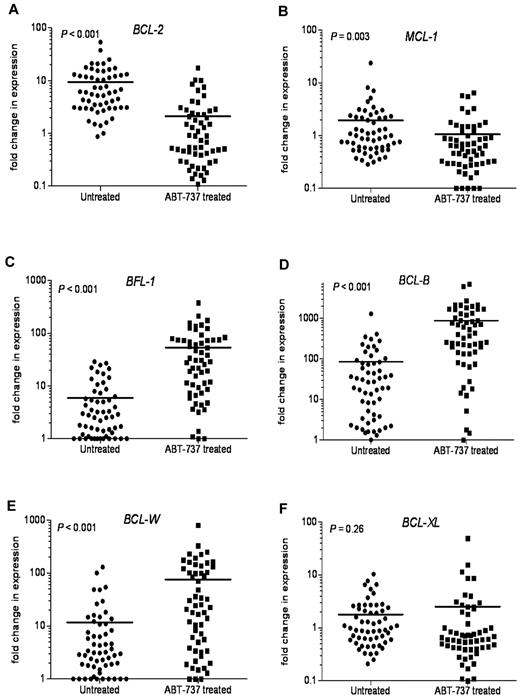
Because chemotherapeutics are known to modulate the expression of BCL-2 family members in hematologic tumors, we next examined the changes in expression of antiapoptotic BCL-2 family mRNAs after ABT-737 treatment. In most primary CLL samples tested, there was a significant decrease in BCL-2 (P < .001) and MCL-1 (P = .003) mRNA expression (Figure 7A-B). In contrast, BFL-1, BCL-B, and BCL-W expression levels were highly up-regulated after ABT-737 treatment (Figure 7C-E). BFL-1 levels were up-regulated 9-fold, (P < .001), with BCL-W levels increased 6.5-fold (P < .001). BCL-B levels were increased more than 10-fold after ABT-737 treatment (P < .001). There were no significant changes in BCL-XL levels (P = .26; Figure 7F).
Changes in the levels of antiapoptotic BCL-2 family transcripts after ABT-737 treatment. Changes in mRNA expression levels of antiapoptotic Bcl-2 family proteins after treatment with ABT-737 (50nM) for 18 hours. Expression was determined by quantitative RT-PCR to compare fold difference in expression with levels before treatment that were normalized to those found in lymphocytes isolated from 6 healthy donors. Fold change for: (A) BCL-2, (B) MCL-1, (C) BFL-1, (D) BCL-B, (E) BCL-W, and (F) BCL-XL levels. P values are shown.
Changes in the levels of antiapoptotic BCL-2 family transcripts after ABT-737 treatment. Changes in mRNA expression levels of antiapoptotic Bcl-2 family proteins after treatment with ABT-737 (50nM) for 18 hours. Expression was determined by quantitative RT-PCR to compare fold difference in expression with levels before treatment that were normalized to those found in lymphocytes isolated from 6 healthy donors. Fold change for: (A) BCL-2, (B) MCL-1, (C) BFL-1, (D) BCL-B, (E) BCL-W, and (F) BCL-XL levels. P values are shown.
Discussion
ABT-737 was rationally designed to bind selectively to the antiapoptotic proteins BCL-2, BCL-XL, and BCL-W, which are known to be overexpressed in lymphoid malignancies.15,16 There is no available marker that directly predicts the sensitivity to ABT-737 based on antiapoptotic protein expression levels. The inconsistency of reports on the correlation of the expression of antiapoptotic proteins with the ABT-737 response might be the result of the difficulty in detecting some of them by Western blotting in primary cells.5,33,34 This may explain the emphasis that has been placed on individual antiapoptotic proteins, such as BCL-2 and MCL-1, with no extensive studies available on the contribution of BFL-1 and BCL-B to the ABT-737 response in primary CLL. Moreover, the primary use of Western blotting in most of these studies further limited the development of quantitative approaches.
Here, we report that the antiapoptotic BCL-2 family mRNAs are expressed at different basal levels in lymphocytes from healthy donors. Therefore, comparing the basal expression levels of the antiapoptotic transcripts in CLL with healthy donors could be misleading. In contrast, normalizing these mRNAs to β-actin provided an equal scale for comparison of their expression in untreated primary CLL and further establishes their contribution to the ABT-737 response. We found that MCL-1, BCL-2, BFL-1, and BCL-B transcripts were more abundant than those of Bcl-xl and BCL-W. However, MCL-1, BFL-1, and BCL-2 appear to be the most critical antiapoptotic factors that account for ABT-737 sensitivity. Applying RT-PCR as a highly sensitive and quantitative assay allowed us to establish a quantitative comparison between the expression levels of BCL-2 family members. BCL-2, MCL-1, and BFL-1 expression was higher in cells sensitive to ABT-737, with BCL-2 expression being the highest. These findings may explain why the sensitivity to ABT-737 in primary CLL was correlated with high Mcl-1 levels in previous reports.33
In the moderately sensitive CLL group, MCL-1 and BFL-1 levels were higher than those of Bcl-2, whereas in the resistant group MCL-1 and BFL-1 expression was much higher compared with those of BCL-2. Our findings indicate that individual or even combined levels of MCL-1 and BFL-1 could not predict ABT-737 sensitivity without accounting for their relative ratio to BCL-2. This probably reflects the molecular abundance of MCL-1, BFL-1, and MCL-1, as well as the cellular balance between low- and high-affinity ABT-737–binding proteins. Importantly, our model directly addresses the wide range of ABT-737 responses in primary CLL that were reported previously.26,34 The antiapoptotic Bcl-2 family expression index in a panel of leukemic tumor cell lines was consistent with our CLL data. This model was also validated in SCLC as another tumor type. Recent data from a phase 1 study of navitoclax (ABT-263) has shown that a high BCL-2 copy number in circulating tumor cells was associated with the best tumor response in SCLC patients. Remarkably, our predictive model showed a higher correlation with ABT-737 response than expression of BCL-2 alone in these SCLC cell lines based on data extracted from the ONCOMINE database.47 There are currently 17 clinical trials (http://clinicaltrials.gov) with the BCL-2–family inhibitor navitoclax, the oral derivative of ABT-737, for CLL, other hematologic malignancies, and solid tumors for which our predictive model could also become informative.
We also studied the expression of proapoptotic BH3-only Bcl-2 members NOXA, BIM, and PUMA. These proapoptotic members are most likely to be sequestered by antiapoptotic proteins as the primed for cell death model predicts.6 This model posits that cells expressing significant amounts of BH3-only proteins, such as Bim, must sequester them with antiapoptotic proteins to stay alive. The sensitive CLL group had increased levels of NOXA, BIM, and PUMA. However, as some resistant and intermediate groups also had high NOXA, BIM, and PUMA levels, we could not establish a predictive value for a BH3-only index and ABT-737 response in the CLL samples we have examined. Nevertheless, it is possible that including their expression in a BCL-2 family index may help to predict clinical response to other therapeutics or in other tumor types.
We have applied both a clinically relevant pharmacologic (flavopiridol) as well as a genetic (shRNA) approach to reduce MCL-1 and BFL-1 levels to improve the ABT-737 response in the resistant cell lines. shRNA-expressing cells, in which expression of either MCL-1 or BFL-1 was down-regulated, were treated with a higher ABT-737 concentration compared with flavopiridol in response to which both MCL-1 and BFL-1 levels were decreased. In addition, there was some compensation by an increase in the levels of alternative apoptosis-resistance determinants, MCL-1 in SHBFL-1 and BFL-1 in SHMCL-1–expressing cells. Nevertheless, these data indicate that clinical agents that reduce MCL-1 and BFL-1 levels can be used in combination with navitoclax to maximize their efficacy.
After ABT-737 treatment, we observed induction of BFL-1, BCL-B, and BCL-W expression and reduction of BCL-2 and MCL-1 levels in most CLL samples. BFL-1 and BCL-B are known to have very low binding affinity for ABT-737; therefore, their increased levels suggest that these proteins may account for resistance as a result of long-term ABT-737 exposure, as a published report indicates.36 Here we show that BFL-1 levels can be up-regulated after ABT-737 treatment in primary CLL not only, as previously reported, as a result of co-culture of primary CLL with fibroblasts that mimics the lymph node environment.33 As this is the first report that reveals the elevated BCL-B levels after ABT-737 treatment, this finding suggests a potential novel role for BCL-B in developing ABT-737 resistance in CLL patients.
In conclusion, we have applied RT-PCR as a highly sensitive and quantitative assay to examine BCL-2 family transcripts in primary CLL to develop a unique predictive marker for ABT-737 sensitivity. Our model provides highly predictive power for ABT-737 sensitivity in primary CLL. This approach requires a small number of primary cells and allows the study of comparative expression of all relevant genes. It can also identify whether CLL patients are dependent on MCL-1/BFL-1 or on BCL-2 as MCL-1–dependent leukemic cells have been reported to be more chemo-sensitive than those that are BCL-2 dependent.50 Establishing this dependency may guide appropriate ABT-737 administration for future development of novel combinations of ABT-737 with other targeted agents.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Saul Rosenberg and Steven Elmore (Abbott Laboratories) for ABT-737.
This work was supported by the National Institutes of Health (research grant CA127264, A.A.) and Saudi Arabia Education Ministry Fellowship (S.A). Elutriation was supported in part by the National Institutes of Health/National Center for Research Resources, Cleveland (Clinical and Translational Science Award UL1RR024989).
National Institutes of Health
Authorship
Contribution: S.A., B.T.H., and A.A. participated in designing the research; S.A., K.S., S.M., and G.C. performed the research and data analysis; J.D.V. assisted with flow cytometry; B.T.H., M.K., and J.P.M. contributed the patient samples and participated in their characterization; L.A.R. provided the statistical support; S.A., B.T.H., J.A.H., and A.A. wrote the paper; and all authors examined the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandru Almasan, Department of Cancer Biology, Lerner Research Institute, Cleveland Clinic, NB40, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: almasaa@cf.org.